Abstract
In vertebrates, mutations in Protein O-mannosyltransferase1 (POMT1) or POMT2 are associated with muscular dystrophy due to a requirement for O-linked mannose glycans on the Dystroglycan (Dg) protein. In this study we examine larval body wall muscles of Drosophila mutant for Dg, or RNA interference knockdown for Dg and find defects in muscle attachment, altered muscle contraction, and a change in muscle membrane resistance. To determine if POMTs are required for Dg function in Drosophila, we examine larvae mutant for genes encoding POMT1 or POMT2. Larvae mutant for either POMT, or doubly mutant for both, show muscle attachment and muscle contraction phenotypes identical to those associated with reduced Dg function, consistent with a requirement for O-linked mannose on Drosophila Dg. Together these data establish a central role for Dg in maintaining integrity in Drosophila larval muscles and demonstrate the importance of glycosylation to Dg function in Drosophila. This study opens the possibility of using Drosophila to investigate muscular dystrophy.
INTRODUCTION
Dystroglycan (Dg) is an extracellular matrix (ECM) receptor for a variety of ligands and it plays important roles in epithelial polarity, muscle viability, neural migration, and cancer progression (Winder, 2001). In skeletal muscle, as part of the dystrophin glycoprotein complex (DGC), Dg spans the sarcolemma and provides a link between the ECM and the cytoskeleton. Disruption of this complex leads to muscle dystrophy (Blake et al., 2002; Deconinck and Dan, 2007). Dg protein is heavily glycosylated in its extracellular mucin-like domain with a variety of glycans, including O-linked mannose and mucin-type O-GalNAc structures (Ervasti and Campbell, 1993; Michele and Campbell, 2003; Combs and Ervasti, 2005). Mutations in glycosyltransferases that are involved in the posttranslational modification of Dg are likewise associated with muscular dystrophy and neural migration defects (Muntoni et al., 2004; Kanagawa and Toda, 2006). These phenotypes seem to be directly due to the impact of these mutations on Dg and reflect the importance of glycosylation and posttranslational processing to Dg function.
The O-mannose glycans present on Dg have received particular attention because these play a role in ligand binding. In particular, Dg lacking O-mannose glycans show reduced binding to laminin (Lam; Chiba et al., 1997; Michele et al., 2002; Michele and Campbell, 2003; Muntoni et al., 2004). The initial step in generating these glycans is the addition of mannose in an O-linkage onto serine or threonine residues of Dg. Biochemical data indicates that this activity is carried out by two proteins, POMT1 and POMT2, which together form a biochemically active complex. Increased protein O-mannosylation in human cultured cells is only observed when both enzymes are transfected together (Manya et al., 2004), and a further study demonstrated physical association between the two enzymes (Akasaka-Manya et al., 2006). Genetic data demonstrates a requirement for both POMT1 and POMT2, as mutation of either protein results in muscular dystrophy and associated neural defects in humans and other mammals (Akasaka-Manya et al., 2004; van Reeuwijk et al., 2005).
Genetic studies in model invertebrates, where genes are often present in single copy and genetic studies are less complicated by redundancy, have proven useful for furthering our understanding of cellular mechanisms involved in complex disorders. The main components of the mammalian DGC are conserved in Drosophila (Greener and Roberts, 2000; Neuman et al., 2001). Studies have identified a roles for Dg in epithelial and oocyte polarity (Deng et al., 2003; Poulton and Deng, 2006; Schneider et al., 2006) and recently age-dependent changes in adult muscles with reduced Dg levels have been identified (Shcherbata et al., 2007). Interestingly, several Drosophila Dg isoforms are generated via alternative splicing (Deng et al., 2003; Schneider et al., 2006). Only one of these contains the full mucin-like domain, and it appears to be subjected to significant levels of glycosylation (Schneider et al., 2006). Dg isoforms that lack the mucin-like domain are required to maintain polarity in the follicular epithelium (Schneider et al., 2006), suggesting that the Dg isoforms can have different functional roles in Drosophila. POMTs are also found in Drosophila (Martin-Blanco and Garcia-Bellido, 1996; Ichimiya et al., 2004; Lyalin et al., 2006) and biochemical analysis of Drosophila POMT1, encoded by the gene rotated abdomen (rt; Martin-Blanco and Garcia-Bellido, 1996) and POMT2, encoded by twisted (tw; Lyalin et al., 2006) has demonstrate that these enzymes possess POMT activity similar to their vertebrate homologues (Ichimiya et al., 2004). When transfected together in insect cells they are able to transfer mannose to a GST-α-Dg substrate generating O-linked mannose (Ichimiya et al., 2004). In addition, reducing the level of either enzyme in vivo by RNA interference (RNAi) resulted in reduced POMT activity of larval extracts toward the Dg substrate (Ichimiya et al., 2004). Mutation of the Drosophila enzymes or reducing their function using RNAi results in rotation of adult abdominal muscles, suggesting the enzymes have some role in muscle function or development (Martin-Blanco and Garcia-Bellido, 1996; Ichimiya et al., 2004; Lyalin et al., 2006). However, it is unclear if these phenotypes are related to a role for the enzymes in Dg modification and function because such a phenotype has not been described in Dg mutants.
In this study we examined Drosophila larval body wall muscles and identified errors in muscle attachment, changes in muscle contraction, and reduced cellular integrity associated with Dg mutant alleles and RNAi-mediated reduction of Dg. We also find similar defects in larvae mutant for rt or tw or double mutant for both and show genetic interactions between rt, tw, and Dg, suggesting that the glycosyltransferases function in the same molecular pathway as Dg. These data are consistent with a nonredundant requirement for POMT enzymes and the O-linked mannose glycans they generate, for proper Dg function in Drosophila.
MATERIALS AND METHODS
Drosophila Stocks and Crosses
Dg248 and Dg323 are previously described P-element excision alleles: Dg248 results in reduced Dg expression, whereas Dg323 is null (Deng et al., 2003; Shcherbata et al., 2007). UAS-Dg-i, UAS-Dg-C, and UAS-Dg-cyt have been previously described (Deng et al., 2003). rt2 and rtp are semilethal P-element insertion alleles as described by Martin-Blanco and Garcia-Bellido (1996). tw1 is viable and carries an amino acid substitution in the conserved POM domain (Lyalin et al., 2006). Lethal and semilethal alleles were balanced over green fluorescent (GFP)-marked balancers; absence of GFP was used to select homozygous mutant larvae. Double mutant stocks were also generated with GFP-marked balances (tw1/FM7 GFP; rt2/TM3 GFP, tw1/FM7 GFP; rtp/TM3 GFP, tw1/FM7 GFP; Dg323/CyO GFP, Dg323/CyO GFP; rt2/TM3 GFP). The following Gal4 drivers used: 24B-Gal4, 69B-Gal4, and P-tub-Gal4. All crosses were maintained at 25°C. For 30°C experiments crosses were set up at 25°C, adults were allowed to lay eggs for 2 d, and vials were then transferred to 30°C.
Muscle Measurements
Larvae were dissected, fixed, and stained with phalloidin; muscles were imaged, and measurements obtained as described previously. All muscle measurements were from muscles 6/7 abdominal segments 3 or 4. Sarcomere measurements were from the central region of muscle 6 abdominal segments 3 or 4. No more than two muscles were measured from a single larvae. Sarcomeres were measured from gray scale intensity plots across phalloidin-stained sarcomeres (see Figures 2 and 3; Haines and Stewart, 2007), sarcomere size being the distance between adjacent peaks. In cases where the graph did not clearly show a single peak for a sarcomere, the center of the high-intensity region was selected by eye as the peak value.
