Abstract
Nucleotides present an important role in ocular physiology which has been demonstrated by recent works that indicate their involvement in many ocular processes. P2Y are important among P2 receptors since they can control tear production, corneal wound healing, aqueous humour dynamics and retinal physiology. Commercial antibodies have allowed us to investigate the distribution of P2Y receptors in the cornea, anterior and posterior chamber of the eye and retina. The P2Y1 receptor was present mainly in cornea, ciliary processes, and trabecular meshwork. The P2Y2 receptors were present in cornea, ciliary processes and retinal pigmented epithelium. P2Y4 was present in cornea, ciliary processes, photoreceptors, outer plexiform layer and ganglion cell layer. The P2Y6 presented almost an identical distribution as the P2Y4 receptor. The P2Y11 was also detectable in the retinal pigmented epithelium. The detailed distribution of the receptors clearly supports the recent findings indicating the relevant role of nucleotides in the ocular function.
Key words: ciliary processes, cornea, eye, ocular surface, P2 purinergic receptors, retina
Introduction
There is a general knowledge of nucleotides acting as extracellular messengers in tissues [1]. Among the tissues/organs which are under investigation, the eye is one that has not been fully investigated; it has been taken into consideration only in recent times, mainly because nucleotides seem to have interesting physiological roles and putative therapeutic applications (for a review, see Pintor [2]).
A quick review of the existing literature in the nucleotide field of the eye will emphasise the importance of metabotropic P2Y receptors in the ocular structure. For instance, P2Y receptors can produce an increase in the proportion of the mucin layer in the tear film [3]. Also, uridine nucleotides can modify chloride currents facilitating the production of the aqueous component of the tear [4, 5]. Inside the eye, P2Y receptors are able to regulate the production and the drainage of the aqueous humour due to their presence in the ciliary processes and trabecular meshwork cells [6, 7]. Finally, metabotropic nucleotide receptors have been described in the neural and non-neural retina [8–11].
Despite the fact that P2Y receptors seem widely distributed in the ocular surface and in other ocular areas, one needs to be aware that frequently the existence of those receptors has been pharmacologically demonstrated in primary cell cultures or in cell lines rather than in the native tissues. Apart from this, another important point is that the cells under investigation may contain more than one P2 purinoceptor subtype. This fact makes difficult the interpretation of the pharmacological data, avoiding very often a clear picture of the P2 receptors present in a tissue. For these reasons, in the present experimental work we present the distribution of P2Y receptors in the rat eye by means of commercial antibodies. We hope that this ‘picture’ will help researchers to better understand the role of nucleotides in the eye.
Materials and methods
Immunohistochemistry
A total of 10 Wistar rats of 13 days postnatal (P13) were sacrificed by rapid decapitation. For the immunohistochemical study, the eyes were removed and were fixed overnight at 4 °C, using 4% paraformaldehyde in phosphate buffer, pH 7.2. After fixation, the eyes were submitted to a cryoprotective process. Sections of 14 µm were made using a Leica 3050 M cryostate. Immunohistochemical studies were performed starting with the following primary antibody dilutions: anti-P2Y1, 1/200; anti-P2Y2, l/500; anti-P2Y4, 1/500; anti-P2Y6, 1/200 and anti-P2Y11, l/1,000. As secondary antibody we used goat anti-rabbit IgG-TRITC from Sigma (T-6778). In the case of double immunostaining, we used as primary antibodies mouse anti-synaptophysin (Sigma, S-5768) 1/250, as a neuronal marker, mouse anti-vimentin (Sigma, V-6630) 1/500, as a marker of the protein vimentin. As secondary antibodies we used goat anti-IgG mouse-FITC (Sigma, F-4014), 1/500 in the case of anti-synaptophysin marker, and goat anti-IgM mouse-FITC (Sigma, F-9259), l/100 for the anti-vimentin marker. Controls were carried out by following the same procedures but the primary antibody was substituted by the same volume of PSS/BSA solution.
Eye sections were analysed by confocal microscopy using a Zeiss Axiovert 200M microscope equipped with a LSM5 Pascal confocal module. Sections were observed with a Zeiss 63 × oil immersion lens, numerical aperture 1.40. FITC was monitored by excitation with the 488-nm wavelength laser, and TRITC was excited at 543-nm wavelength. All the images were managed with the LSM5 Pascal software.
Western blotting
For Western blot analysis, the eyes were rapidly removed and the different parts were placed on ice and subsequently homogenised with lysis buffer that contains HEPES 50 mM pH 7.5, Triton 2.5% (w/v), EDTA 10 mM, PMSF 0.2 mM and leupeptin 5 µg/ml. Protein samples (40 µg) were separated by SDS-PAGE (10% acrylamide gel) using the Bio-Rad Mini-Protein® 3-Cell System. Proteins were transferred to nitrocellulose membranes. Following transfer, the membranes were washed, blocked and incubated. The dilutions of primary antibodies were as follows: anti-P2Y1, 1/200; anti-P2Y2, 1/500; anti-P2Y4, l/200; anti-P2Y6, l/200 and anti-P2Y11, l/1000. As secondary antibody mouse anti-rabbit IgG coupled to horseradish peroxidase, from Sigma (A-2074) at 1/1000 dilution were used. Blots were developed using the Enhanced Chemiluminiscence detection system (Amersham).
