Abstract
Ca2+/calmodulin (CaM)-dependent protein kinase II (CaMKII) is a major mediator of cellular Ca2+ signaling. Several inhibitors are commonly used to study CaMKII function, but these inhibitors all lack specificity. CaM-KIIN is a natural, specific CaMKII inhibitor protein. CN21 (derived from CaM-KIIN amino acids 43–63) showed full specificity and potency of CaMKII inhibition. CNs completely blocked Ca2+-stimulated and autonomous substrate phosphorylation by CaMKII and autophosphorylation at T305. However, T286 autophosphorylation (the autophosphorylation generating autonomous activity) was only mildly affected. Two mechanisms can explain this unusual differential inhibitor effect. First, CNs inhibited activity by interacting with the CaMKII T-site (and thereby also interfered with NMDA-type glutamate receptor binding to the T-site). Because of this, the CaMKII region surrounding T286 competed with CNs for T-site interaction, whereas other substrates did not. Second, the intersubunit T286 autophosphorylation requires CaM binding both to the “kinase” and the “substrate” subunit. CNs dramatically decreased CaM dissociation, thus facilitating the ability of CaM to make T286 accessible for phosphorylation. Tat-fusion made CN21 cell penetrating, as demonstrated by a strong inhibition of filopodia motility in neurons and insulin secrection from isolated Langerhans' islets. These results reveal the inhibitory mechanism of CaM-KIIN and establish a powerful new tool for dissecting CaMKII function.
INTRODUCTION
Ca2+/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional protein kinase best known for its critical role in learning and memory (for review, see Lisman and McIntyre, 2001; Soderling et al., 2001; Hudmon and Schulman, 2002; Lisman et al., 2002). CaMKII is highly expressed in the brain (Erondu and Kennedy, 1985), but at least one of its four isoforms (α, β, γ, and δ) has been found in every cell type examined (Tobimatsu and Fujisawa, 1989; Bayer et al., 1999; Tombes et al., 2003). Numerous cellular functions of CaMKII have been described previously, both in and outside the nervous system. These include regulation of various ion channels (Worrell and Frizzell, 1991; Wang and Best, 1992; Roeper et al., 1997; Derkach et al., 1999; Dzhura et al., 2000), gene expression (Nghiem et al., 1994; Ramirez et al., 1997; Meffert et al., 2003), cell cycle/proliferation control (Baitinger et al., 1990; Patel et al., 1999; Matsumoto and Maller, 2002; Illario et al., 2003), apoptotic and excitotoxic cell death (Laabich and Cooper, 2000; Fladmark et al., 2002), cell morphology (Wu and Cline, 1998; Fink et al., 2003), and filopodia motility (Fink et al., 2003). CaMKII also has been implicated in regulation of insulin secretion (for review, see Easom, 1999); however, this conclusion is largely based on experiments using KN inhibitors, which also affect the Ca2+ channels required for secretion (see below).
CaMKII forms multimeric holoenzymes (Bennett et al., 1983; Kanaseki et al., 1991; Kolodziej et al., 2000; Morris and Torok, 2001; Hoelz et al., 2003; Rosenberg et al., 2005), and a Ca2+/calmodulin (CaM)-dependent intersubunit autophosphorylation at T286 renders the kinase active even after dissociation of Ca2+/CaM (Hanson et al., 1994; Rich and Schulman, 1998). Phosphorylation of T286, which is located in the regulatory region, relieves autoinhibition by preventing binding of the region around T286 to the T-site, which is adjacent to the substrate binding S-site (see kinase domain model in Figure 3A) (Bayer et al., 2001, 2006; Rosenberg et al., 2005). The subsequent Ca2+-independent or autonomous activity has been regarded as a form of “molecular memory,” and it is important in several neuronal functions of the kinase (Giese et al., 1998; Lisman and McIntyre, 2001; Lisman et al., 2002). Additionally, T286 phosphorylation traps CaM on CaMKII (Meyer et al., 1992), and regulates CaMKII binding to other proteins (for reviews, see Bayer and Schulman, 2001; Colbran, 2004), such as syntaxin (Ohyama et al., 2002), densin-180 (Strack et al., 2000; Walikonis et al., 2001), NR1 (Leonard et al., 2002), NR2A (Gardoni et al., 1999), NR2B (Strack and Colbran, 1998; Bayer et al., 2001), and F-actin (Fink et al., 2003; O'Leary et al., 2006). Among the other autophosphorylation sites, the functional consequences of T305/306 phosphorylation are understood best. T305/306 autophosphorylation can occur in an intrasubunit reaction, blocks CaM binding (Colbran and Soderling, 1990), accelerates CaMKII dissociation from synaptic sites (Shen et al., 2000), and also plays a role in learning (Elgersma et al., 2002).
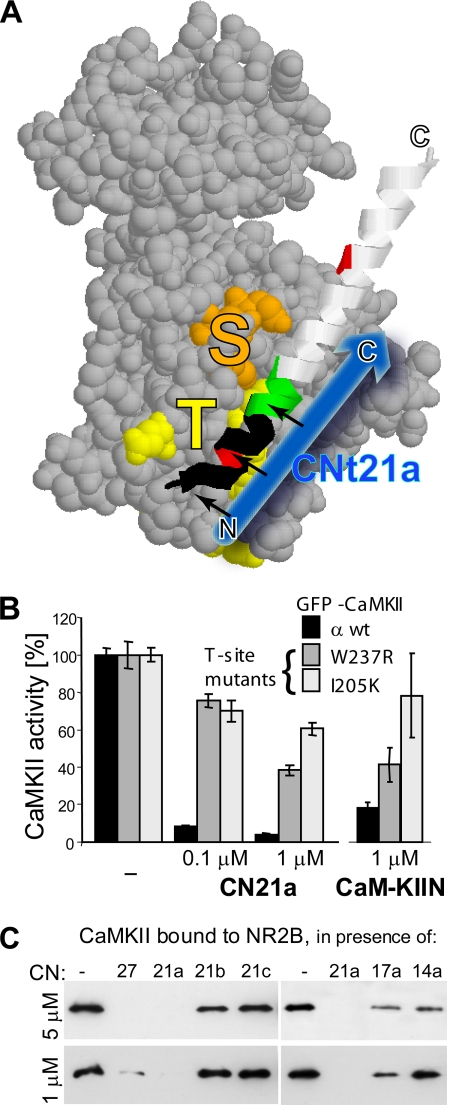
Figure 3.
CaM-KIIN and CN21 interact with the CaMKII T-site. (A) Model of the CaMKII kinase domain (Rosenberg et al., 2005) with the substrate binding S-site (orange), the T286-region binding T-site (yellow), and the regulatory region (ribbon). The blue arrow indicates the proposed orientation of CN21a binding to the T-site (when the regulatory region is displaced after activation) (B) Compared with GFP-CaMKIIα wild type (wt), the activity of the CaMKII T-site mutants W237R and I205K were significantly less affected by CN21a (p < 0.001; n = 6) or CaM-KIIN (p < 0.02; n = 4), as assessed by standard kinase assays with the peptide substrate syntide2. Results are normalized to maximal kinase activity without inhibitor. Error bars show SEM (C) CN21 efficiently blocked Ca2+/CaM-induced CaMKII binding to immobilized GST-NR2B-c, an interaction that occurs at the CaMKII T-site (Bayer et al., 2001, 2006). Bound CaMKII was eluted and detected by Western blot. C- but not N-terminal truncations of CN21 also inhibited binding to NR2B.
CaMKII inhibitors such as KN62, KN93, and peptides derived from the autoinibitory region of CaMKII, such as AIP or AC3-I, are useful tools for examining functions of the kinase. However, the KN drugs cannot discriminate between CaMKII and CaMKIV (Enslen et al., 1994), and they inhibit voltage-gated K+ and Ca2+ channels (Li et al., 1992; Ledoux et al., 1999). Moreover, the KN drugs interfere competitively with activation by CaM, and thus they do not inhibit autonomous activity of the kinase (Tokumitsu et al., 1990; Sumi et al., 1991). The CaMKII-derived peptide inhibitors are widely thought to be more specific. However, such peptides also inhibit other CaM-dependent kinases and protein kinase (PK) A (Smith et al., 1990; Hvalby et al., 1994), and their potency is low (Chen et al., 2001). The natural CaMKII inhibitor protein CaM-KIIN provides a promising alternative, because it potently inhibits CaMKII but not CaMKI, CaMKIV, PKA, or PKC (Chang et al., 1998, 2001). Two CaM-KIIN isoforms are highly homologous to each other and colocalize with microtubules in neurons; both bind selectively to CaMKII only in its activated states (Chang et al., 1998, 2001). CaM-KIIN–derived peptides could provide superior CaMKII inhibitors, especially if they are short enough to be synthesized easily.