Figure 2.
Sarcomere size is altered in Dg mutants. Phalloidin-stained sarcomeres from muscle 6 (A) yw, (B) Dg248, and (C) Dg323. The graphs (A′–C′) show gray scale intensity across the dashed line drawn on the sarcomeres in A–C. Six sarcomeres are in the 48-μm-wide region in yw compared with seven in the Dg248 mutant and five in Dg323. Red bars indicate one sarcomere. (D) Scatter plot of sarcomere size in the different genotypes, where the horizontal line represents the mean value of the data. Dg248 sarcomeres are smaller then yw but more variable. Dg323/Dg248 and Dg323 show a large variation in sarcomere size and larger sarcomeres compared with yw.
Figure 3.
Sarcomere size changes associated with Dg RNAi knockdown. (A) Graph shows gray scale intensity of phalloidin-stained sarcomeres shown below. Sarcomeres are aligned on the left of the graph but rapidly become out of phase with each other because the tub-Gal4::UAS-Dg-i sarcomeres are smaller than yw controls. (B) Scatter plot displays sarcomeres size across the different genotypes, where the horizontal line represents the mean value of the data. Sarcomere size was measured as the peak-to-peak distance from graphs as shown in A. Note the increased variance in the 24B-Gal4::UAS-Dg-i sample compared with controls and the decrease in size of the tub-Gal4::UAS-Dg-i sarcomeres. n > 100 sarcomeres of each genotype.
Surface area of the 3rd instar larval muscles 6/7 from which sarcomere measurements were taken was as follows (μm2 mean ± SEM, n = number muscles): in Figure 3B, 25°C yw: 76334 ± 2191, n = 22; UAS-Dg-i/+: 76674 ± 2281, n = 21; 24B-Gal4/+: 74794 ± 1579, n = 22; P-tub-Gal4/+: 74398 ± 3260, n = 18; 24B-Gal4::UAS-Dg-i: 72544 ± 2006, n = 22; P-tub-Gal4::UAS-Dg-i: 71404 ± 2677, n = 14; 69B-Gal4::UAS-Dg-i: 79663 ± 2124, n = 12; in Figure 4B: 30°C yw: 71010 ± 2149, 24; UAS-Dg-i/+: 77368 ± 1554, n = 20; 24B-Gal4/+: 80932 ± 1478, n = 16; 24B-Gal4::UAS-Dg-i: 76610 ± 1973, n = 26; and in Figure 7F: tw1: 77698 ± 1789, n = 50; rt2 64747 ± 1926, n = 19; rt2/rtp: 70809 ± 3759, n = 22; tw1;rt2: 60834 ± 1884, n = 29; tw1; rt2/rtp: 62877 ± 1725, n = 17.
Figure 4.
Strong knockdown of Dg in muscles results in large sarcomeres. (A) Western blot with Dg antibody detects several bands (marked with arrowheads) in yw (lane 1) larvae muscle preparation; these bands are reduced in 24B-Gal4::UAS-Dg-i at 25°C (lane 2) and are further reduced when these larvae develop at 30°C (lane 3). Bottom panel, β-tubulin loading control. (B) Sarcomere size of controls and 24B-Gal4::UAS-Dg-i all at 30°C. 24B-Gal4::UAS-Dg-i 30°C are consistently larger then controls. N > 100 sarcomeres each genotype. *p < 0.001. (C) α-actinin (green)- and phalloidin (red)-stained sarcomeres. The pattern of c-actinin and F-actin are normal despite the larger sarcomeres in the 24B-Gal4::UAS-Dg-i 30°C muscles.
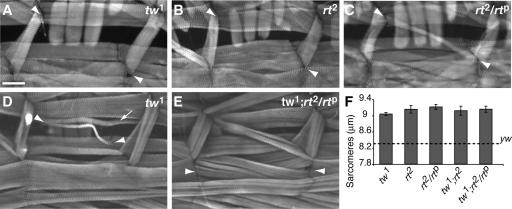
Figure 7.
rt and tw and double tw;rt mutants show defects in muscle attachment and large sarcomeres. (A) tw1 larvae showing absence of muscle 5; arrowheads indicated normal location of this muscle. (B) rt2 mutant showing absence of muscle 5 (arrowheads show normal location). (C) rt2/rtp muscle 5 (arrowheads) is very thin. (D) tw1, muscle 5 (arrowheads) is very thin and is attached at an abnormal location. Muscle 4 has split fibers (arrow) (E) tw1; rt2/rtp muscles 6 is missing (arrowheads). (F) tw, rt, and tw;rt double mutants all have large sarcomeres compared with yw. Images A–E are shown to scale. Scale bar, (A) 100 μm.
Antibody Staining
Third instar larvae were dissected along the dorsal midline in zero-calcium HL3 saline (Stewart and McLean, 2004). On removal of the digestive tract, fat bodies, and main trachea, the preparations were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 10 min. The tissue was then washed in PBS, 0.1% triton, 0.5% bovine serum albumin (PBT), and stained in PBT + 5% normal goat serum, overnight a 4°C or for anti-Dlg and anti-horseradish peroxide (HRP) at room temperature for 1 h. The following antibodies were used: rabbit anti-Dg (1:800; Deng et al., 2003), guinea pig anti-Lam (1:1000; gift T. Volk), rabbit anti-Dys (1:1000; Schneider et al., 2006), mouse anti-Dlg (1:200; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), Texas Red goat anti-HRP (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:100, and rat anti-α-actinin (Babraham Bioscience Technologies, Cambridge, United Kingdom) at 1:10. Secondary antibodies were made in goat and purchased from Molecular Probes (Eugene, OR); anti-rabbit Alexafluor488, anti-guinea pig Alexafluor633, anti-mouse Alexafluor633, and anti-rat Alexafluor488. Antibody staining was, where appropriate, followed by lectin (see below) or phalloidin staining. Tetramethylrhodamine isothiocyanate-Phalloidin (Sigma, St. Louis, MO) or fluorescein isothiocyanate-phalloidin (Sigma) was used at 1 μg/ml for 30 min.
Stained preparations were mounted in 90% glycerol in PBS, or where indicated Vectashield with propidium iodide (Vector Laboratories, Burlingame, CA). Images of antibody stained muscle sarcomeres were collected on a Zeiss LSM 510 confocal microscope(Thornwood, NY) using F-Flura 40×/1.3 oil lens with 2× zoom. The pinhole was set to 70 μm, with a resulting confocal section thickness of 0.6 μm. Confocal images were exported to imageJ (NIH). Low-magnification images of whole muscles stained with antibodies or with phalloidin were taken with a 10× lens on a Nikon E600FN microscope (Melville, NY) equipped with a Hamamatsu Orca ER CCD camera (Bridgewater, NJ). Images then were obtained from the camera with SimplePCI (Compix, Cranberry Township, PA) software and exported to imageJ. Where comparisons were made (Figures 4C, 5a, 6, a and b, and 6, c and d) samples were stained in the same tube, mounted together on a slide and imaged with the same settings.
Figure 5.