Chemicals
Antibodies against the P2Y receptors were purchased from Alomone Labs (Israel), except P2Y11 which was a gift from Dr D. Cousens (Glaxo-Smithkline). Anti-synaptophysin and anti-vimentin antibodies were from Sigma (St. Louis, Missouri, USA). Other reagents were analytical grade from Merck (Darmstadt, Germany).
Results
P2Y receptors in the cornea
The cornea is the most external and transparent part of the eye. This eye region is formed by five to six different layers. The most relevant ones include the epithelium, the most superficial one; the stroma, the thickest; and endothelium the inner one. The distribution of the purinergic receptors present in the cornea can be carried out by making slices and performing immunohistochemistry, as described in Materials and methods. The analysis of the P2Y receptors present in corneal slices, by means of the available P2Y receptor antibodies, showed that P2Y1, P2Y2, P2Y4 and P2Y6 antibodies labelled this part of the eye. Among the different areas of the cornea, the one which offered positive staining for all receptors was the epithelial layer (Figure 1), although the intensity of the labelling was not the same for all the tested antibodies and for the different areas examined (see Table 1). For example, the stroma showed a minor label in all the cases (it only contains some keratinocytes) while the endothelium (the most inner part of the cornea) gave positive results with the P2Y2, P2Y4 and P2Y6 antibodies (Table 1).
Figure 1.
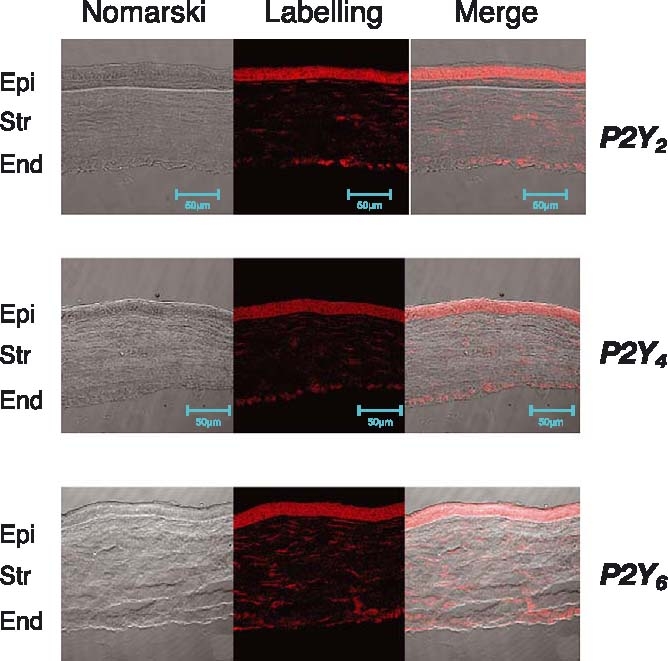
Presence of P2Y receptors in the cornea. Corneal sections labelled as indicated in Materials and methods, presented positive staining to P2Y2 (upper panel), P2Y4 (mid panel) and P2Y6 (lower panel), in the epithelium, and in some cases in the endothelium.
Table 1.
Distribution of P2Y receptors in the anterior segment of the eye.
| Receptor | Corneal epithelium | Corneal endothelium | Pigmented epithelial cells | Non-pigmented epithelial cells | Trabecular meshwork | Iris |
|---|---|---|---|---|---|---|
| P2Y1 | ++ | + | + | ++ | ++ | + |
| P2Y2 | +++ | + | ++ | ++ | ++ | + |
| P2Y4 | ++ | + | + | ++ | − | ++ |
| P2Y6 | ++ | + | − | − | − | − |
| P2Y11 | − | − | − | + | − | − |
−, No labelling, +, low labeling, ++, moderate labelling, +++, strong labelling.
P2Y receptors in the iris and ciliary body
The presence of P2Y1, P2Y2 and P2Y4 was demonstrated (Table 1) in the iris, which is the structure that works as a diaphragm, modifying the amount of light entering the eye. In particular, one of the better staining was achieved with the P2Y4 receptor antibody (Figure 2). Concerning the structure of the iris, formed by the dilator muscle and the sphincter, we tried to see whether or not there were differences between these two parts concerning the P2Y4 receptor. We have concluded that there were no differences between these two regions of the iris.
Figure 2.
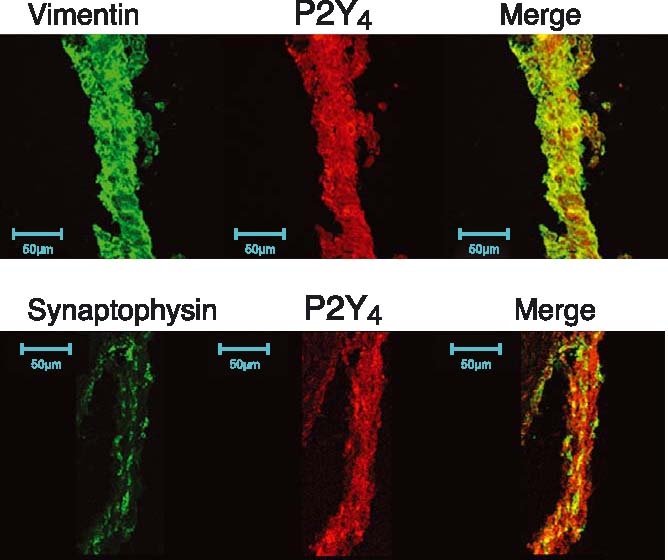
Presence of P2Y4 receptors in the iris. Iris sections presented positive staining to P2Y4 receptor antibody (in red). Vimentin (to label intermediate filaments) or synaptophysin (nerve terminals) in green demonstrated some co-localisation between P2Y4 receptors and the other markers.