Here, we identify the minimal region of CaM-KIINα that retains full potency and specificity of CaMKII inhibition (CN21) (the homologous CaM-KIINβ region differs at one residue only). CN21 efficiently blocked substrate- and T305 autophosphorylation of CaMKII, but surprisingly it only mildly affected T286 autophosphorylation. Identification of the T-site as the CaM-KIIN interaction site on CaMKII provided two mechanisms for this novel differential inhibitor effect: CaM-KIIN was competitive with the region around T286, and strengthened the CaM binding required for presentation of T286 as a substrate. Tat-fused CN21 was cell-penetrating and inhibited filopodia motility and insulin secretion, thus providing a powerful new tool for studying cellular CaMKII function.
MATERIALS AND METHODS
Peptides and Proteins
CaMKIIα and β were purified from a baculovirus/Sf9 cell expression system, CaM was purified after bacterial expression (Bayer et al., 2001; Singla et al., 2001). 2-Chloro-(ε-amino-Lys75)-[6-(4-N,N-diethylaminophenyl)-1,3,5-triazin-4-yl] calmodulin (TA-CaM) was kindly provided by Dr. Katalin Torok (Torok et al., 2001; Tzortzopoulos and Torok, 2004). Green fluorescent protein (GFP)-CaMKIIα wild-type and mutants were expressed in Cos-7 cells, and extracts were prepared in 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 7.2, 10% glycerol, 1 mM EGTA, 1 mM dithiothreitol, and protease inhibitors (Roche Diagnostics, Indianapolis, IN) (O'Leary et al., 2006). Then, 0.2 g/ml rat liver was homogenized in the same buffer and spun for 10 min at 10,000 × g. Glutathione transferase (GST)-NR2B-c (amino acids 1120-1482 of the cytoplasmic NR2B C terminus) was expressed in bacteria (Bayer et al., 2001). CaM-KIIN, MAP2, AC2, syntide2 (Sigma-Aldrich, St. Louis, MO), calmodulin binding domain (CBD; Calbiochem, San Diego, CA), tat fusion peptides (Global Peptide Services, Ft. Collins, CO), and other CN peptides (Caltech Synthesis Core. Pasadena, CA) were obtained commercially.
CaMKII Activity Assays
Standard CaMKII assays were done for 1 min at 30°C (Bayer et al., 2001, 2002), with 20 nM CaMKII (subunit, not holoenzyme concentration), 50 mM PIPES, pH 7.2, 0.1 mg/ml bovine serum albumin (BSA), 10 mM MgCl2, 100 μM [γ-32P]ATP (∼1 Ci/mmol), 1 mM CaCl2, 1–2 μM CaM, and 30–60 μM AC2 peptide (or syntide2). The reactions were spotted onto Whatman P81 phosphocellulose paper rectangles (∼2 × 2.5 cm). To remove free radioactivity, the paper rectangles were rinsed and washed for 30 min under agitation in 0.5% phosphoric acid or water. After two more rinses, an additional 30-min wash typically did not release any more measurable radioactivity. Radioactivity of the bound peptides was quantified in a Beckman 6000TA scintillation counter (Beckman Coulter, Fullerton, CA) by the Cherenkov method. Any changes of the standard protocol were done as indicated. For assays of the GFP-CaMKIIα mutants, kinase amounts were normalized by GFP fluorescence in the extract, and total protein was adjusted with Cos-7 extracts.
Kinase Panels
A panel of different kinases was tested using a kinase profiling service (Upstate Biotechnology, Charlottesville, VA). Forty-minute reactions at room temperature contained 0.1% BSA, and they were started by addition of 10 mM Mg-acetate and [γ-33P]ATP, stopped by 0.1% phosphoryic acid, spotted on filter mats, and washed 3 × 5 min in 75 mM phosphoric acid and 1× in methanol before drying and scintillation counting. CaMKII and -IV were activated by 5 mM CaCl2 and 1.7 μM CaM in 40 mM HEPES, pH 7.4; substrate was 30 μM KKLNRTLSVA. Buffers and substrates for the other kinases are stated in parentheses: PKA (8 mM 3-(N-morpholino)propanesulfonic acid [MOPS], pH 7, 0.2 mM EDTA; 30 μM Kemptide), PKCα (20 mM HEPES, pH 7.2, 0.3% Triton X-100, 0.1 mg/ml phosphatidylserine, 10 μg/ml diacylglycerol; 0.1 mg/ml histone H1), JNK1α1 (50 mM Tris-HCl, pH 7.5, 0.1 mM EGTA, 0.1% β-mercaptoethanol; 3 μM ATF), MAPK1 and raf (25 mM Tris-HCl, pH 7.5, 0.02 mM EGTA; 250 μM proprietary substrate and 0.66 mg/ml myelin basic protein, respectively). Reactions in presence of 5 μM CN21a were done in duplicate and normalized to four parallel reactions without inhibitor.
CaMKII Auto- and Protein Phosphorylation
Mitogen-activated prtoein (MAP)2 (10 nM; ∼140 nM phosphorylation sites; Schulman, 1984) was used instead of substrate peptide, and reaction times were 5 min, unless indicated otherwise. CaMKII concentration was 100 nM subunits (=8.3 nM holoenzymes). Auto- and MAP2 phosphorylation were assessed by immunoblot analysis (Bayer et al., 2002) with phospho-T286- or -T305–specific antibodies (1:500; PhosphoSolutions, Aurora, CO) and an anti-phospho-Thr antibody (1:500; Zymed Laboratories, South San Francisco, CA), respectively. Total CaMKIIα was detected with CBα2 antibody (1:2000; Invitrogen, Carlsbad, CA). Protein was separated on 10% poly-acrylamide SDS gels, and electroblotted onto Protran 0.2-μm pore nitrocelluse filters (Whatman Schleicher and Schuell, Keene, NH) or onto polyvinylidene difluoride (PVDF) filters (PerkinElmer-Cetus, Boston, MA). Alternatively, for quantitative analyses, a vacuum-driven slot-blot manifold (Whatman Schleicher and Schuell) was used for transfer onto PVDF membranes. PVDF membranes were air-dried for 15 min, and then wetted in methanol. Blots were blocked in 5% milk in Tris-buffered saline, pH 7.6, with 0.1% Tween 20 (TBS-T). Antibodies were incubated for 45–60 min at room temperature in 2.5% milk in TBS-T; for the anti-phospho-T305 antibody, 2.5% BSA was used instead. After secondary antibody incubation (anti-mouse or anti-rabbit horseradish peroxidase conjugate; 1:4000; GE Healthcare, Chalfont St. Giles, United Kigndom), detection was done using the Western Lightning system (PerkinElmer-Cetus) and exposure to Hyperfilm (GE Healthcare). For quantitative analysis, chemoluminescence was captured using a ChemiImager 4400 imaging system (Alpha Innotech, San Leandro, CA) instead of film. Only nonsaturated images were analyzed, using AlphaEase software (Alpha Innotech).
Crude liver extracts or NR2B-c were phosphorylated with 100 nM autophosphorylated CaMKII, 2 μM CaM, and 40 μM [γ-32P]ATP (∼2 Ci/mmol). Pre-autophosphorylation (of 300 nM CaMKII) was done for 5 min on ice in presence of 3 mM CaCl2, 6 μM CaM, and 120 μM unlabeled ATP. After addition of [γ-32P]ATP and inhibitors as indicated, 2-min reactions at 30°C were started by addition of substrate protein extract (1/3 to 1/12 of total reaction volume) and stopped with 25 mM EDTA. Phosphorylation was detected by autoradiography after gel electrophoresis.
CaMKII Binding to NR2B-c
CaMKII binding to NR2B-c was assessed as described previously (Bayer et al., 2001, 2006). Briefly, GST-NR2B-c was immobilized on anti-GST–coated microtiter plates, then overlaid with 100 nM CaMKII in presence of Ca2+/CaM and 1 or 5 μM of various CN peptides. After extensive wash, protein was eluted from the plates by boiling in SDS loading buffer. Eluted CaMKII was detected by Western blot as described above.