Antibody against Dg reveals staining through the sarcomeres in larval muscles. (A) Antibody against the C-terminal Dg epitope reveals staining in larval muscles. Strong Dg puncta are present on the muscle, and staining is present at the sarcomeres. Staining is reduced in 24B-Gal4::UAS-Dg-i muscles. (B) An example image of Dg puncta (green) on the muscle surface, the muscle nuclei are stained in red with PI. (C–F) Z-stack of seven 0.6-μm sections through sarcomeres. T-tubules marked with (C) Dlg (blue), (D) Dg (green), and E is merge of panels C and D. (F) Overlap showing location of F-actin bands of muscle contractile apparatus labeled with phalloidin (red).
Figure 6.
Lectin that recognizes N-terminal Dg overlaps Laminin staining in the larval muscles. (A and B) Dg antibody (blue), VVA (green), and HRP (red). (A) 24B-Gal4–driven expression of a Dg-C construct leads to strong accumulation of Dg and VVA at postsynaptic regions. (B) 24B-Gal4–driven expression of a truncated Dg, which lacks the extracellular domain; Dg antibody reveals strong accumulation of Dg, but VVA staining is not detected. (C and D) Images are single confocal sections; VVA (green), anti-Lam (blue), and F-actin (red). (C) yw muscles Lam and VVA overlap at the sarcomeres. (D) 24B-Gal4::UAS-Dg-i 30°C muscle Lam staining appears normal, but VVA staining is strongly reduced compared with C.
Lectin Staining
Larvae were dissected, fixed and washed as for antibody staining and incubated with 4 μg/ml lectin in PBT for 30 min at room temperature. Lectins used were as follows: fluorescein-Vicia villosa agglutinin (VVA), rhodamine-Sophora japonica agglutinin (SJA), fluorescein-Griffonia simplicifolia lectin II (GSL-II), rhodamine-wheat germ agglutinin (WGA), and fluorescein-Sambucus nigra lectin (SNL), all from Vector Laboratories.
Western Blot
Six 3rd instar larvae of each genotype were dissected along the dorsal midline in zero calcium HL3 saline. Digestive tract, fat bodies, main trachea, and CNS were removed. Tissue was transferred to 12 μl of buffer containing 50 mM Tris¤ pH 7.4, and 1% Triton with complete proteases inhibitors (Roche, Indianapolis, IN). The sample was homogenized with a microtissue grinder and incubated at 4°C for 20 min. Twelve microliters of 2× SDS loading buffer +dithiothreitol was added, and the samples were boiled for 5 min. Fifteen microliters of each sample was separated on a 6% SDS-polyacrylamide gel and Western-transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was incubated with rabbit anti-Dg (1:3000) and detected with an HRP-coupled secondary antibody, followed by chemiluminescent detection (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). Four microliters of each sample was loaded on 10% SDS-polyacrylamide gel, blotted, and incubated with β-tubulin (1:100 MabE7, Developmental Studies Hybridoma Bank) for loading control.
Physiology
Physiology assays were carried out in HL3 saline (Stewart et al., 1994) with 0.75 mM calcium, as previously described (Stewart and McLean, 2004). Excitatory junctional potentials (EJPs) were analyzed using the cursor options of Clampfit 10.0 (Molecular Devices, Sunnyvale, CA), and miniature EJPS (mEJPs) were analyzed with the template search option. Sample size was 7 for each genotype.
Statistics
One-way ANOVA tests were used for statistical comparisons except where indicated. Tests were carried with Graphpad 4.0 (Prism Software, San Diego, CA), with p < 0.05 chosen as the level of significance.
RESULTS
Muscle Attachment Defects in Dystroglycan Mutants
To determine if Dg plays a role in Drosophila muscles, we examined the musculature of larvae carrying mutant alleles of Dg. Dg alleles have previously been generated and described (Deng et al., 2003; Shcherbata et al., 2007); we examined larvae homozygous for a null allele, Dg323, a hypomorph, Dg248, and Dg323/Dg248 larvae. These Dg mutants survive until early 3rd instar; we dissected late second instar larvae to reveal the neuromuscular system and stained it with fluorescently labeled phalloidin to visualize the muscles. In wild-type larvae, muscles are arranged in a stereotypical pattern and are attached to the underlying epidermis via a link with tendon cells at defined locations (Bate, 1990; Prokop et al., 1998; Volk, 1999; Figure 1A). In abdominal segments 2–7, 30 individual muscles are present in each hemisegment. We found that in Dg323, Dg248, or Dg323/Dg248 larvae one or more muscles were frequently missing or mis-attached (Figure 1, B–F, and Table 1). In some larvae a single muscle from one hemisegment was affected, whereas in other cases more than one hemisegment was affected. No consistent pattern as to which hemisegment or muscle was affected could be determined. Occasionally additional muscle tissue was present (Figure 1C). Some examples of muscle mis-attachment occurred in hemisegments that lacked a muscle and may result from rearrangement of the remaining muscles. In other examples, mis-attachment was observed despite a normal number of muscles in the hemisegment. Together these results demonstrate that Dg plays a role in normal muscle attachment in Drosophila.
Figure 1.
Dg mutants have defects in muscle attachment. Phalloidin-stained musculature of 2nd instar larvae. (A) yw, wild-type arrangement of muscles in part of a hemisegment, and muscles referred to in text are labeled by number according to standard nomenclature. (B–F) Examples of muscle attachment defects in Dg mutants. (B) Dg248, muscle 5 normally located between the arrowheads, is missing. (C) Dg323/Dg248, muscle 5 is underneath, rather than above muscle 4, as indicated by arrowhead. Extra muscle tissue is also present (arrow). (D) Dg248, muscles 6 and 7 cross over and swap their normal attachment sites. Arrowhead on the left side of the panel indicates a normal attachment site for muscle 6, and the muscle at this site is attachment to the normal muscle 7 location on the right-and side of the panel (arrowhead on right). The muscle at the normal muscle 7 attachment site on the left (arrow) crosses under the other muscle to attach on the right-hand side, as indicated by the arrow. (E) Dg323, muscle 5 attachment site is shifted slightly from its normal position indicated by arrow. (F) Dg323/Dg248, muscle 6 is absent, as indicated by arrowheads. All images are shown to scale. Scale bar, (A) 50 μm.
Table 1.
Frequency of muscle attachment defects in 2nd instar Dg mutants
| Muscle defect | yw | Dg248 | Dg323//Dg248 | Dg323 |
|---|---|---|---|---|
| M5 or M8 | ||||
| Abnormal attachment | 1 | 5 | 2 | 1 |
| Missing | 0 | 2 | 0 | 0 |
| Split or very thin | 1 | 1 | 2 | 1 |
| M6, 7, 12, and 13 | ||||
| Abnormal attachment | 0 | 2 | 0 | 2 |
| Missing | 0 | 2 | 3 | 0 |
| n/a | ||||
| Extra muscle tissue | 0 | 1 | 0 | 1 |
| Total defects | 2 | 13 | 7 | 5 |
| Hemisegments examined | 114 | 137 | 99 | 56 |
| Defects/hemisegment | 0.018 | 0.095 | 0.071 | 0.089 |
Dystroglycan Mutant Muscles Have Altered Sarcomere Size
In the course of examining the muscle attachment phenotypes we also noticed that some muscles appeared to be shorter and wider than normal (see below). To examine the underlying muscle sarcomeres we stained the muscles with phalloidin which labels the F-actin present in the muscle contractile machinery resulting in broad stripes along the muscles (Figure 2). The center of each F-actin band, the Z-band, stains more intensely with phalloidin. The spacing of the F-actin bands can be analyzed by graphing gray scale intensity across phalloidin-stained sarcomeres, and Z-bands are represented as peaks. The distance between peaks can be used as a measure of sarcomere size, and the width of the curve can be used to assess the width of the F-actin band (Figure 2; Haines and Stewart, 2007). We examined the spacing of the F-actin bands along muscle 6, segments 3 or 4; when the banding pattern across the entire length of the muscle was intact (yw, n = 8; Dg248, n = 8; Dg323/Dg248, n = 8; Dg323, n = 6).