The ciliary body (ciliary processes) is the part of the eye where the aqueous humour is synthesised. The physiology of this area is relevant since it is one of the ocular structures contributing to keep the right pressure within the eye. An augment of this intraocular pressure can trigger a pathology termed glaucoma.
The ciliary body contains two types of epithelial cells, non-pigmented and pigmented ciliary epithelial cells. The non-pigmented cells are facing the posterior chamber where the release of the aqueous humour occurs. The pigmented epithelial cells are just underneath the non-pigmented and they limit the stroma that contains a fenestrated endothelium, which supplies this ocular region. With the intermediate filament antibody vimentin, it is possible to visualise the non-pigmented epithelium and lens capsule (see Figure 3). This antibody helps to identify these two regions and provides interesting information when it is applied together with the P2Y receptor antibodies.
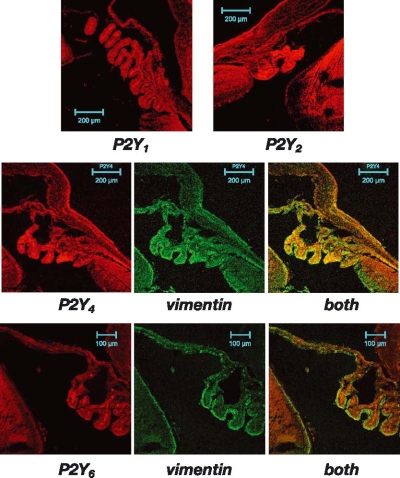
Figure 3.
P2Y receptors in the ciliary body and related areas. Positive labelling to P2Y1, P2Y2 (upper panel), P2Y4 (mid panel) and P2Y6 (lower panel) were observed in the ciliary body (in red). It is noteworthy that the distribution of the receptor depends on the particular P2Y subtype. P2Y2 labelled all the ciliary body while P2Y6 mainly labelled the stromal area. Vimentin (in green) labels the non-pigmented epithelial cells as well as the lens capsule and helps to understand the distribution of some of the P2Y receptors.
The studies performed with the P2Y receptor antibodies demonstrated that P2Y1, P2Y2, P2Y4, and P2Y6 gave positive labelling in this region (Figure 3). Nevertheless, the distribution was not the same for each antibody. P2Y1, P2Y2 and P2Y4 marked both, non-pigmented and pigmented epithelial cells. In these cases, it was possible to see that the stroma was also labelled. A different picture was obtained with the P2Y6 receptor antibody. P2Y6 antibody is concentrated exclusively in the stromal area and is not a positive labelling in the epithelia (Figure 3).
Another important area close to the ciliary processes is the trabecular meshwork. This area is responsible for the drainage of the aqueous humour, thereby regulating intraocular pressure. The immunohistochemical study of this area permitted us to detect the presence of P2Y1 and P2Y2 receptors, but it was not possible to visualise any of the other tested (Table 1).
P2Y receptors in the retina
The retina has also been investigated for the presence of P2Y receptors. Sections of the retina were incubated with P2Y receptor antibodies as well as with vimentin, which behaves as a glial cell marker (Müller cells marker) in this region, and synaptophysin, which is a neural marker.
Taking together the combination P2Y/vimentin and P2Y/synaptophysin allowed us to not only see the presence of the purinoceptors, but also made it possible for us to allocate them with specific areas of the retina.
Three P2Y receptor antibodies labelled the retina, the P2Y2, P2Y4, and P2Y6, (Table 2). P2Y2 presented a strong labelling in the non-neural retina, i.e. in the retinal pigmented epithelium (RPE), while other areas were not significantly stained (Figure 4). It was also possible to observe inmunolabelling against the P2Y11 antibody on the RPE, this being the only area where this receptor seems to be present (Figure 4). The RPE was also labelled with the P2Y4 antibody, although other areas of the neural retina were also positive. In this sense, the outer segments of the photoreceptors, the outer plexifom layer and the ganglion cell layer, were stained (Figure 5). The P2Y6 presented a similar distribution pattern labelling the outer segments of the photoreceptors, outer plexiform layer and ganglion cell layer. Moreover, the inner plexiform layer was also labelled with the P2Y6 receptor antibody in clear contrast with the P2Y4 (Table 2).
Table 2.