TA-CaM Dissociation
TA-CaM dissociation was assessed by increased TA-CaM (30 nM) fluorescence after dissociation from CaMKII or its CBD (150 nM) during a chase with unlabeled CaM (60 μM) (Torok et al., 2001; Tzortzopoulos and Torok, 2004). Buffer contained 50 mM HEPES, pH 7.4, 150 mM KCl, 2 mM MgCl2, 2 mM Mg-ADP, 2 mM CaCl2, and 0.1 mg/ml BSA. Fluorescence was measured in a time scan (1-s samples) at 365-nm excitation and 415-nm emission wavelength on a spectrofluorometer (Fluorolog3; HORIBA Jobin Yvon, Edison, NJ) and was corrected for photobleach (Supplemental Figure 9).
Imaging of Neuronal Filopodia Motility
Imaging of neuronal filopodia motility was done similarly to that described previously (Fink et al., 2003). Hippocampal cultures were prepared from newborn Sprague Dawley rats (Harlan, Indianapolis, IN) as described previously (Bayer et al., 2001), plated onto poly-d-lysine–coated glass-bottomed dishes (MatTek, Ashland, MA) at a density of ∼2.5 × 106 cells/cm2, and maintained in Neurobasal A medium with penicillin/streptomycin (50 U/ml), GlutaMAX (2 mM), and B27 supplement (Invitrogen). Glial growth was inhibited by 5-fluoro-2′-deoxy-uridine and uridine (70 and 140 μM, respectively). After 5 d in vitro, neurons were transfected with a GFP expression construct (Clontech, Palo Alto, CA) by using Lipofectamine 2000 (Invitrogen), as described previously (Bayer et al., 2001, 2006). On the next day, neurons were imaged in culture medium on a Zeiss Axiovert 200M system equipped with a 40× oil immersion objective, CoolSnap HQ charge-coupled device camera (Roper Scientific, Trenton, NJ), Xenon lamp LB-LS/17 (Sutter Instrument, Novato, CA), and climate control set to 30°C and 5% CO2. Fluorescence Images were acquired and analyzed using SlideBook software (Intelligent Imaging Innovations, Denver, CO). Sixteen images were taken in 20-s time intervals, at 100-ms exposure time and bin factor 2. Subtraction image (Δ image) stacks were generated by subtracting one stack (first image deleted) from its duplicate (last image deleted). Then, Δ image stacks were converted into average images in pseudocolor, for better visualization of motility. Quantification of the pixel intensity yielded a relative motility index, expressed as Δ image intensity before treatment set as 100%. Intensity cutoff masks eliminated most background pixels not located within neurons. Neurons were imaged before and after 20-min incubation with either tatCN21 or tatRev (5 μM). Incubation was done on the imaging setup; data from experimental days on which mock incubation without peptide affected motility were discarded.
Insulin Secretion
Langerhans' islets acutely isolated from adult male Wistar rats (Harlan) were obtained from the islet core facility of the Barbara Davis Center at University of Colorado Denver. On 24-well plates, 10 islets were pooled per well in 20 mM HEPES, 25 mM NaHCO3, 114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.16 mM MgSO4, 2.5 mM CaCl2, and 0.2% BSA; adjusted to pH 7.2. Within 90 min after isolation, insulin secretion was stimulated with 11 mM glucose. Inhibitors or EGTA were added 30 min before stimulation. Insulin secreted into the medium during 90-min stimulation was measured using an ELISA kit (CrystalChem, Downers Grove, IL). Two independent islet preparations showed inhibition of glucose-stimulated insulin secretion by tatCN21.
RESULTS
The Minimal Inhibitory Region of CaM-KIIN Retains CaMKII Specificity
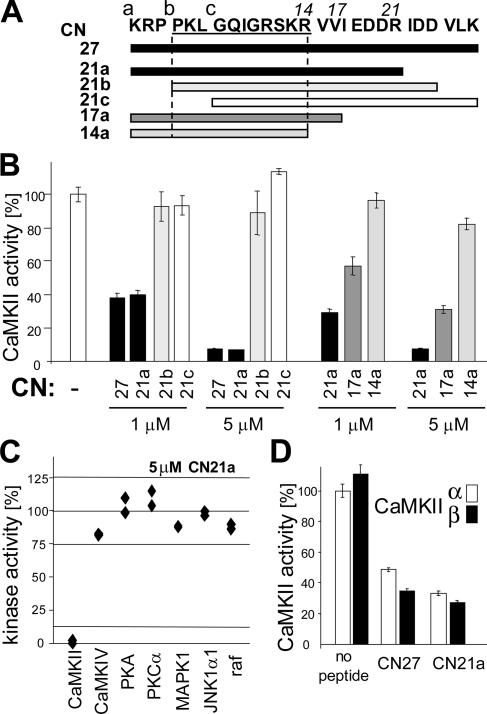
Three overlapping 21 amino acids long peptides were derived from the previously identified inhibitory region of CaM-KIIN (Chang et al., 1998) (Figure 1A). Their effect on AC2 substrate phosphorylation by CaMKII was then assayed in vitro (Figure 1B). The N-terminal peptide CN21a showed the full inhibitory effect observed with the full-length CaM-KIINtide (here named CN27). The other 21mer peptides, CN21b and CN21c, had minimal or no effect. C-terminal truncations of CN21a by four and seven amino acids significantly impaired inhibition, although CN17a still clearly affected CaMKII activity (Figure 1B). Thus, the full inhibitory activity is contained in CN21a (CaM-KIIN 43–63). CN21a also blocked phosphorylation of crude liver protein extracts and of bacterially expressed GST-NR2B-c (Supplemental Figure 10).
Figure 1.
CN21a contains the full CaMKII inhibitory potency and specificity of CaMK-IINtide. (A) The CN peptides used in this study are indicated relative to the sequence of CaMK-IINtide (Chang et al., 1998) (here termed CN27). Darker bars indicate greater CaMKII inhibitory potential found in this study. (B) CN21a contains the full inhibitory potency. Deletion of the three N-terminal amino acids abolishes inhibiton, whereas C-terminal deletion reduces it. Ca2+/CaM-induced CaMKII activity was measured by 32P incorporation into the peptide substrate AC2. (C) CN21a specificity was tested on a panel of different kinases (at 5 μM, 50-fold IC50). Kinase activity without inhibitor was normalized to 100% for each kinase. CaMKII activity was completely blocked, whereas activities of the other kinases were not affected. Individual data points of duplicate assays are shown. (D) CN27 and CN21 inhibited both major brain CaMKII isoforms, α and β. Error bars indicate SEM.
CaM-KIIN efficiently inhibits CaMKIIα but not CaMKIV, PKA or PKC (Chang et al., 1998). CN21a retained this specificity, because it had no inhibitory effect on CaMKIV, PKA, PKC, MAPK1, JNK1α1, or Raf even at 5 μM (Figure 1C). The CaMKIIα and β isoforms were inhibited to a similar degree (Figure 1D). Thus, CN21a selectively inhibited CaMKII, but not in an isoform-specific manner.
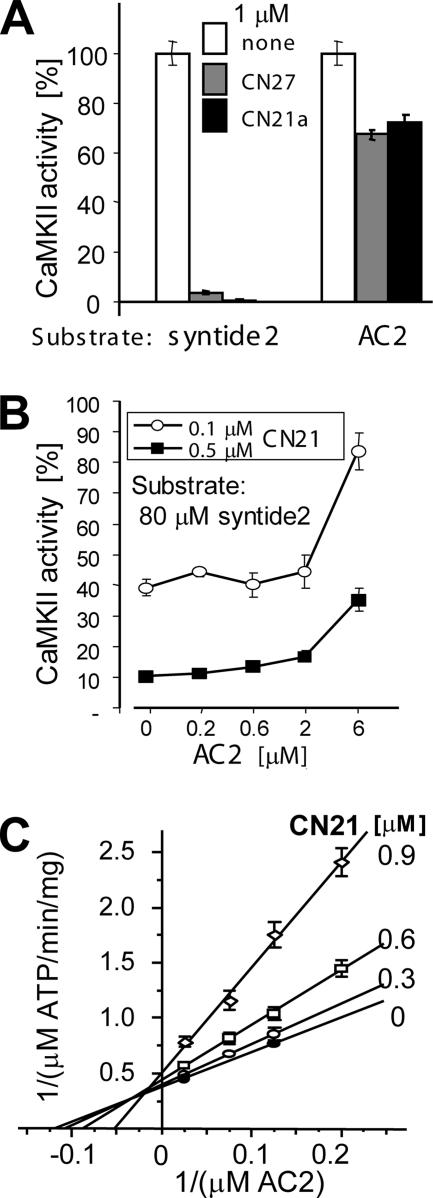
Different CN Effects on Two Peptide Substrates
Surprisingly, the concentration of half maximal inhibition (IC50) for CN27 and CN21a was substrate dependent (Figure 2A). AC2 and syntide2 are commonly used peptide substrates of CaMKII (Chang et al., 1998; Chen et al., 2001; Bayer et al., 2002; Lu et al., 2003; Zhang et al., 2005). Although 1 μM CN27 or CN21a reduced phosphorylation of AC2 only by approximately half, phosphorylation of syntide2 was completely blocked (Figure 2A). For syntide2, the IC50 of CN21a was ∼0.1 μM (Figure 2B). Addition of AC2 to the syntide2 phosphorylation reaction significantly reduced the inhibitory effect of CN21a in a concentration-dependent manner, indicating a competitive effect of AC2 with the CN inhibitor (Figure 2B). This is in contrast to the noncompetitive inhibition of syntide2 phosphorylation (Chang et al., 2001). Competition of CN21a with AC2 was further corroborated using a double-reciprocal plot (Lineweaver and Burk, 1934): Increasing CN21a concentrations affected apparent km (x-axis intersection: −1/km) much more strongly than apparent Vmax (y-axis intersection: 1/Vmax) (Figure 2C).