First, we found that yw larvae showed very consistent sarcomere sizes of ∼8 μm (8.01 ± 0.07 μm, n = 40 sarcomeres from eight muscle; Figure 2D). In contrast, Dg248 mutants the F-actin bands appeared closer together than in controls; on average Dg248 sarcomeres were 7.3 ± 0.07 μm (n = 53 sarcomeres from eight muscles), but the width of the F-actin band was unaffected (Figure 2, B and B′). This could suggest the muscles are hypercontracted. We would expect hypercontracted muscles to be wider and shorter then controls, and so we measured the width and length of the muscles, together with overall muscle surface area. The width of muscle 6/7 combined from Dg248 mutants was larger then controls, whereas muscle length was smaller (width ± SEM in μm; length ± SEM in μm; n = no. of muscles; yw: 61.3 ± 1.2, 195.2 ± 6.3, n = 41; Dg248: 66.8 ± 1.6, 166.8 ± 2.6, n = 27). This resulted in a significantly different width/length ratios for the Dg248 mutants compared with controls (p < 0.001; width/length ± SEM; yw: 0.33 ± 0.011, n = 41; Dg248: 0.41 ± 0.014, n = 27). No significant difference in surface area was found (p > 0.05; area ± SEM μm2, yw: 12844 ± 577, n = 41; Dg248: 11566 ± 239, n = 27). This change in muscle shape, but no change in surface area is consistent with the Dg248 muscles being hypercontracted.
Further examination of the Dg248 data revealed that, along with the reduction in the mean value, the sarcomere measurements were more variable. For example, the coefficient of variation (SD/mean) was 5.6% for yw samples compared with 7.8% for Dg248 samples. This greater variability is also evident from the scatter plot shown in Figure 2D.
We next examined stronger Dg combinations, including Dg323/Dg248 and Dg323 mutants. The mean value for all the data from Dg323/Dg248 muscles was similar to yw: 8.26 ± 0.15 μm, n = 39 sarcomeres from eight muscles. However the data were obviously more variable than in the yw sample, with a coefficient of variation of 12% and 14 sarcomeres larger than the largest measured from the yw sample. For Dg323, the mean value for all the data was increased to 8.7 ± 0.12 μm, n = 35 sarcomeres from six muscles, and the coefficient of variation was 8.4%. In Dg323 samples there were 18 sarcomeres larger than the largest yw sarcomere.
Analysis of variance was performed on the sarcomere size data from the yw, Dg248, Dg323/Dg248, and Dg323 groups to test for differences among the mean values. This revealed significant variation between the groups (p < 0.0001). Posttests revealed that only yw compared with Dg323/Dg248 was not different (p > 0.05), whereas all other combinations were different from each other (p < 0.01). Furthermore, Bartlett's test for equal variance revealed a significant (p < 0.001) difference in the sample variability between the genotypes.
Together these results show Dg alleles can significantly affect sarcomere size. The weakest allele causes sarcomeres to become smaller, whereas increasing allelic strength results in progressively larger sarcomeres, but also a higher degree of variability in the mutant lines. Because Dg323 is a null allele, these data indicate the strongest loss-of-function phenotype is longer sarcomeres.
RNAi Knockdown of Dg Alters Sarcomere Size
To overcome the lethality associated with the Dg mutant alleles and to investigate the requirement for Dg in different tissues, we turned to an RNAi approach. We expressed a UAS-coupled RNAi construct against Dg with a mesoderm driver (24B-Gal4), a ubiquitous driver (P-tub-Gal4), and a pan-ectodermal driver (69B-Gal4). Although 24B-Gal4 UAS-Dg-i was viable, some lethality during larval stages was observed with 69B-Gal4 and P-tub-Gal4; however in each case adult flies could be obtained.
We examined 3rd instar larval muscles to determine if they showed a change in sarcomere size similar to those we had observed in the Dg mutants. Driving expression of the UAS-Dg-i construct with P-tub-Gal4 resulted in sarcomeres that were significantly smaller than controls (p < 0.001; Figure 3, A and B). With 24B-Gal4 we observed a more variable phenotype. Some muscles had small sarcomeres and others were large. Generally, individual larvae showed either small or large sarcomeres in each of its muscles. We did not observe both small and large sarcomeres in a single muscle. This variable phenotype resulted in the 24B-Gal4:UAS-Dg-i having a similar mean sarcomere size as controls but a larger variation (Figure 3B). This increased variation resulted in a coefficient of variation value of 14.35% for 24B-Gal4:UAS-Dg-i compared with 7.03% for yw and 5.21 and 6.21% for UAS-Dg-i/+ and 24B-Gal4/+, respectively. Furthermore, Bartlett's test for equal variance revealed a significant (p < 0.001) difference in the sample variability between the genotypes.
Expressing UAS-Dg-i with 69B-Gal4 did not affect sarcomeres size (Figure 3B). We did not observed a difference between the overall size of the muscles across the different genotypes, as measured by surface area of muscle 6 and 7 combined (p > 0.05). Together these results demonstrate that loss of Dg in muscle results in altered sarcomere size in 3rd instar larval muscles and that both smaller and larger sarcomeres can result from reduced Dg levels. This is consistent with our analysis of the 2nd instar Dg mutant muscles where we observed examples of smaller and larger sarcomeres. In the Dg mutants we had found that the hypercontraction phenotype was predominant among larvae homozygous for the Dg hypomorphic allele Dg248, whereas wider, more relaxed sarcomeres were more common in Dg323/Dg248 or homozygous null Dg323 individuals. This suggests that more severe loss of Dg may tend to result in larger sarcomeres, and weaker Dg knockdown could result in hypercontraction.
To test this idea we took advantage of the temperature dependency of the Gal4 system and enabled the 24B-Gal4::UAS-Dg-i larvae to develop at 30°C, rather than at 25°C as in our experiments described above. This resulted in a stronger decrease of Dg protein on Western blots compared with 24B-Gal4::UAS-Dg-i larvae raised at 25°C (Figure 4A). Although the 24B-Gal4::UAS-Dg-i larvae raised at 25°C had shown a high degree of variance among sarcomeres the variance for the 24B-Gal4::UAS-Dg-i, sarcomeres at 30°C were similar to controls (Figure 4B). The coefficient of variation value was 8.6% for 24B-Gal4::UAS-Dg-i raised at 30°C compared with 8.0% for yw, 6.5% for 24B-Gal4/+, and 5.2% for UAS-Dg-i/+ when raised at 30°C. Although no significant change in variation between the samples was observed, the mean sarcomere size was significantly larger for the 24B-Gal4::UAS-Dg-i compared with the control groups (p < 0.001; Figure 4B). Together these results indicate that strong 24B-Gal4-driven Dg RNAi knockdown results in sarcomeres that are consistently larger than controls (Figure 4B) and together with our results from RNAi knockdown experiments at 25°C suggests that the larger sarcomere phenotype is the result of a more severe loss of Dg compared with the variable or hypercontracted phenotype.