Distribution of P2Y receptors in the retina.
| Receptor | Reinal pigmented epithelium | Photoreceptors | Inner plexiform layer | Outer plexiform layer | Ganglion cell layer |
|---|---|---|---|---|---|
| P2Y1 | − | − | − | − | − |
| P2Y2 | +++ | + | − | − | − |
| P2Y4 | + | +++ | − | ++ | ++ |
| P2Y6 | − | +++ | + | ++ | ++ |
| P2Y11 | − | − | − | − | − |
−, No labelling, +, low labeling, ++, moderate labelling, +++, strong labelling.
Figure 4.
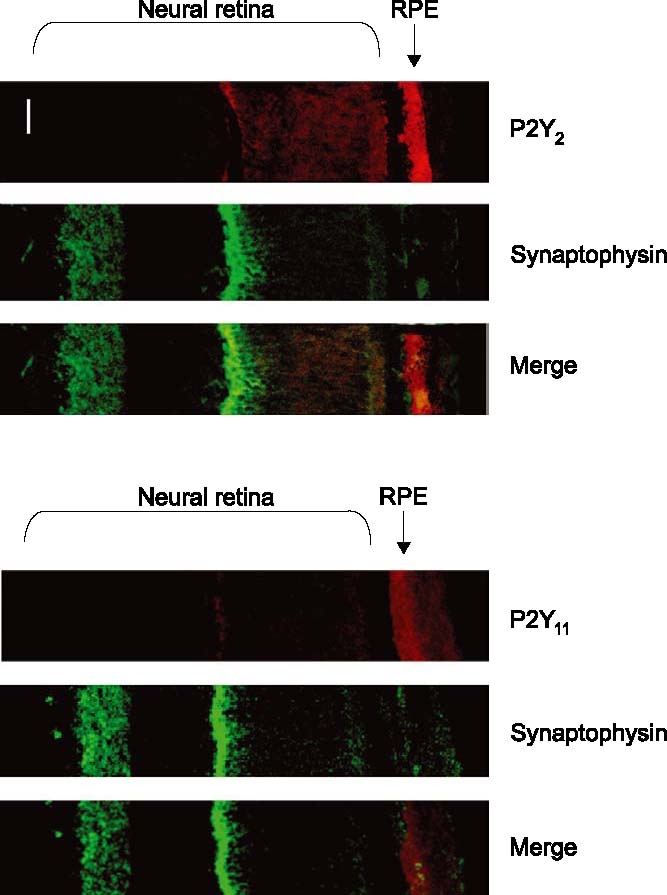
Presence of P2Y2 and P2Y11 in the RPE. Labelling to the P2Y2 receptor was obtained in the retinal pigmented epithelium (upper pictures). Combination with synaptophysin labelling permits a better identification of the retinal areas. P2Y11 receptors were also present in the RPE as observed in the three lower pictures. Scale bar, 10 µm.
Figure 5.
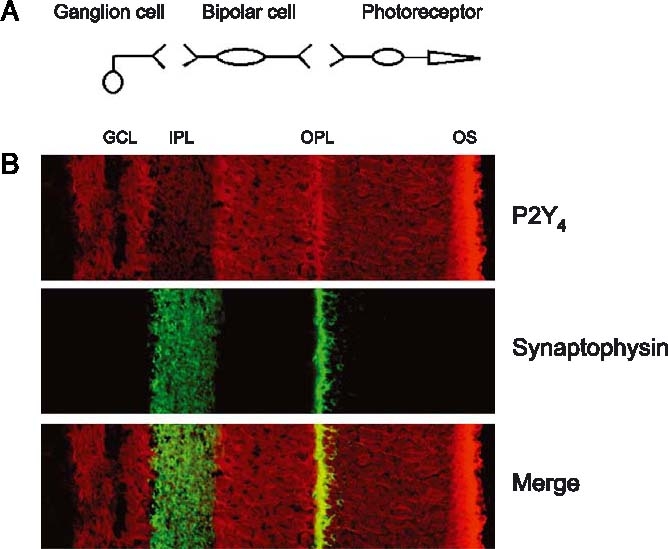
Distribution of P2Y4 receptors in the retina. (A) Schematic diagram showing the main cells in the retina, the synapses, and how they are related. (B) The presence of P2Y4 receptors in the retina. Upper panel, labelling with the P2Y4 receptor antibody presenting positive staining in the external segments of photoreceptors (OS), outer plexiform layer (OPL) and ganglion cell layer (GCL). Mid panel, synaptophysin staining labelling the synaptic layer outer plexifom layer (OPL) and inner plexiform layer (IPL). Lower panel, combination of the upper and mid panels.
Studies on the presence of P2Y receptors in Müller cells were performed with vimentin. These studies demonstrated the presence of P2Y4 and P2Y6 receptors in this glial cells (Figure 6). It was not possible to observe any co-localisation (results not shown) when the other P2Y receptors were assayed together with the vimentin.
Figure 6.