Figure 2.
CNs differentially inhibit phosphorylation of two peptide substrates. (A) CN peptides (1 μM) blocked CaMKII phosphorylation of the peptide substrate syntide2 (derived from a phosphorylation site on glycogen synthase), but only reduced phosphorylation of AC2 (derived from the autophosphorylation site around T286). (B) AC2 interferes with CaMKII inhibition by CN21. Kinase assays were done in presence of 0.1 or 0.5 μM CN21 with 80 μM syntide2 as the principle substrate; AC2 was added at the indicated concentration. Kinase activity is shown as percentage of maximal activity without CN21 peptide. Error bars indicate SEM (C) Inhibition by CN21 is competitive with AC2. Standard kinase assays were performed with 10 nM CaMKII, 1 μM CaM, and varying concentrations of AC2 (40, 13.33, 8, and 5 μM) and CN21 (0, 0.3, 0.6, and 0.9 μM). In a Lineweaver–Burk plot, increasing the CN21 concentration has a much stronger effect on apparent −1/km (x-axis intersection) than on apparent 1/Vmax (y-axis intersection), indicating inhibition by a largely competitive mechanism. The r2 values of regression for all inhibitor series were >0.994.
CaM-KIIN and CN21a Require the CaMKII T-Site for Inhibition
Why is CN-mediated inhibition of AC2 phosphorylation competitive (Figure 2, B and C), whereas inhibition of other substrates is not (Chang et al., 2001)? AC2 is derived from the CaMKII autoregulatory region around T286, and thus it can interact not only with the CaMKII S-site (substrate binding site) but also with the T-site (which interacts with the T286 region in the basal, inactive state of the kinase) (Bayer et al., 2001) (Figure 3A). Therefore, we hypothesized that CN may bind to the T-site of CaMKII, leading to competition for binding to the T-site (with AC2) but not the S-site (all substrates). This model was tested using GFP-CaMKIIα wild type and two T-site mutants, W237R and I205K (Yang and Schulman, 1999; Bayer et al., 2006). Indeed, both CN21a and CaM-KIIN inhibited the T-site mutants much less than CaMKII wild type (Figure 3B). CN21a (1 μM) reduced phosphorylation of syntide2 by CaMKII wild type to <4%, whereas the W237R and I205K and mutants retained ∼35 and 60% of their maximal activity, respectively (Figure 3B). At 0.1 μM CN21a, both mutants showed ∼75% of their maximal activity, whereas activity of CaMKII wild type was reduced to ∼10% (Figure 3B). In an independent experiment, 1 μM CaM-KIIN yielded similar results: W237R and I205 retained ∼40 and 80% of their maximal activity, respectively, whereas the activity of CaMKII wild type was reduced to below 20% (Figure 3B). These results indicate that inhibition by CNs requires interaction with the T-site of CaMKII, thereby explaining the T286-region-specific competition.
CN Peptides Inhibit CaMKII Binding to NR2B
If CN peptides bind to the CaMKII T-site, they should interfere with CaMKII binding to the N-methyl-d-aspartate (NMDA)-type glutamate receptor subunit NR2B, which binds to the same site (Bayer et al., 2001, 2006). Indeed, CN21a efficiently inhibited binding of CaMKII to immobilized GST-NR2B C terminus (Figure 3C). CN21a (1 μM) was sufficient to completely block CaMKII binding to NR2B (Figure 3C), whereas much higher concentrations of peptides derived from the binding site on NR2B or the CaMKII T286-region are required to produce the same effect (>30 μM; Bayer et al., 2006). The C-terminal truncations of CN21a (CN17 and 14) also affected CaMKII binding to NR2B, but these effects were less pronounced (Figure 3C).
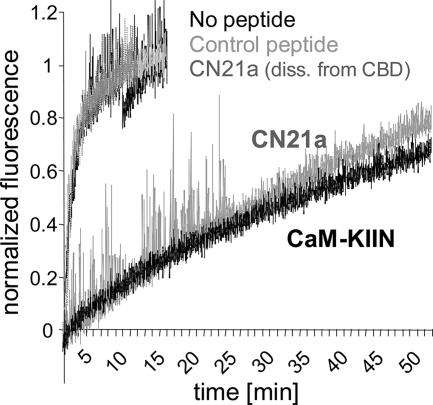
CaM-KIIN and CN21a Trap CaM on CaMKII
CaMKII T286 autophosphorylation or binding to NR2B dramatically reduces the CaM off-rate, which can effectively result in trapping of CaM on CaMKII (Meyer et al., 1992; Bayer et al., 2001). We hypothesized that CaM-KIIN and CN21a should have a similar effect, if CaM trapping is indeed mediated by displacement of the CaMKII autoregulatory region from the T-site. To follow CaM dissociation, we used TA-CaM, which shows increased fluorescence after dissociation from CaMKII or a peptide derived from the CaMKII calmodulin binding domain (CBD) (Torok et al., 2001; Tzortzopoulos and Torok, 2004). Dissociation of TA-CaM was initiated by a chase with an excess of unlabeled CaM, and the change in fluorescence was followed over time (Figure 4). Both CN21a and CaM-KIIN dramatically reduced the CaM off-rate from CaMKII. A control peptide (CN21c) had no effect. The direct action of CN21a was on CaMKII and not on CaM, because CN21a did not slow down dissociation of TA-CaM from a CBD peptide (Figure 4).
Figure 4.
CaM-KIIN and CN21a (5 μM) slowed down CaM dissociation from CaMKII (150 nM). Dissociation of TA-CaM (30 nM) was monitored by its increased fluorescence (1-s sample times) during a chase with excess unlabeled CaM (60 μM). The control peptide CN21c did not slow down dissociation. CN21a slowed dissociation from CaMKII, but not from a peptide derived from the CaMKII calmodulin binding domain (CBD), demonstrating a CaMKII directed effect.
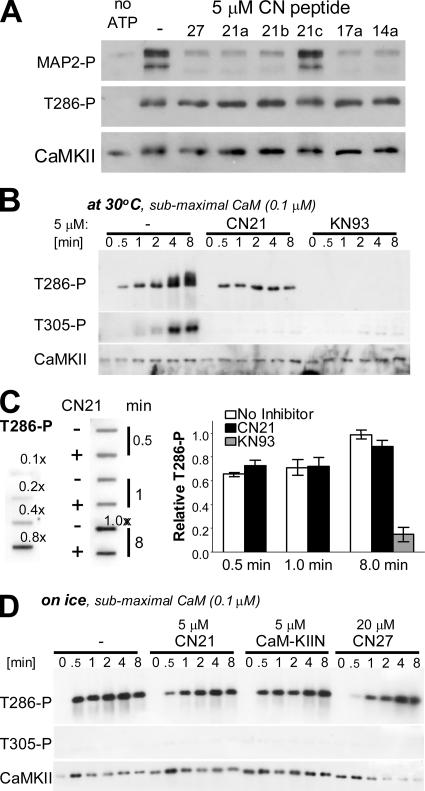
CaM-KIIN and CNs Block Substrate- and T305 Autophosphorylation, but Only Mildly Affect T286 Autophosphorylation
Intersubunit, intraholoenzyme autophosphorylation at T286 generates Ca2+/CaM-independent (autonomous) activity of CaMKII. T286 autophosphorylation requires CaM binding to both the “kinase subunit” (for activation) and the “substrate subunit” (to make T286 accessible for phosphorylation). Based on two mechanistic actions of CNs, we hypothesized that they should affect T286 autophosphorylation much less than phosphorylation of exogenous substrates: 1) the T286-region competed with CNs for T-site binding (even at 6 μM of the T286-derived AC2; Figures 2 and 3); and 2) CNs strengthened CaM binding to CaMKII (Figure 4) and thereby should facilitate the substrate-directed CaM effect in autophosphorylation. Indeed, even 5 μM CN21a or CN27 failed to block T286 phosphorylation (Figure 5A). By contrast, phosphorylation of MAP2 present in the same reaction was strongly inhibited. This suggests that CNs do not block generation of the autonomous state of CaMKII by autophosphorylation, even though they efficiently inhibit substrate phosphorylation by Ca2+/CaM-stimulated and autophosphorylated CaMKII.