As with the smaller sarcomeres, we did not observe a difference in the width of the phalloidin-stained band (Figure 4C). To further examine the 24B-Gal4::UAS-Dg-i 30°C muscles, we used an antibody against the Z-band marker α-actinin and found that this protein was localized normally. The apparently normal arrangement of F-actin and the Z-band maker in the 24B-Gal4::UAS-Dg-i muscles suggests that despite the changes in sarcomere size the contractile machinery remains intact. Further the Dg RNAi knockdown and Dg mutant larvae appear to crawl normally, suggesting that the ability of the muscles to contract is not significantly compromised. We conclude the simplest explanation for the change in sarcomere size that we observe is altered muscle contraction.
The 3rd instar UAS-Dg-i larvae were also examined for muscle attachment defects; however, driving expression with 24B-Gal4, 69B-Gal4, or P-tub-Gal4 did not result in a significant number of attachment defects when compared with the Gal4 drivers alone. This suggests that the temporal or spatial knockdown of Dg achieved with these drivers is insufficient to disrupt this aspect of Dg function.
Electrical Properties of Dg RNAi Muscles
In vertebrate muscles lacking components of the DGC membrane, fragility leading to microlesions in the membrane have been suggested as early events that begin a cycle of muscle damage (Deconinck and Dan, 2007). We used intracellular recording techniques to measure membrane resistance, membrane potential, and both spontaneous mEJPs and nerve-stimulated EJPs synaptic release to determine if the muscle membrane function or synaptic function was compromised in the 24B-Gal4::UAS-Dg-i muscles. We found that the resting membrane potential was similar for all genotypes (Table 2), but that there was a significant difference in membrane resistance between the controls (UAS-Dg-i/+) and 24B-Gal4::UAS-Dg-i larvae that were raised at either 25°C and at 30°C (p < 0.05 for both comparison; Table 2). However, rather than decreased membrane resistance as we expected if membrane were more permeable because of lesions, we found a significantly higher resistance in 24B-Gal4::UAS-Dg-i muscles. In line with the increased membrane resistance, the EJP amplitude was also significantly increased. No change in mEJP amplitude was detected, although the mean value was increased in 24B-Gal4::UAS-Dg-i muscles at both temperatures. Together these results indicate a change in membrane function in the 24B-Gal4::UAS-Dg-i muscles compared with controls.
Table 2.
Membrane resistance is increased in Dg RNAi knockdown muscles
| 25°C |
30°C |
|||
|---|---|---|---|---|
| Dg-i/+ | 24B-Gal4::Dg-i | Dg-i/+ | 24B-Gal4::Dg-i | |
| Membrane potential (mV) | −69 ± 2.5 | −67 ± 1.1 | −65 ± 2.0 | −67 ± 1.4 |
| Membrane resistance (mΩ) | 5.8 ± 0.45 | 8.3 ± 0.61a | 3.7 ± 0.89 | 5.8 ± 0.37a |
| mEJP frequency (Hz) | 2.62 ± 0.36 | 2.26 ± 0.42 | 1.13 ± 0.14 | 0.70 ± 0.05 |
| mEJP amplitude (mV) | 0.89 ± 0.05 | 1.1 ± 0.11 | 0.73 ± 0.10 | 0.85 ± 0.03 |
| EJP amplitude (mV) | 34.42 ± 4.2 | 44.41 ± 2.5a | 20.29 ± 2.3 | 32.86 ± 1.4a |
| Quantal content | 44.32 ± 5.7 | 46.46 ± 2.7 | 30.76 ± 5.1 | 44.11 ± 2.9 |
Each parameter was analyzed by two-way ANOVA (genotype × temperature); ap < 0.05; two-way ANOVA.
Dystroglycan Is Associated with the Transverse-Tubule Network in Larval Muscles
Dg expression has previously been reported in muscle fibers in Drosophila embryos by in situ hybridization (Dekkers et al., 2004); however, its subcellular location has not been reported. Because we have identified phenotypes when Dg levels are reduced in muscles with RNAi, we sought to establish the subcellular localization of Dg in the Drosophila larval muscles using an anti-Dg antibody (Deng et al., 2003). Drosophila Dg seems not to be cleaved into α and β subunits but remains as a single polypeptide (Deng et al., 2003), and this antibody recognizes an epitope in the intracellular domain (Deng et al., 2003). Dg staining was observed in puncta on the top of the muscle cells (Figure 5, A and B). In the internal area of the muscles Dg staining was present in repeating stripes through the sarcomeres (Figure 5D). Given the transmembrane domain in Dg, we reasoned that this could represent Dg at the transverse (T)-tubule membrane. These invaginations of the plasma membrane penetrate deep into the muscle and are involved in excitation contraction coupling. In vertebrate cardiac muscle Dg, its associated protein Dystrophin (Dys), and Lam are found in regions where T-tubules are located (Klietsch et al., 1993; Kaprielian et al., 2000), and the staining pattern of Dys in Drosophila larval muscles has been suggested to be T-tubule associated (van der Plas et al., 2006). We stained muscles with an antibody against the known T-tubules marker Discs Large (Dlg; Razzaq et al., 2001) and found that the Dlg and Dg signals are localized in a similar pattern across the sarcomeres (Figure 5, C–F). The similar pattern but the lack of specific colocalization suggests that these proteins are localized to different regions of the T-tubule network.
N-terminal Dystroglycan Extends into the T-tubule Lumen
If Dg is associated with the T-tubule membrane, we would expect that its N-terminal domain would extend into the T-tubule lumen, allowing Dg to interact with extracellular ligands. As vertebrate α-Dg is heavily glycosylated in its mucin domain we investigated the possibility that lectins could be used to specifically recognize the extracellular region of Dg in Drosophila muscles via glycan structures. Therefore, we first needed to validate potential lectins as tools to probe Dg localization.
To identify potential Dg binding lectins¤ we overexpressed a UAS-Dg (Deng et al., 2003) construct using 24B-Gal4; this full-length construct encodes the Dg-C isoform that contains the entire mucin domain (Deng et al., 2003; Schneider et al., 2006). In this overexpression situation, anti-Dg antibody reveals high levels of Dg present in the sarcomeres and strong accumulation at the postsynaptic side of the neuromuscular junction (Figure 6A). Therefore, lectins that bind Dg should also show ectopic postsynaptic staining in the UAS-Dg muscles. No change in staining pattern was observed with WGA, SJA, or GSL-II (data not shown); however, background staining may interfere with our ability to detect an increase in staining at the neuromuscular junction with these lectins. SNL, which has specificity toward sialic acid, showed some increase in postsynaptic staining, but Vicia villosa Agglutinin (VVA), which binds N-acetylgalactosamine–terminated glycoconjugates, showed a dramatic accumulation at postsynaptic sites in 24B-Gal4::UAS-Dg-C muscles, but no significant postsynaptic staining in yw controls (Figure 6A and data not shown; compare to Figure 6B).
To confirm that VVA was binding to the N-terminal region of Dg, we expressed a truncated Dg construct that lacks the entire extracellular domain, UAS-Dg-cyt (Deng et al., 2003). The Dg antibody recognized this truncated protein, and the antibody showed strong staining in the sarcomeres and in postsynaptic regions, similar to overexpression of the full-length construct (Figure 6B); however, no VVA staining was detected at postsynaptic regions in these muscles. These results demonstrate that VVA can recognize the extracellular domain of Dg.