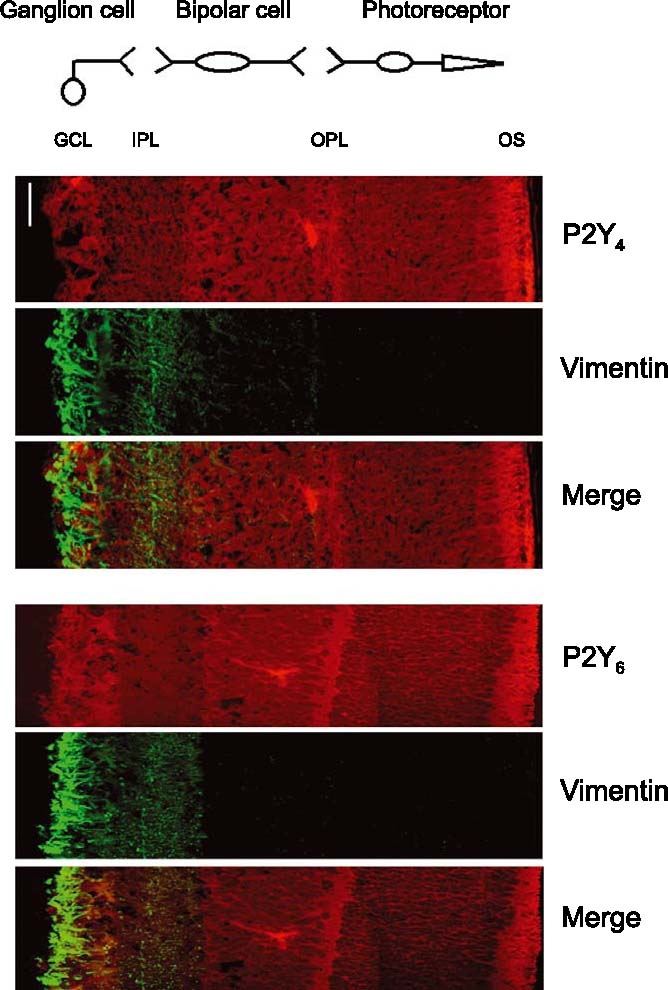
Study on the co-localisation of P2Y4 and P2Y6 receptors with vimentin (Müller cells). Upper pictures represent the labelling with the P2Y4 receptor antibody (upper), with the vimentin (mid) antibody and a combination of both (lower). Lower pictures represent the labelling of P2Y6 receptors (upper), vimentin (mid) and a combination of both (lower). Scale bar, 10 µ.
Western blot analysis
Western blots were carried out by taking samples of the different areas under study following the protocol described in Materials and methods. Using this technique, we were able to confirm the existence of those P2Y receptors previously shown in the immunohistochemical studies. It is important to point out that we had some problems in the retinal preparations to get a positive band against the P2Y6 receptor. We still do not know the reason why it is not possible to obtain a positive Western blot for this receptor. In contrast, it was possible to see the mentioned band in the corneal extracts (Figure 7). P2Y1, P2Y2, P2Y4 and P2Y6 receptors presented molecular weights of 42, 50, 92 and 97 kDa, respectively. The other antibodies, like the one for the P2Y11, did not reveal any positive band.
Figure 7.
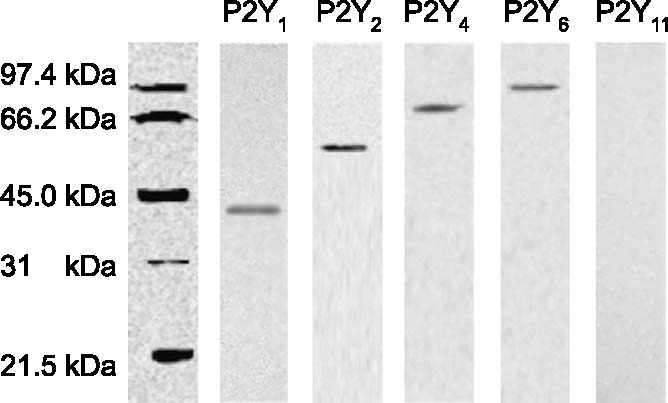
Western blot for the P2Y receptors in corneal extracts. Analysis of the P2Y receptors present in the cornea by Western blot analysis following the protocol described in Materials and methods. The molecular weights of the identified bands fit quite well with those described in the literature.
Discussion
P2Y receptors in the cornea and sclera
Metabotropic receptors for nucleotides in the cornea have been identified by means of in situ hybridisation techniques. P2Y2 receptors are predominantly expressed in the cornea, sclera, goblet cells and meibomian glands of monkeys and rabbits [12]. In our experiments, we describe P2Y2 receptors and the presence of P2Y1, P2Y4 and P2Y6. The differences between our results and those described in the literature can be due to the animal model used. In the rabbit corneal epithelium the possible existence of P2Y4 and P2Y6 receptors cannot be discarded. Experiments studying the effect of nucleotides on corneal wound healing demonstrate that, apart from the involvement of a P2Y2 receptor in the re-epithelialisation process, the participation of P2Y4/P2Y6 receptors needs to be taken into consideration [13].
In the endothelium, the existence of receptors for ATP has been demonstrated. These receptors are of the P2Y subtype, although there are not a detailed description of the particular subtype [14]. We may suggest, according to our results, that P2Y2 and P2Y4 are good candidates to fulfill the results described by Srinivas, who clearly identified different responses, therefore suggesting the presence of more than one P2Y receptor subtype.