Figure 5.
CaM-KIIN and CN21 block substrate- and T305 but not T286 autophosphorylation. (A) CN peptides (5 μM) blocked CaMKII substrate- but not T286 autophosphorylation when stimulated with 1 μM CaM (5-min reaction time). Phosphorylation of MAP2 and CaMKII autophosphorylation at T286 were assessed by Western analysis. Only CN21c failed to block MAP2 phosphorylation, indicating that CN21a amino acids 4–14 from the core inhibitory region. Importantly, none of the CN peptides blocked CaMKII T286 autophosphorylation. (B) Time course of CaMKII autophosphorylation stimulated by 0.1 μM CaM at 30°C. CN21 and KN93 (5 and 10 μM, respectively) blocked T305 and other autophosphorylation that result in a band-shift of CaMKII. By contrast, T286 was essentially completely blocked by KN93, but not by CN21. Total CaMKII and autophosphorylation at T286 and T305 were detected by Western analysis. (C) Slot-blot analysis (left) was performed for quantification (right) of T286 autophosphorylation in the experiment shown in B. T286 autophosphorylation was normalized to the degree seen after 8-min reaction without inhibitor; dilutions of this reaction were used as standard. Slot-blot avoids differences in the area of the signal caused by the band-shift seen only in absence of inhibitor. CN21 had no significant effect on the T286 autophosphorylation measured (p > 0.25). Error bars show SEM of triplicates. (D) Reactions as in B (submaximal CaM) were slowed down further by low temperature (on ice). Under these conditions, CN inhibitors slowed down T286 autophosphorylation, but they still did not completely block it. T305 and other autophosphorylation that result in band-shift were not detected under these conditions.
Can CaM-KIIN slow down T286 autophosphorylation? To test this, reactions were stimulated with submaximal CaM (0.1 μM) at 30°C and stopped at different time points (Figure 5B) (essentially the same results were obtained at 1 μM CaM; Supplemental Figure 11). Both CN21a and KN93 (5 and 10 μM, respectively) blocked T305 and other slow secondary autophosphorylation reactions that result in a band-shift of CaMKII (Figure 5B). Thus, CNs can block autophosphorylation in principle. However, T286 autophosphorylation was almost completely blocked only by KN93, but not by CN21a (Figure 5B). Thus, T286 autophosphorylation is not inherently hard to inhibit, and T286 protection is specific to CN inhibitors. In Figure 5B, CN21a seems to somewhat reduce T286 phosphorylation, even if it does not block it. However, this appearance is because the CaMKII band stays much tighter in the presence of CN21a, because it inhibits the secondary autophosphorylation reactions that cause a band-shift. Thus, we quantified T286 autophosphorylation after performing a slot-blot, which keeps the area of signal constant (Figure 5C). Indeed, CN21a did not significantly affect T286 autophosphorylation (Figure 5C).
Detection of any slowing of T286 autophosphorylation by CN inhibitors (5 μM; ∼50-fold IC50) required subphysiological conditions, i.e., combination of submaximal CaM (0.1 μM) with low temperature (on ice) (Figure 5D). A more obvious slowing down of the reaction was obtained by further increasing inhibitor concentrations (20 μM CN27; ∼200-fold IC50) (Figure 5D). However, even this degree of inhibition is qualitatively different from the essentially complete block of substrate- and T305 autophosphorylation by CN inhibitors and from the block of T286 autophosphorylation by KN93 seen in Figure 5, A–C. Together, CN inhibitors completely block substrate and T305 autophosphorylation, but only mildly inhibit T286 autophosphorylation by CaMKII.
CN21a Truncations and a Bulky Protein Substrate
MAP2 is an excellent CaMKII substrate and contains >10 different phosphorylation sites (Schulman, 1984). MAP2 phosphorylation by CaMKII was efficiently blocked not only by CN21a, but also by the C-terminally truncated CN17 and 14, and by the N-terminally shifted CN21b but not by CN21c (Figure 5A). This indicated that the eleven CaMK-IIN amino acids 46–56 (CN21a amino acids 4–14; Figure 1A) form a core inhibitory region that was sufficient to block access of the bulky protein substrate MAP2, but not of smaller peptide substrates.
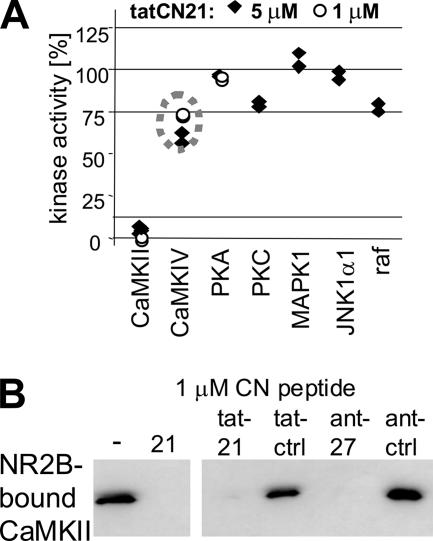
tatCN21 Retains Inhibition of CaMKII Activity and NR2B Binding
To generate a cell-penetrating CaMKII inhibitor, the tat sequence was fused to the N terminus of CN21a. In contrast to a previously used CaMK-IIN–derived inhibitor (antCN27; Fink et al., 2003; Illario et al., 2003; Meffert et al., 2003; Sanhueza et al., 2007), the resulting 32-amino acid-long tatCN21 can be easily synthesized by any routine peptide facility. tatCN21 (1 μM) completely blocked CaMKII activity, whereas even 5 μM tatCN21 had little or no effect on other kinases tested (Figure 6A). Importantly, this included basophilic kinases such as PKA and PKC (as the tat peptide is rich in basic Arg and Lys residues). However, 5 μM tatCN21 did have a mild effect on CaMKIV activity (∼35% reduction), consistent with a previously reported similar mild effect by CaMK-IIN (Chang et al., 1998). Thus, tatCN21 is much more specific than any other reported CaMKII inhibitor, but use at concentrations significantly higher than 5 μM should be avoided. Both tatCN21 and antCN27 (1 μM) blocked CaM-induced CaMKII binding to immobilized NR2B C terminus (Figure 6B), as seen for CN21a and CN27 (Figure 3C).
Figure 6.
tatCN21 retains inhibition of CaMKII activity and binding to NR2B. (A) Effect of tatCN21 was tested on a panel of different kinases. Kinase activity without inhibitor was normalized to 100% for each kinase. tatCN21 (1 μM) completely blocked CaMKII activity, whereas even 5 μM tatCN21 had little or no effect on the other kinases. A mild but clear effect was observed only on CaMKIV (∼35%). Individual data points of duplicate assays are shown. (B) Like CN21a, both tatCN21 and antCN27 efficiently blocked Ca2+/CaM-induced CaMKII binding to immobilized GST-NR2B-c. Control peptides did not affect CaMKII binding. Bound CaMKII was eluted and detected by Western blot.