We next examined VVA staining in wild-type muscles and observed staining in broad stripes in the sarcomeres overlapping the phalloidin signal (Figure 6C). This staining is reduced in 24B-Gal4::UAS-Dg-i 30°C muscles (Figure 6D). Altogether these results demonstrate that Dg is a major VVA-reactive protein in the larval muscles, and suggest that Drosophila Dg is modified with GalNAc-terminated glycans. The VVA staining pattern establishes the location of N-terminal Dg in broad stripes that appear to overlap phalloidin. This staining pattern likely represents N-terminal Dg projecting into the T-tubule lumen.
Laminin and Dystrophin are T-Tubule Associated
The N-terminal region of Dg interacts with a number of extracellular ligands and in muscles a major ligand is Lam. We sought to establish if Dg could potentially interact with Lam in Drosophila larval muscles. Using an antibody against Lam, we found that this protein is present in repeating stripes through the sarcomeres where it overlaps with the VVA signal (Figure 6C).
In vertebrates binding between Dg and Lam provides a link between the membrane and the extracellular matrix (Blake et al., 2002); a second link between the intracellular C-terminal domain of Dg and the actin-binding protein Dys serves to anchor the complex to the cytoskeleton (Blake et al., 2002). A previous study demonstrated that Dys is found in repeating stripes in the Drosophila larval muscles and suggested the possibility that Dys could be associated with T-tubules (van der Plas et al., 2006). Altogether the location and proximity of the core DGC proteins, Dg and Dys, and the presence of Lam in the Drosophila muscles indicated that a T-tubule DGC-like complex is present in the larval muscles.
Mutants in POMTs Display Muscle Attachment Defects and Altered Sarcomere Size
Having established that Dg is required for normal muscle integrity and provided evidence that Dg is localized to T-tubules, along with the other known members of the DGC complex, we next examined the importance of glycosylation to Dg function. Mutations in POMT1 or POMT2 are associated with muscular dystrophy in vertebrates because of a requirement for O-linked mannose glycans on the Dg protein (Akasaka-Manya et al., 2004; van Reeuwijk et al., 2005). We investigated whether POMTs may be required for Dg function in Drosophila by examining larvae mutant for either rt (POMT1) or tw (POMT2) or doubly mutant for both. Muscle attachment defects have previously been reported in rt mutants (Martin-Blanco and Garcia-Bellido, 1996), and our analysis found muscle attachment errors in single rt and tw mutants and in double mutants (Figure 7, A–E). These defects occurred at a frequency similar to that seen in the Dg mutants (Table 3). Examples of muscle absence, mis-attachment, and extra or split muscle fibers were observed (Figure 7, A–E). To determine if these mutants showed changes in muscle contraction, sarcomere size was measured from 3rd instar larval muscles. tw and rt mutants and tw;rt double mutant sarcomeres were all larger than controls (Figure 7F) and were similar in size to the sarcomeres of 24B-Gal4::UAS-Dg-i larvae that had been raised at 30°C. Together these data are consistent with a requirement for O-linked mannose glycans on Drosophila Dg and support biochemical data that indicate a nonredundant role for these enzymes in modifying Dg (Ichimiya et al., 2004).
Table 3.
Frequency of muscle attachment defects in 3rd instar rt and tw mutants
| Muscle defect | yw | tw1 | rt2 | rt2/rtp | tw1;rt2 | tw1;rt2/rtp |
|---|---|---|---|---|---|---|
| M5 or M8 | ||||||
| Abnormal attachment | 0 | 5 | 5 | 0 | 3 | 2 |
| Missing | 0 | 5 | 1 | 1 | 3 | 1 |
| Split or very thin | 0 | 10 | 1 | 5 | 4 | 8 |
| M6, 7, 12, and 13 | ||||||
| Abnormal attachment | 0 | 2 | 0 | 0 | 1 | 0 |
| Missing | 1 | 2 | 0 | 0 | 3 | 2 |
| n/a | ||||||
| Extra muscle tissue | 2 | 4 | 1 | 3 | 3 | 4 |
| Total defects | 3 | 28 | 8 | 9 | 17 | 17 |
| Hemisegments examined | 129 | 219 | 60 | 82 | 128 | 147 |
| Defects/hemisegment | 0.016 | 0.128 | 0.133 | 0.110 | 0.132 | 0.116 |
Genetic Interactions between POMTs and Dystroglycan
To further establish a link between POMTs and Dg, we generated larvae that carried mutations in tw or rt in combination with Dg alleles and examined the musculature for the attachment and sarcomere size phenotypes. Both tw and rt interacted with Dg. tw/+; Dg248/+ 3rd instar larvae, showing muscle attachment defects (0.110/hemisegment, n = 82), but normal sized sarcomeres (8.34 ± 0.09 μm, n = 73). A strong interaction was observed between Dg and rt, the Dg323/+; rtp/+ combination was semilethal, and larvae that survived to 3rd instar showed muscle attachment defects (0.073/hemisegment, n = 82) and large sarcomeres (9.045 ± 0.07 μm, n = 47). These interactions support a role for the glycosyltransferases in the same molecular pathway as Dg.
We next examined double mutant combinations. We found that tw1/Y;Dg323/Dg323 or Dg323/Dg323; rt2/rt2 animals showed an identical phenotype to the Dg323/Dg323 single mutants. The larvae survived until early 3rd instar and tw1/Y;Dg323/Dg323 (n = 4), and Dg323/Dg323; rt2/rt2 (n = 6) larvae showed changes in sarcomere size and muscle attachment defects. Attachment defects were observed at a frequency of 0.094/hemisegment (n = 32) for tw1/Y;Dg323/Dg323 and at 0.091/hemisegment (n = 33) for Dg323/Dg323; rt2/rt2. The lack of additional phenotypes in these double mutant combinations, compared with the Dg323/Dg323 single mutants, argues that Dg is the major functional substrate for these glycosyltransferases in Drosophila.
DISCUSSION
The DGC is known to play a central role in maintaining integrity in a variety of different muscle types. In this study we established that Dg is required in Drosophila larval muscles to maintain integrity. We have identified changes in muscle contraction associated with reduced Dg function. Second instar Dg248 larvae had short and wide muscles, and a decrease in sarcomere size, together indicating that the muscles are hypercontracted (Figure 2). Among larvae null for Dg we found that sarcomere size was more variable between individuals, with some muscles having smaller sarcomeres and others larger (Figure 2). Among 3rd instar larvae knockdown for Dg we also observed changes in sarcomere size. When an RNAi construct against Dg was driven with the ubiquitous driver P-tub-Gal4, we found that sarcomeres were consistently smaller than controls (Figure 3). Driving the RNAi construct with the mesoderm driver 24B-Gal4 resulted in a variable phenotype, with both small and large sarcomere being observed. This phenotype shifted to exclusively larger sarcomeres when these larvae developed at 30°C, likely due to a greater decrease of Dg protein (Figures 3B and 4). Because the large sarcomere phenotype was seen in null Dg larvae and with strong RNAi knockdown compared with smaller sarcomeres in the Dg248 individuals and with weaker RNAi knockdown of Dg, we conclude that the large sarcomere phenotype is related to more severe loss of Dg than with hypercontraction.
Because we observed changes in muscle sarcomere size with RNAi driven with the ubiquitous driver and the mesoderm driver but not with the pan-ectodermal driver, we conclude that the sarcomere size changes are due to loss of Dg in muscles. Together our results demonstrate that reduced levels of Dg alter muscle contraction and that Dg plays a role in Drosophila muscles.