P2Y4 receptors have been also identified by in situ hybridisation techniques in the conjunctiva, and a role on the CI− movement on conjunctival epithelial cells has been suggested. Hosoya et al. [15] indicate that P2Y2 and P2Y4 receptors control the ion flux across the conjunctiva. This ion flux can be measured by activating P2Y2 receptors with UTP. This receptor stimulation produces a clear movement of CI− ions and the concomitant fluid transport [16].
Conjunctival goblet cells can modulate the release of mucins by means of P2Y2 receptors, as suggested by Dartt [17] and Murakami and co-workers [18].
P2Y receptors in the iris ciliary body
There is not much information about the presence of P2 receptors in the iris. We have observed the presence of P2Y1, P2Y2 and mainly P2Y4. Previous functional studies demonstrated the presence of P2Y receptors, although nothing indicated the receptor subtype. In these studies, Fuder and co-workers [19] demonstrated the effect of ATP on the iris, and how this effect is mediated by a P2Y-like receptor [20]. P2Y receptors present in the iris may regulate the pupil size by either controlling the muscle contraction/relaxation or by modulating the sympathetic nervous system which controls the iris physiology [20].
Concerning the ciliary body, the presence of P2Y receptors has been demonstrated in pigmented and non-pigmented epithelial cells by measuring IP3 generation and cytosolic Ca2+ levels [21]. Other experiments performed by Shaindullah and Wilson [22] also demonstrate that the effects of UTP and ATP are equipotent and that the receptors they activate may belong to the P2Y2 purinoceptor subtype. In our case, we have been able to identify different P2Y receptors; nevertheless, we were able to see a differential distribution of the receptors in the ciliary processes. P2Y1, P2Y2 and P2Y4 showed immunoreactivity in the ciliary non-pigmented and pigmented epithelium. The existence of P2Y2 receptors in non-pigmented and pigmented epithelial cells has been described by in situ hybridisation techniques [12]. This P2Y2 receptors and also P2Y1 have been described in the rabbit ciliary body epithelium by means of functional studies that reveal the metabotropic nature of these receptors [7]. These receptors, when activated, can increase cytosolic Ca2+ levels plus PGE2 and cAMP. This combination produces the activation of chloride channels which reduce the aqueous humour formation and a reduction in IOP [23]. The P2Y6 receptor appears only in the stroma of the ciliary processes. This fact could be due to the existence of a fenestrated blood vessel network in this area. Since P2Y6 receptors have been described in the endothelium of blood vessels [24], it could be the case that our results are showing positive immunoreactivity to the P2Y6 receptor present in the vessels that can modify the blood flow in this area.
Concerning the trabecular meshwork, our results, indicating the presence of P2Y1 P2Y2 and P2Y4 in the rat slices, cope quite well with the results described recently by Crosson and co-workers [6]. They also described the presence of P2Y11 in a human trabecular meshwork cells line [6]. We were not able to see a positive immunoreactivity against this receptor in the rat eye slices.
P2Y receptors in the retina
The distribution in the retina concerning the P2Y receptor is complicated, mainly due to the great cellular heterogeneity. The presence of P2Y2 receptors has been demonstrated in the retinal pigmented epithelium (RPE) in situ hybridisation [12], and functional studies [11]. These studies fit well with our results, which indicate strong immunoreactivity to the P2Y2 receptor. The role of this P2Y2 receptor seems to be very interesting from the therapeutic point of view The presence of this receptors on the apical membrane of RPE cells is very important for the re-absorption of fluids present in the inter-retinal space [25]. This fact has invited us to think about the possibility of using selective P2Y2 agonists for the treatment of pathologies such as the retinal detachment.
In the neural retina, and comparing our P2Y2 results with others previously published, we were unable to see labelling in other retinal areas apart from the photoreceptors. This is in clear contrast with the results of Cowlen et al. [11], who described the presence of P2Y2 mRNA in the inner nuclear layer and ganglion cell layer.
The other P2Y receptor which showed imunoreactivity in the neural retina was the P2Y6 receptor, which was present at the synaptic location (the plexiform layers) as indicated by the co-localisation with synaptophysin. Only P2X1 receptors have been described as associated to the plexiforms layers in the retina; these include P2X1 [26–29].
Vinmentin antibody is a useful tool to identify Müller cells in the retina [30]. In this sense, we have been able to identify P2Y4 and P2Y6 co-localisation with vimentin in retinal slices, indicating the presence of these two purinoceptors subtypes in Müller cells. Reports about the presence of P2Y receptors in glial cells have been done [31, 32]. The existence of a P2Y receptors in Müller cells produces the typical increase in the cytosolic Ca2+ levels [33]. Recently, Bringmann and co-workers [34] have demonstrated how P2Y receptors can modify physiological processes such as K+ turnover in Müller cells, which is a crucial aspect in the neurophysiology of the retina.