tatCN21 Inhibits Motility in Hippocampal Neurons
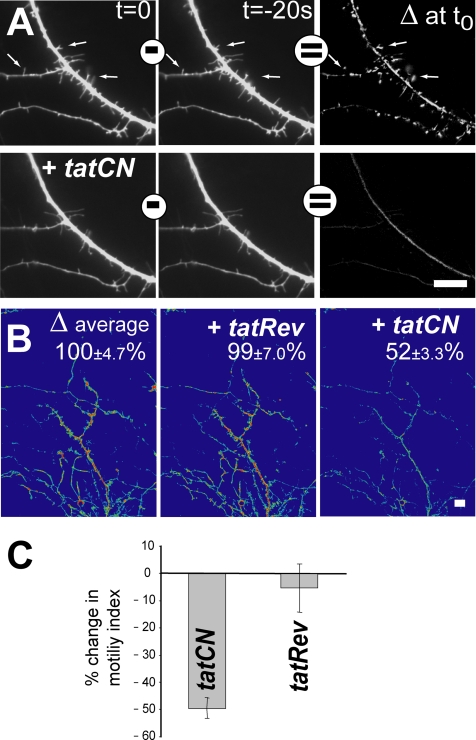
CaMKII activity regulates motility of filopodia and small dendritic branches in hippocampal neurons (Fink et al., 2003; Jourdain et al., 2003). This CaMKII function was used to test the effect of extracellular tatCN21 application on an intracellular process. Motility was assessed by time series imaging of cultured hippocampal neurons that express GFP (Figure 7; see Supplemental Movies), essentially as described previously (Fink et al., 2003). Briefly, subtraction images (Δ images) were created by subtracting pixel intensities of one image from an image taken 20 s later, and then they were used to quantify relative motility before and after a 20-min treatment with peptide. tatCN21 but not tatRev (the reverse sequence control), inhibited motility (Figure 7). It also resulted in a net loss of filopodia (Figure 7A), consistent with a stronger effect of CaMKII inhibition on filopodia extension than retraction (Fink et al., 2003). Figure 7A shows an area of high motility that was dramatically reduced by the inhibitor (see Supplemental Movies). Averages of larger areas (110 × 148 μm), as shown in Figure 7B, were used for the quantification in Figure 7C. Reduced motility is not due to toxicity of tatCN21, as assessed by a cell death assay based on lactate dehydrogenase release 20–24 h after tatCN21 addition (unpublished observation). Thus, extracellularly applied tatCN21 affects an intracellular function that is regulated by CaMKII.
Figure 7.
tatCN21 inhibits motility in hippocampal neurons. Images of GFP-expressing hippocampal neurons (5–6 d in vitro) were acquired at 30°C in 20-s intervals to assess motility. After the first set of 16 images, neurons were incubated with tatCN21a or with control peptide for 20 min; then a second set of images was taken. Bars, 10 μm. (A) Example images of the same dendrite area before and 20 min after addition of tatCN21, at different times of acquisition as indicated (also see Supplemental Movies). Δ images were created by subtracting pixel intensities of one image from the one taken 20 s later. Shown Δ image intensities are fourfold exaggerated compared with the original captures on the left. (B) Average of Δ images in pseudocolor visualize motility in a larger area (110 × 148 μm). Error indicates SEM of the Δ images used for the average projections shown. (C) Quantification of the change in motility after application of tatCN21 or tatRev control based on Δ image average projections. Error bars show SEM (n = 7 neurons from three independent cultures). In three cases, neurons were treated first with tatRev and then tatCN21, as shown in B.
tatCN21 Inhibits Insulin Secretion from Isolated Langerhans' Islets
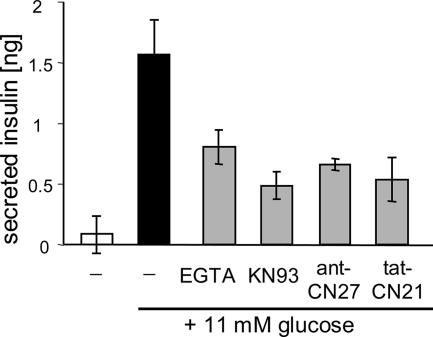
CaMKII has been implicated in regulation of insulin secretion, however, largely based on experiments with KN93, which also directly affects Ca2+ channels required for insulin secretion (for review, see Easom, 1999). Insulin secretion from isolated rat Langerhans' islets was stimulated by 11 mM glucose (Figure 8). Stimulated insulin secretion was significantly inhibited not only by extracellular EGTA and KN93 but also by the CaM-KIIN–derived fusion peptides tatCN21 and antCN27 (Figure 8). These results corroborate previous indication of CaMKII involvement in insulin secretion. Together, the results show that tatCN21 is cell penetrating and can affect CaMKII function in cells (Figures 7 and 8).
Figure 8.
tatCN21 inhibits glucose-induced insulin secretion. Insulin secretion from acutely isolated rat Langerhans' islets (10/well) was stimulated by 11 mM glucose. This secretion was inhibited by extracellular EGTA (0.5 mM; instead of 2.5 mM CaCl2), KN93 (10 μM), antCN27 (5 μM), and tatCN21 (5 μM) (p < 0.025; for KN93 and tatCN21, p < 0.01). Error bars show SEM (n = 4; for antCN27, n = 3). The CN peptides inhibited secretion also in an independent experiment (which did not include the standard for determining absolute insulin amount; data not shown).
DISCUSSION
The results of this study reveal the inhibitory mechanism of the natural CaMKII-specific inhibitory protein CaM-KIIN (Chang et al., 1998, 2001) and establish a powerful new tool for dissecting cellular CaMKII functions. CN21a (now also termed CN21) was identified as the minimal 21-amino acid region of CaM-KIIN that contains the full inhibitory potency and specificity. CN21 interacted with the T-site of CaMKII to achieve inhibition, block CaMKII binding to NR2B, and significantly enhance binding of CaM. CN21 efficiently blocked substrate- and T305 autophosphorylation, but remarkably (and in contrast to KN93) only mildly affected T286 autophosphorylation. Thus, although CN21 blocked general stimulated and autonomous CaMKII activity, it did not block the autophosphorylation at T286 that generates the Ca2+/CaM-independent autonomous state. To our knowledge, this is the first mechanistic characterization of such differential effect of a kinase inhibitor, in this case for an inhibitor that is naturally expressed in cells.
How does the differential effect of CN inhibitors on substrate- versus T286 autophosphorylation work? CaM-KIIN inhibits phosphorylation of syntide2 in a noncompetitive manner (Chang et al., 1998). By contrast, according to our results, inhibition was competitive with the substrate peptide AC2. Syntide2 should only interact with the substrate binding site (S-site), whereas AC2 can interact with the S-site and the autoregulatory T286 binding site (T-site), because AC2 is derived from the region around T286 (Figure 3A; Bayer et al., 2001, 2006). Mutations in the CaMKII T-site greatly reduced inhibition by CaM-KIIN and CN21, indicating that these inhibitors interact with the T-site to achieve inhibition. Thus, CNs competed with AC2 and the T286 region for T-site binding. Consistent with such T-site binding, CaM-KIIN binds only to activated forms of CaMKII (Chang et al., 1998), because the T-site is only accessible upon CaMKII activation. Also, CN21 prevented CaMKII binding to NR2B, which is known to occur at the T-site (Bayer et al., 2001, 2006). The unusual competition between CN and the CaMKII T286 region for T-site binding, together with high T286 concentration, may prevent block of T286 autophosphorylation by CN. The “local concentration” of T286 within the space occupied by a holoenzyme (Kolodziej et al., 2000; Hoelz et al., 2003; Rosenberg et al., 2005) is 6 mM. This should indeed allow highly efficient competition, because the T286 region-derived AC2 peptide competed even at a 1000× lower concentration. High local substrate concentration alone is not sufficient to escape inhibition by CN, because CN blocked autophosphorylation at T305. This is consistent with the model, because substrates that do not interact with the T-site (including CaMKII T305) do not compete with CNs, even at high concentrations (also see Chang et al., 1998). Also, T286 autophosphorylation is not difficult to inhibit in principle, because an essentially complete block was achieved by the less potent inhibitor KN93. Thus, partial protection of T286 from CN inhibition indeed requires a special mechanism, such as the demonstrated competitive effect. Perhaps even more importantly, T286 (but not T305) autophosphorylation occurs intersubunit and requires CaM binding not only to the kinase but also to the substrate subunit (Hanson et al., 1994; Rich and Schulman, 1998). Although CNs may reduce activity of the kinase subunit, they did significantly strengthen CaM binding, thus enhancing the substrate-directed effect of CaM in T286 autophosphorylation. The observed CaM trapping by CNs is likely a direct effect of displacing the region around T286 from the T-site, because T286 autophosphorylation or binding to NR2B have similar effects (Meyer et al., 1992; Bayer et al., 2001). Consistent with our model, although CNs did not block T286 autophosphorylation (in contrast to KN93), they were able to slow down the reaction. However, clear detection even of a slowing of the reaction required high CN concentration (200-fold IC50) and much subphysiological temperature and CaM concentration. At first glance, protection of T286 from CN block seems contradictory with a previous report (Chang et al., 1998); however, detection of inhibition in that study also required high CaMK-IIN concentrations at much subphysiological temperature and also did not result in complete block of T286 phosphorylation (Chang et al., 1998).