Consistent with our findings is that both hypercontraction and overstretching of sarcomeres is seen in vertebrate dystrophic muscles before they progress to a more advanced degeneration phenotype (Cullen and Fulthorpe, 1975). A tendency to hypercontract is also associated with disruption of DGC proteins in Caenorhabditis elegans (Bessou et al., 1998; Grisoni et al., 2002). These findings suggest that contractile changes are a common early response to lack of Dg function in different types of muscle. Further studies will focus on understanding whether these changes in muscle contraction stem from developmental changes in the muscles or are the result of early changes associated with muscle degeneration.
In vertebrate muscles lacking DGC components, the mechanisms leading to muscle dystrophy remain unclear; however, membrane fragility is likely involved (Blake et al., 2002; Deconinck and Dan, 2007). Mechanical stress from muscle contraction is thought to lead to membrane microlesions and compromised muscle membrane function. Our electrophysiological analysis showed an increase in membrane resistance and greater EJP amplitude in 24B-Gal4::UAS-Dg-i muscles. The increase in passive membrane resistance could be due to down-regulation of the leakage channel activity by the muscle. The increased resistance would help to maintain excitation-contraction coupling. Interestingly, a similar increase in muscle membrane resistance has been reported in mice mutant for dystrophin (mdx; De Luca et al., 1997).
In vertebrate cardiac muscle T-tubule–associated Dys, Dg¤ and Lam have been reported (Klietsch et al., 1993; Kaprielian et al., 2000). This location differs from the sarcolemma localized DGC found in vertebrate skeletal muscle (Blake et al., 2002). The inherited muscular dystrophies are associated with dilated cardiomyopthy, and differences in the degree of dysfunction between cardiac and skeletal muscle in individuals with muscular dystrophy has lead to the suggestion that the DGC may have alternative cellular roles in these muscle types (Lapidos et al., 2004; Goodwin and Muntoni, 2005). Our finding that Dg and Lam are T-tubule associated in Drosophila larval muscles suggests these Drosophila muscles may provide a good model for investigating the cellular function of T-tubule–associated DGC. Studying the role of the DGC in different types of muscle will increase our overall understanding of how this complex functions at both a molecular and cell biological level.
Lam and adhesion molecules such as intergrins play important roles in muscle attachment in Drosophila (Brown, 1994; Yarnitzky and Volk, 1995; Prokop et al., 1998; Delon and Brown, 2007). Muscles detach from the epidermis and round up or stick nonspecifically to other muscles or the ECM in Drosophila carrying mutant alleles of these genes (Brown, 1994; Prokop et al., 1998). Given the role of Dg in ECM adhesion in other cellular contexts (Winder, 2001) and the role of Lam in muscle attachment in Drosophila (Prokop et al., 1998), the muscle attachment phenotypes we have observed in the Dg mutants most likely result from weakening of the connection between muscle and epidermal tendon cell to which it connects or these cells and the ECM. Such a weakness could explain the random nature of the phenotypes. Failure of a muscle to maintain its connection with the tendon cell could result in loss of the muscle or result in the muscle forming a link with another muscle or tendon resulting in a mis-attachment phenotype.
In vertebrates, Dg function is regulated by glycosylation (Michele and Campbell, 2003; Muntoni et al., 2004; Barresi and Campbell, 2006). We observed changes in sarcomere size and defects in muscle attachment in Drosophila mutant for genes encoding POMT1 and POMT2. We also found that both these mutants interact with Dg mutant alleles in transheterozygous combinations. The similarity of the rt and tw muscle phenotypes to those associated with reduced Dg function and the interactions between mutant alleles of rt, tw, and Dg provide strong evidence that rt and tw are required for Dg-dependent processes in Drosophila. Given the O-mannosyltransferase activity of the rt and tw gene products toward Dg (Ichimiya et al., 2004) and the genetic evidence from vertebrates that loss of POMTs results in hypoglycosylation of Dg and subsequent disruption to Dg function, these data provide compelling evidence supporting a functional requirement for O-mannose glycans on Dg in Drosophila. The adult abdomen in tw and rt mutant flies is rotated. We have not observed this phenotype in our experiments with Gal4-driven Dg RNAi knockdown. Possibly the abdominal rotation is due to loss of Dg function in these mutants and the lack of this phenotype in the RNAi flies is due to insufficient knockdown of Dg. It also remains possible that rt and tw have another substrate, and loss of glycan structures on this protein results in the rotated abdomen phenotype.
Altogether the phenotypes we have identified by manipulating Dg, tw, and rt demonstrate that Dg plays a central role in maintaining cell integrity in the Drosophila larval muscles and that glycosylation of Dg is important to its function. This report therefore opens the possibility of using genetic analysis of the highly accessible neuromuscular system available in this model organism to analyze the mechanisms by which loss of DGC function leads to muscle dystrophy.
ACKNOWLEDGMENTS
The authors thank H. Ruohola-Baker (University of Washington), J. Poulton (Florida State University), W. M. Deng (Florida State University), M. Schneider (Lund University, Lund, Sweden), and T. Volk (Weizmann Institute of Sciences, Rehovot, Israel) for generously providing flies lines and antibodies that were used in this study; the Bloomington stock center for fly stocks; and the Developmental Studies Hybridoma Bank for antibodies. We also thank Ken Irvine for helpful advice on the manuscript. This work was supported by grants to B.A.S. from the Natural Sciences and Engineering Research Council of Canada, the Mizutani Foundation for Glycoscience Research, a Premiers Research Excellence Award, and the Canada Research Chairs program.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-01-0047) on September 19, 2007.