Very recently, Fries and co-workers [35] described the expression of P2Y receptors in the rat retina. The use of in situ hybridization techniques demonstrated the presence of P2Y1, 4 and 6 in the inner layers of the retina. We have similar results with the P2Y4 and P2Y6 receptor antibodies; nevertheless, we do not get labelling with the P2Y2. Also, and in clear contrast with the results presented by these authors, we do not observe labelling with the P2Y1 and P2Y2 in the ganglion cell layer but we do find the P2Y4 receptor. This group also describes immunoreactivity to the P2Y1 and P2Y4 in the inner plexiform layer. In our case, the only receptor located in this retinal area is the P2Y6. The differences among our results and the ones described by Fries et al., can be, in part, due to the strain and age of the animals (they used Brown Norway, we used Wistar; they used adults, we used young animals). Changes in the results can also be due to the methodologies used by both groups, which differ slightly.
In summary, P2Y receptors are widely distributed in the eye. Some of the receptors were identified as P2Y although they have not been fully characterised to know which of the P2Y subtype is present in each area. We hope that this work will help researchers to apply better pharmacological tools when investigating these receptors in the different ocular structures.
Acknowledgement
This work has been supported by research grants from the Ministerio de Educación y Ciencia SAF2003-00338, and SAF2004-06119-C2-01 and Fondos FEDER UCOM03-33-024. We thank Mr D. Forsblade for his help in the preparation of this manuscript.
References
- 1.Burnstock G, Williams M. P2 purinergic receptors: Modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295(3):862–869. [PubMed] [Google Scholar]
- 2.Pintor J. Purinergic signalling in the eye. In: Burnstock G, Sillito A, editors. Nervous Control of the Eye. London: Harwood Academic Publishers; 2000. pp. 171–210. [Google Scholar]
- 3.Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998;67(3):341–346. doi: 10.1006/exer.1998.0520. [DOI] [PubMed] [Google Scholar]
- 4.Ueda H, Hosoya K, Shiue MHI, et al. UTP induced net Cl− secretion in the pigmented rabbit conjuntiva is mediatic by Ca2+ signalling mechanisms. Invest Ophthalmol Vis Sci. 1999;40:S90. [Google Scholar]
- 5.Shiue M, Kim KJ, Lee V. Regulation of fluid secretion in the pigmented rabbit conjuntiva. Invest Ophthalmol Vis Sci. 1999;40:S90. [Google Scholar]
- 6.Crosson CE, Yates PW, Bhat AN, et al. Evidence for multiple P2Y receptors in trabecular meshwork cells. J Pharmacol Exp Ther. 2004;309(2):484–489. doi: 10.1124/jpet.103.060319. [DOI] [PubMed] [Google Scholar]
- 7.Farahbakhsh NA, Cilluffo MC. P2 purinergic receptor-coupled signaling in the rabbit ciliary body epithelium. Invest Ophthalmol Vis Sci. 2002;43(7):2317–2325. [PubMed] [Google Scholar]
- 8.Laasberg T. Ca2(+)-mobilizing receptors of gastrulating chick embryo. Comp Biochem Physiol C. 1990;97(l):9–12. doi: 10.1016/0742-8413(90)90164-5. [DOI] [PubMed] [Google Scholar]
- 9.Lohman F, Drews U, Donie F, et al. Chick embryo muscarinic and purinergic receptors activate cytosolic Ca2+ via phosphatidylinositol metabolism. Exp Cell Res. 1991;197:326–329. doi: 10.1016/0014-4827(91)90441-V. [DOI] [PubMed] [Google Scholar]
- 10.Sakaki Y, Fukuda Y, Yamashita M. Muscarinic and purinergic Ca2+ mobilizations in the neural retina of early embryonic chick. Int J Dev Neurosci. 1996;14(6):691–699. doi: 10.1016/S0736-5748(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 11.Peterson WM, Meggyesy C, Yu K, et al. Extracellular ATP activates calcium signaling, ion, and fluid transport in retinal pigment epithelium. J Neurosci. 1997;17(7):2324–2337. doi: 10.1523/JNEUROSCI.17-07-02324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowlen MS, Zhang VZ, Warnock L, et al. Localization of ocular P2Y2 receptor gene expression by in situ hybridisation. Exp Eye Res. 2003;77(1):77–84. doi: 10.1016/S0014-4835(03)00068-X. [DOI] [PubMed] [Google Scholar]
- 13.Pintor J, Bautista A, Carracedo G, et al. UTP and diadenosine tetraphosphate accelerate wound healing in the rabbit cornea. Ophthalmic Physiol Opt. 2004;24(3):186–193. doi: 10.1111/j.1475-1313.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 14.Srinivas SP, Yeh JC, Ong A, et al. Ca2+ mobilization in bovine corneal endothelial cells by P2 purinergic receptors. Curr Eye Res. 1998;17(10):994–1004. doi: 10.1076/ceyr.17.10.994.5242. [DOI] [PubMed] [Google Scholar]
- 15.Hosoya K, Ueda H, Kim KJ, et al. Nucleotide stimulation of Cl(−) secretion in the pigmented rabbit conjunctiva. J Pharmacol Exp Ther. 1999;291(l):53–59. [PubMed] [Google Scholar]