How do CNs inhibit CaMKII activity? CaM-KIIN and CN21 interact with the noncatalytic T-site of CaMKII, and this may prevent substrate access to the immediately adjacent catalytic S-site. But why, then, does the T-site binding peptide AC2 not inhibit CaMKII activity? In contrast to CN21, AC2 bound to the T-site does not block the S-site (Figure 3A). Indeed, a CaMKII truncation to L300 (the C terminus of AC2) is constitutively active, whereas a slightly less extensive truncation to N304 results in permanent S-site block, due to loss of the CaM binding site (Yamagata et al., 1991; Cruzalegui et al., 1992) (also see Figure 3A). Thus, T-site binding inhibitors have to be sufficiently long to prevent substrate access to the neighboring S-site. Consistent with this notion, our results showed that further truncations of CN21 were still able to block phosphorylation of a large protein substrate, even though they allowed at least partial access of small peptide substrates (CN21 amino acids 1–14 were sufficient to inhibit MAP2 phosphorylation; full-length CN21 was required to block access of small peptide substrates). This suggests that the CN21 N-terminus interacts with the T-site, whereas the C terminus extends to block S-site access (Figure 3A). However, this primary effect on substrate access does not preclude additional, more subtle effects on ATP binding pocket conformation.
Currently used CaMKII inhibitors are demonstrated to have nonspecific effects (Smith et al., 1990; Li et al., 1992; Enslen et al., 1994; Hvalby et al., 1994; Chang et al., 1998; Ledoux et al., 1999). CN21 will be a useful tool, due to its specificity. Additionally, the differences in the inhibitory actions of CN21 and KN93 may allow further dissection of the functions of different modes of CaMKII action. KN93 interferes with activation by CaM, thus blocking the CaM-dependent substrate- and T286 autophosphorylation (Tokumitsu et al., 1990; Sumi et al., 1991), but not autonomous activity once generated by autophosphorylation. By contrast, CN inhibitors blocked both CaM-stimulated and autonomous activity, even though they did not block T286 autophosphorylation. Therefore, these peptides could be used to determine functions of T286 autophosphorylation and autonomous CaMKII activity, such as their proposed roles in maintenance of long-term potentiation of synaptic strength and in metaplasticity (for review, see Tompa and Friedrich, 1998; Lisman and McIntyre, 2001). Indeed, a recent study using antCN27 indicated a possible role of CaMKII in maintenance of synaptic memory (Sanhueza et al., 2007). The characterization of the CN inhibitory mechanism suggests involvement of either autonomous activity or NR2B binding of CaMKII.
The neuronal functions of endogenous CaM-KIIN are currently unknown. However, its specific dendritic shaft localization (Chang et al., 1998) suggests inhibition of CaMKII only in dendrites but not in synapses or filopodia. CaMKIIβ localizes to neuronal filopodia and regulates their motility (Fink et al., 2003). The inhibitory and targeting regions of CaM-KIIN are likely on different parts of the protein: extracellular application of its inhibitory region made cell-penetrating by tat fusion (tatCN21) inhibited filopodia motility in hippocampal neurons, and its effect is thus not restricted to the dendritic shaft. Cell penetration of tatCN21 was further demonstrated by its inhibition of insulin secretion from Langerhans' islets. KN93 has similar effects (Wasmeier and Hutton, 1999), also in α-toxin–permeabilized β-cells (Bhatt et al., 2000) that circumvent requirement of voltage-dependent Ca2+ channels, which are directly affected by KN93 (Li et al., 1992). TatCN21 made it possible to corroborate these findings in intact islets stimulated by glucose. Tat fusion peptides have proven useful not only in tissue culture but also in vivo, and they can even cross the blood-brain barrier (Aarts et al., 2002). With a length of 32 amino acids, tatCN21 can be easily generated by any routine peptide synthesis facility (in contrast to the 42 amino acid antCN27). Thus, tatCN21 provides a powerful, readily accessible, and easy-to-use tool for studying cellular CaMKII function.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mark Dell'Acqua (University of Colorado Health Sciences Center) and members of his laboratory, especially Drs. Jessica Gorski, Karen Smith, and Eric Horne, for critical comments. We thank Dr. Katalin Torok (St. George's University of London) for kindly providing TA-CaM, and Philipp Pratt (Barbara Davis Center) for providing isolated islets. We thank Dr. Howard Schulman (Stanford; PPD Biomarker Discovery Sciences, Menlo Park, CA) for insightful discussion and support. R.S.V. and K.D.D. were supported by National Institutes of Health grant T32 GM-07635. The research was supported by National Institutes of Health grant NS-050120, DK-070735, and NS-052644 and American Heart Association grant 0430196N (to K.U.B.).
Note added in proof.
The University of Colorado is currently seeking patent protection for the peptide inhibitors described in this study.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0185) on October 17, 2007.

 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Aarts M., Liu Y., Liu L., Besshoh S., Arundine M., Gurd J. W., Wang Y. T., Salter M. W., Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J. Cell Biol. 1990;111:1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K. U., De Koninck P., Leonard A. S., Hell J. W., Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- Bayer K. U., De Koninck P., Schulman H. Alternative splicing modulates the frequency-dependent response of CaMKII to Ca(2+) oscillations. EMBO J. 2002;21:3590–3597. doi: 10.1093/emboj/cdf360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K. U., LeBel E., McDonald G. L., O'Leary H., Schulman H., De Koninck P. Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J. Neurosci. 2006;26:1164–1174. doi: 10.1523/JNEUROSCI.3116-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K. U., Lohler J., Schulman H., Harbers K. Developmental expression of the CaM kinase II isoforms: ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res. Mol. Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- Bayer K. U., Schulman H. Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem. Biophys. Res. Commun. 2001;289:917–923. doi: 10.1006/bbrc.2001.6063. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J. Biol. Chem. 1983;258:12735–12744. [PubMed] [Google Scholar]
- Bhatt H. S., Conner B. P., Prasanna G., Yorio T., Easom R. A. Dependence of insulin secretion from permeabilized pancreatic beta-cells on the activation of Ca(2+)/calmodulin-dependent protein kinase II. A re-evaluation of inhibitor studies. Biochem. Pharmacol. 2000;60:1655–1663. doi: 10.1016/s0006-2952(00)00483-4. [DOI] [PubMed] [Google Scholar]
- Chang B. H., Mukherji S., Soderling T. R. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. H., Mukherji S., Soderling T. R. Calcium/calmodulin-dependent protein kinase II inhibitor protein: localization of isoforms in rat brain. Neuroscience. 2001;102:767–777. doi: 10.1016/s0306-4522(00)00520-0. [DOI] [PubMed] [Google Scholar]
- Chen H. X., Otmakhov N., Strack S., Colbran R. J., Lisman J. E. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J. Neurophysiol. 2001;85:1368–1376. doi: 10.1152/jn.2001.85.4.1368. [DOI] [PubMed] [Google Scholar]
- Colbran R. J. Targeting of calcium/calmodulin-dependent protein kinase II. Biochem J. 2004;378:1–16. doi: 10.1042/BJ20031547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbran R. J., Soderling T. R. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J. Biol. Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- Cruzalegui F. H., Kapiloff M. S., Morfin J. P., Kemp B. E., Rosenfeld M. G., Means A. R. Regulation of intrasteric inhibition of the multifunctional calcium/calmodulin-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1992;89:12127–12131. doi: 10.1073/pnas.89.24.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V., Barria A., Soderling T. R. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhura I., Wu Y., Colbran R. J., Balser J. R., Anderson M. E. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat. Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- Easom R. A. CaM kinase II: a protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes. 1999;48:675–684. doi: 10.2337/diabetes.48.4.675. [DOI] [PubMed] [Google Scholar]
- Elgersma Y., Fedorov N. B., Ikonen S., Choi E. S., Elgersma M., Carvalho O. M., Giese K. P., Silva A. J. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- Enslen H., Sun P., Brickey D., Soderling S. H., Klamo E., Soderling T. R. Characterization of Ca2+/calmodulin-dependent protein kinase IV. Role in transcriptional regulation. J. Biol. Chem. 1994;269:15520–15527. [PubMed] [Google Scholar]
- Erondu N. E., Kennedy M. B. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci., 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C. C., Bayer K. U., Myers J. W., Ferrell J. E., Jr, Schulman H., Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Fladmark K. E., Brustugun O. T., Mellgren G., Krakstad C., Boe R., Vintermyr O. K., Schulman H., Doskeland S. O. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- Gardoni F., Schrama L. H., van Dalen J. J., Gispen W. H., Cattabeni F., Di Luca M. AlphaCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 1999;456:394–398. doi: 10.1016/s0014-5793(99)00985-0. [DOI] [PubMed] [Google Scholar]