REFERENCES
- Akasaka-Manya K., Manya H., Endo T. Mutations of the POMT1 gene found in patients with Walker-Warburg syndrome lead to a defect of protein O-mannosylation. Biochem. Biophys. Res. Commun. 2004;325:75–79. doi: 10.1016/j.bbrc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Akasaka-Manya K., Manya H., Nakajima A., Kawakita M., Endo T. Physical and functional association of human protein O-mannosyltransferases 1 and 2. J. Biol. Chem. 2006;281:19339–19345. doi: 10.1074/jbc.M601091200. [DOI] [PubMed] [Google Scholar]
- Barresi R., Campbell K. P. Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Bate M. The embryonic development of larval muscles in Drosophila. Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- Bessou C., Giugia J. B., Franks C. J., Holden-Dye L., Segalat L. Mutations in the Caenorhabditis elegans dystrophin-like gene dys-1 lead to hyperactivity and suggest a link with cholinergic transmission. Neurogenetics. 1998;2:61–72. doi: 10.1007/s100480050053. [DOI] [PubMed] [Google Scholar]
- Blake D. J., Weir A., Newey S. E., Davies K. E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Brown N. H. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development. 1994;120:1221–1231. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- Chiba A., Matsumura K., Yamada H., Inazu T., Shimizu T., Kusunoki S., Kanazawa I., Kobata A., Endo T. Structures of sialylated O-linked oligosaccharides of bovine peripheral nerve alpha-dystroglycan. The role of a novel O-mannosyl-type oligosaccharide in the binding of alpha-dystroglycan with laminin. J. Biol. Chem. 1997;272:2156–2162. doi: 10.1074/jbc.272.4.2156. [DOI] [PubMed] [Google Scholar]
- Combs A. C., Ervasti J. M. Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation. Biochem. J. 2005;390:303–309. doi: 10.1042/BJ20050375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. J., Fulthorpe J. J. Stages in fibre breakdown in Duchenne muscular dystrophy. An electron-microscopic study. J. Neurol. Sci. 1975;24:179–200. doi: 10.1016/0022-510x(75)90232-4. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S., Camerino D. C. Electrical properties of diaphragm and EDL muscles during the life of dystrophic mice. Am. J. Physiol. 1997;272:C333–C340. doi: 10.1152/ajpcell.1997.272.1.C333. [DOI] [PubMed] [Google Scholar]
- Deconinck N., Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr. Neurol. 2007;36:1–7. doi: 10.1016/j.pediatrneurol.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Dekkers L. C., van der Plas M. C., van Loenen P. B., den Dunnen J. T., van Ommen G. J., Fradkin L. G., Noordermeer J. N. Embryonic expression patterns of the Drosophila dystrophin-associated glycoprotein complex orthologs. Gene Expr. Patterns. 2004;4:153–159. doi: 10.1016/j.modgep.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Delon I., Brown N. H. Integrins and the actin cytoskeleton. Curr. Opin. Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Deng W. M., Schneider M., Frock R., Castillejo-Lopez C., Gaman E. A., Baumgartner S., Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–184. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Campbell K. P. Dystrophin-associated glycoproteins: their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol. Cell Biol. Hum. Dis. Ser. 1993;3:139–166. doi: 10.1007/978-94-011-1528-5_6. [DOI] [PubMed] [Google Scholar]
- Goodwin F. C., Muntoni F. Cardiac involvement in muscular dystrophies: molecular mechanisms. Muscle Nerve. 2005;32:577–588. doi: 10.1002/mus.20352. [DOI] [PubMed] [Google Scholar]
- Greener M. J., Roberts R. G. Conservation of components of the dystrophin complex in Drosophila. FEBS Lett. 2000;482:13–18. doi: 10.1016/s0014-5793(00)02018-4. [DOI] [PubMed] [Google Scholar]
- Grisoni K., Martin E., Gieseler K., Mariol M. C., Segalat L. Genetic evidence for a dystrophin-glycoprotein complex (DGC) in Caenorhabditis elegans. Gene. 2002;294:77–86. doi: 10.1016/s0378-1119(02)00762-x. [DOI] [PubMed] [Google Scholar]
- Haines N., Stewart B. A. Functional roles for β 1,4-N-acetlygalactosaminyltransferase-A in Drosophila larval neurons and muscles. Genetics. 2007;175:671–679. doi: 10.1534/genetics.106.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T., Manya H., Ohmae Y., Yoshida H., Takahashi K., Ueda R., Endo T., Nishihara S. The twisted abdomen phenotype of Drosophila POMT1 and POMT2 mutants coincides with their heterophilic protein O-mannosyltransferase activity. J. Biol. Chem. 2004;279:42638–42647. doi: 10.1074/jbc.M404900200. [DOI] [PubMed] [Google Scholar]
- Kanagawa M., Toda T. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J. Hum. Genet. 2006;51:915–926. doi: 10.1007/s10038-006-0056-7. [DOI] [PubMed] [Google Scholar]
- Kaprielian R. R., Stevenson S., Rothery S. M., Cullen M. J., Severs N. J. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101:2586–2594. doi: 10.1161/01.cir.101.22.2586. [DOI] [PubMed] [Google Scholar]
- Klietsch R., Ervasti J. M., Arnold W., Campbell K. P., Jorgensen A. O. Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ. Res. 1993;72:349–360. doi: 10.1161/01.res.72.2.349. [DOI] [PubMed] [Google Scholar]
- Lapidos K. A., Kakkar R., McNally E. M. The dystrophin glycoprotein complex: signaling strength and integrity for the sarcolemma. Circ. Res. 2004;94:1023–1031. doi: 10.1161/01.RES.0000126574.61061.25. [DOI] [PubMed] [Google Scholar]
- Lyalin D., Koles K., Roosendaal S. D., Repnikova E., Van Wechel L., Panin V. M. The twisted gene encodes Drosophila protein O-mannosyltransferase 2 and genetically interacts with the rotated abdomen gene encoding Drosophila protein O-mannosyltransferase 1. Genetics. 2006;172:343–353. doi: 10.1534/genetics.105.049650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manya H., Chiba A., Yoshida A., Wang X., Chiba Y., Jigami Y., Margolis R. U., Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. USA. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blanco E., Garcia-Bellido A. Mutations in the rotated abdomen locus affect muscle development and reveal an intrinsic asymmetry in Drosophila. Proc. Natl. Acad. Sci. USA. 1996;93:6048–6052. doi: 10.1073/pnas.93.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele D. E., et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Michele D. E., Campbell K. P. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J. Biol. Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- Muntoni F., Brockington M., Torelli S., Brown S. C. Defective glycosylation in congenital muscular dystrophies. Curr. Opin. Neurol. 2004;17:205–209. doi: 10.1097/00019052-200404000-00020. [DOI] [PubMed] [Google Scholar]
- Neuman S., Kaban A., Volk T., Yaffe D., Nudel U. The dystrophin/utrophin homologues in Drosophila and in sea urchin. Gene. 2001;263:17–29. doi: 10.1016/s0378-1119(00)00584-9. [DOI] [PubMed] [Google Scholar]
- Poulton J. S., Deng W. M. Dystroglycan down-regulation links EGF receptor signaling and anterior-posterior polarity formation in the Drosophila oocyte. Proc. Natl. Acad. Sci. USA. 2006;103:12775–12780. doi: 10.1073/pnas.0603817103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A., Martin-Bermudo M. D., Bate M., Brown N. H. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev. Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Razzaq A., Robinson I. M., McMahon H. T., Skepper J. N., Su Y., Zelhof A. C., Jackson A. P., Gay N. J., O'Kane C. J. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Khalil A. A., Poulton J., Castillejo-Lopez C., Egger-Adam D., Wodarz A., Deng W. M., Baumgartner S. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–3815. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbata H. R., Yatsenko A. S., Patterson L., Sood V. D., Nudel U., Yaffe D., Baker D., Ruohola-Baker H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007;26:481–493. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart B. A., Atwood H. L., Renger J. J., Wang J., Wu C. F. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J. Comp. Physiol. [A] 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Stewart B. A., McLean J. R. Population density regulates Drosophila synaptic morphology in a Fasciclin-II-dependent manner. J. Neurobiol. 2004;61:392–399. doi: 10.1002/neu.20096. [DOI] [PubMed] [Google Scholar]
- van der Plas M. C., Pilgram G. S., Plomp J. J., de Jong A., Fradkin L. G., Noordermeer J. N. Dystrophin is required for appropriate retrograde control of neurotransmitter release at the Drosophila neuromuscular junction. J. Neurosci. 2006;26:333–344. doi: 10.1523/JNEUROSCI.4069-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J., et al. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J. Med. Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk T. Singling out Drosophila tendon cells: a dialogue between two distinct cell types. Trends Genet. 1999;15:448–453. doi: 10.1016/s0168-9525(99)01862-4. [DOI] [PubMed] [Google Scholar]
- Winder S. J. The complexities of dystroglycan. Trends Biochem. Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T., Volk T. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev. Biol. 1995;169:609–618. doi: 10.1006/dbio.1995.1173. [DOI] [PubMed] [Google Scholar]