- 16.Li Y, Kuang K, Yerxa B, et al. Rabbit conjunctival epithelium transports fluid, and P2Y2(2) receptor agonists stimulate Cl(−) and fluid secretion. Am J Physiol Cell Physiol. 2001;281(2):C595–C602. doi: 10.1152/ajpcell.2001.281.2.C595. [DOI] [PubMed] [Google Scholar]
- 17.Dartt DA. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog Retin Eye Res. 2002;21(6):555–576. doi: 10.1016/S1350-9462(02)00038-1. [DOI] [PubMed] [Google Scholar]
- 18.Murakami T, Fujihara T, Nakamura M, et al. P2Y(2) receptor elicits PAS-positive glycoprotein secretion from rabbit conjunctival goblet cells in vivo. J Ocul Pharmacol Ther. 2003;19(4):345–352. doi: 10.1089/108076803322279390. [DOI] [PubMed] [Google Scholar]
- 19.Fuder H, Brink A, Meincke M, et al. Purinoceptor-mediated modulation by endogenous and exogenous agonists of stimulation-evoked [3H]noradrenaline release on rat iris. Naunyn-Schmiedeberg’s Arch Pharmacol. 1992;345(4):417–423. doi: 10.1007/BF00176619. [DOI] [PubMed] [Google Scholar]
- 20.Fuder H, Muth U. ATP and endogenous agonists inhibit evoked [3H]-noradrenaline release in rat iris via A1 and P2Y-like purinoceptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;348(4):352–357. doi: 10.1007/BF00171333. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki Y, Nakano T, Sears M. Calcium signals from intact rabbit ciliary epithelium observed with confocal microscopy. Curr Eye Res. 1997;16:166–l75. doi: 10.1076/ceyr.16.2.166.5095. [DOI] [PubMed] [Google Scholar]
- 22.Shaindullah M, Wilson WS. Mobilisation of intracellular calcium by P2Y2 receptors in cultured, non-transformed bovine ciliary epithelial cells. Curr Eye Res. 1996;16:1006–1016. doi: 10.1076/ceyr.16.10.1006.9018. [DOI] [PubMed] [Google Scholar]
- 23.Fleischauer JC, Mitchell CH, Peterson-Yantorno K, et al. PGE(2), Ca(2+), and cAMP mediate ATP activation of Cl(−) channels in pigmented ciliary epithelial cells. Am J Physiol Cell Physiol. 2001;281(5):C1614–C1623. doi: 10.1152/ajpcell.2001.281.5.C1614. [DOI] [PubMed] [Google Scholar]
- 24.Malsmjo M, Hou M, Pendergast W, et al. Potent P2Y6, receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003;3(l):4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maminishkis A, Jalickee S, Blaug SA, et al. The P2Y(2) receptor agonist INS37217 stimulates RPE fluid transport in vitro and retinal reattachment in rat. Invest Ophthalmol Vis Sci. 2002;43(11):3555–3566. [PubMed] [Google Scholar]
- 26.Yazulla S, Studholme KM. Vanilloid receptor like 1 (VRL1) immunoreactivity in mammalian retina: Colocalization with somatostatin and purinergic P2X1 receptors. J Comp Neurol. 2004;474(3):407–418. doi: 10.1002/cne.20144. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood D, Yao WP, Housley GD. Expression of the P2X2, receptor subunit of the ATP-gated ion channel in the retina. Neuroreport. 1997;8(5):1083–1088. doi: 10.1097/00001756-199703240-00004. [DOI] [PubMed] [Google Scholar]
- 28.Ishii K, Kaneda M, Li H, et al. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. Comp Neurol. 2003;459(3):267–277. doi: 10.1002/cne.10608. [DOI] [PubMed] [Google Scholar]
- 29.Puttssery T, Fletcher EL. Synaptic localization of P2X7 receptors in the rat retina. J Comp Neurol. 2004;472(l):13–23. doi: 10.1002/cne.20045. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan DK, Lasater EM. Glial and neuronal markers in bass retinal horizontal and Muller cells. Brain Res. 1990;537(1−2):131–140. doi: 10.1016/0006-8993(90)90349-G. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Wakakura M. P1/P2-purinergic receptors on cultured retinal Müller cells. Jpn J Pharmacol. 1997;42:33–40. doi: 10.1016/s0021-5155(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 32.Morogiwa K, Quan M, Murakami M, et al. P2 purinoceptor expression and functional changes of hypoxia-activated cultured rat retinal microglia. Neurosci Lett. 2000;282:153–156. doi: 10.1016/S0304-3940(00)00887-9. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Holtzclaw LA, Russell JT. Müller cell Ca2+ waves evoked by purinergic receptor agonists in slices of rat retina. J Neurophysiol. 2001;85(2):986–994. doi: 10.1152/jn.2001.85.2.986. [DOI] [PubMed] [Google Scholar]
- 34.Bringmann A, Pannicke T, Weick M, et al. Activation of P2Y receptors stimulates potassium and cation currents in acutely isolated human Muller (glial) cells. Glia. 2002;37(2):139–152. doi: 10.1002/glia.10025. [DOI] [PubMed] [Google Scholar]
- 35.Fries J, Wheeler-Schilling TH, Guenther E, et al. Expression of P2Y1, P2Y2, P2Y4 and P2Y6 receptor subtypes in the rat retina. Invest Ophthalmol vis Sci. 2004;45(10):3410–3417. doi: 10.1167/iovs.04-0141. [DOI] [PubMed] [Google Scholar]