- Giese K. P., Fedorov N. B., Filipkowski R. K., Silva A. J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Meyer T., Stryer L., Schulman H. Dual role of calmodulin in autophosphorylation of multifunctional CaM kinase may underlie decoding of calcium signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- Hoelz A., Nairn A. C., Kuriyan J. Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mol. Cell. 2003;11:1241–1251. doi: 10.1016/s1097-2765(03)00171-0. [DOI] [PubMed] [Google Scholar]
- Hudmon A., Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- Hvalby O., et al. Specificity of protein kinase inhibitor peptides and induction of long-term potentiation. Proc. Natl. Acad. Sci. USA. 1994;91:4761–4765. doi: 10.1073/pnas.91.11.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illario M., Cavallo A. L., Bayer K. U., Di Matola T., Fenzi G., Rossi G., Vitale M. Calcium/calmodulin-dependent protein kinase II binds to Raf-1 and modulates integrin-stimulated ERK activation. J. Biol. Chem. 2003;278:45101–45108. doi: 10.1074/jbc.M305355200. [DOI] [PubMed] [Google Scholar]
- Jourdain P., Fukunaga K., Muller D. Calcium/calmodulin-dependent protein kinase II contributes to activity-dependent filopodia growth and spine formation. J. Neurosci., 2003;23:10645–10649. doi: 10.1523/JNEUROSCI.23-33-10645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaseki T., Ikeuchi Y., Sugiura H., Yamauchi T. Structural features of Ca2+/calmodulin-dependent protein kinase II revealed by electron microscopy. J. Cell Biol. 1991;115:1049–1060. doi: 10.1083/jcb.115.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej S. J., Hudmon A., Waxham M. N., Stoops J. K. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. J. Biol. Chem. 2000;275:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- Laabich A., Cooper N. G. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res. Mol. Brain Res. 2000;85:32–40. doi: 10.1016/s0169-328x(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Ledoux J., Chartier D., Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J. Pharmacol. Exp. Ther. 1999;290:1165–1174. [PubMed] [Google Scholar]
- Leonard A. S., Bayer K. U., Merrill M. A., Lim I. A., Shea M. A., Schulman H., Hell J. W. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and alpha-actinin. J. Biol. Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- Li G., Hidaka H., Wollheim C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62, comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol. Pharmacol. 1992;42:489–498. [PubMed] [Google Scholar]
- Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56:658–666. [Google Scholar]
- Lisman J., Schulman H., Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lisman J. E., McIntyre C. C. Synaptic plasticity: a molecular memory switch. Curr. Biol., 2001;11:R788–R791. doi: 10.1016/s0960-9822(01)00472-9. [DOI] [PubMed] [Google Scholar]
- Lu C. S., Hodge J. J., Mehren J., Sun X. X., Griffith L. C. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron. 2003;40:1185–1197. doi: 10.1016/s0896-6273(03)00786-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Maller J. L. Calcium, calmodulin, and CaMKII requirement for initiation of centrosome duplication in Xenopus egg extracts. Science. 2002;295:499–502. doi: 10.1126/science.1065693. [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Chang J. M., Wiltgen B. J., Fanselow M. S., Baltimore D. NF-kappaB functions in synaptic signaling and behavior. Nat. Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Meyer T., Hanson P. I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Morris E. P., Torok K. Oligomeric structure of alpha-calmodulin-dependent protein kinase II. J. Mol. Biol. 2001;308:1–8. doi: 10.1006/jmbi.2001.4584. [DOI] [PubMed] [Google Scholar]
- Nghiem P., Ollick T., Gardner P., Schulman H. Interleukin-2 transcriptional block by multifunctional Ca2+/calmodulin kinase. Nature. 1994;371:347–350. doi: 10.1038/371347a0. [DOI] [PubMed] [Google Scholar]
- Ohyama A., et al. Regulation of exocytosis through Ca2+/ATP-dependent binding of autophosphorylated Ca2+/calmodulin-activated protein kinase II to syntaxin 1A. J. Neurosci. 2002;22:3342–3351. doi: 10.1523/JNEUROSCI.22-09-03342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary H., Lasda E., Bayer K. U. CaMKII{beta} association with the actin-cytoskeleton is regulated by alternative splicing. Mol. Biol. Cell. 2006;17:4656–4665. doi: 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Holt M., Philipova R., Moss S., Schulman H., Hidaka H., Whitaker M. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- Ramirez M. T., Zhao X. L., Schulman H., Brown J. H. The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J. Biol. Chem. 1997;272:31203–31208. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- Rich R. C., Schulman H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1998;273:28424–28429. doi: 10.1074/jbc.273.43.28424. [DOI] [PubMed] [Google Scholar]
- Roeper J., Lorra C., Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J. Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg O. S., Deindl S., Sung R. J., Nairn A. C., Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Sanhueza M., McIntyre C. C., Lisman J. E. Reversal of synaptic memory by Ca2+/calmodulin-dependent protein kinase II inhibitor. J. Neurosci. 2007;27:5190–5199. doi: 10.1523/JNEUROSCI.5049-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J. Cell Biol. 1984;99:11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Teruel M. N., Connor J. H., Shenolikar S., Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat. Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- Singla S. I., Hudmon A., Goldberg J. M., Smith J. L., Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
- Smith M. K., Colbran R. J., Soderling T. R. Specificities of autoinhibitory domain peptides for four protein kinases. Implications for intact cell studies of protein kinase function. J. Biol. Chem. 1990;265:1837–1840. [PubMed] [Google Scholar]
- Soderling T. R., Chang B., Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 2001;276:3719–3722. doi: 10.1074/jbc.R000013200. [DOI] [PubMed] [Google Scholar]
- Strack S., Colbran R. J. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S., Robison A. J., Bass M. A., Colbran R. J. Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J. Biol. Chem. 2000;275:25061–25064. doi: 10.1074/jbc.C000319200. [DOI] [PubMed] [Google Scholar]
- Sumi M., Kiuchi K., Ishikawa T., Ishii A., Hagiwara M., Nagatsu T., Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Tobimatsu T., Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J. Biol. Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- Tombes R. M., Faison M. O., Turbeville J. M. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Tompa P., Friedrich P. Synaptic metaplasticity and the local charge effect in postsynaptic densities. Trends Neurosci. 1998;21:97–102. doi: 10.1016/s0166-2236(97)01176-4. [DOI] [PubMed] [Google Scholar]
- Torok K., Tzortzopoulos A., Grabarek Z., Best S. L., Thorogate R. Dual effect of ATP in the activation mechanism of brain Ca(2+)/calmodulin-dependent protein kinase II by Ca(2+)/calmodulin. Biochemistry. 2001;40:14878–14890. doi: 10.1021/bi010920+. [DOI] [PubMed] [Google Scholar]
- Tzortzopoulos A., Torok K. Mechanism of the T286A-mutant alphaCaMKII interactions with Ca2+/calmodulin and ATP. Biochemistry. 2004;43:6404–6414. doi: 10.1021/bi036224m. [DOI] [PubMed] [Google Scholar]
- Walikonis R. S., Oguni A., Khorosheva E. M., Jeng C. J., Asuncion F. J., Kennedy M. B. Densin-180 forms a ternary complex with the (alpha)-subunit of Ca2+/calmodulin-dependent protein kinase II and (alpha)-actinin. J. Neurosci. 2001;21:423–433. doi: 10.1523/JNEUROSCI.21-02-00423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992;359:739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Wasmeier C., Hutton J. C. Secretagogue-dependent phosphorylation of phogrin, an insulin granule membrane protein tyrosine phosphatase homologue. Biochem. J. 1999;341:563–569. [PMC free article] [PubMed] [Google Scholar]
- Worrell R. T., Frizzell R. A. CaMKII mediates stimulation of chloride conductance by calcium in T84 cells. Am. J. Physiol. 1991;260:C877–C882. doi: 10.1152/ajpcell.1991.260.4.C877. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Cline H. T. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Yamagata Y., Czernik A. J., Greengard P. Active catalytic fragment of Ca2+/calmodulin-dependent protein kinase II. Purification, characterization, and structural analysis. J. Biol. Chem. 1991;266:15391–15397. [PubMed] [Google Scholar]
- Yang E., Schulman H. Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 1999;274:26199–26208. doi: 10.1074/jbc.274.37.26199. [DOI] [PubMed] [Google Scholar]
- Zhang R., et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat. Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.