Abstract
Phosphorylation of the regulatory light chain of myosin II (MLC20) at the activation sites promotes both the motor activity and the filament formation of myosin II, thus playing an important role in various cell motile processes. In contrast, the physiological function of phosphorylation of MLC20 at the inhibitory sites is unknown. Here we report for the first time the function of the inhibitory site phosphorylation in the cells. We successfully produced the antibodies specifically recognizing the phosphorylation sites of MLC20 at Ser1, and the platelet-derived growth factor (PDGF)-induced change in the phosphorylation at the Ser1 was monitored. The phosphorylation of MLC20 at the Ser1 significantly increased during the PDGF-induced actin cytoskeletal reorganization. PDGF disassembled the stress fibers, and this was attenuated with the expression of unphosphorylatable MLC20 at the Ser1/Ser2 phosphorylation sites. The present results suggest that the down-regulation of myosin II activity achieved by the phosphorylation at the Ser1/Ser2 sites plays an important role in the normal reorganization of actomyosin filaments triggered by PDGF receptor stimulation.
INTRODUCTION
Cell migration plays a key role in both the physiological and the pathophysiological function of the cells including development, wound healing, immunity, and metastasis (Lauffenburger and Horwitz, 1996). Reorganization of actomyosin filaments is an essential process for these cell behaviors. It has been thought that myosin II plays a fundamental role in various types of cellular motility.
In vitro biochemical studies have revealed that the function of smooth muscle and nonmuscle myosin II is regulated by the phosphorylation of MLC20 (Sellers, 1991; Tan et al., 1992). A number of studies have shown that the phosphorylation of MLC20 at Thr18 and Ser19 activates its motor activity and increases filament stability. On the other hand, it has been known that MLC20 can be phosphorylated at other sites different from the activation sites. Originally, it was found that protein kinase C (PKC) phosphorylates Ser1/Ser2 and Thr9 of MLC20. This phosphorylation decreases the affinity of myosin II phosphorylated at the activation sites for actin and the affinity of MLC20 for myosin light-chain kinase (MLCK; Nishikawa et al., 1984; Bengur et al., 1987; Ikebe et al., 1987; Ikebe and Reardon, 1990). In other words, the phosphorylation of MLC20 at these sites inhibits rather than activates myosin II.
It was reported that the phosphorylation of MLC20 at the Thr9 inhibits myosin II motor activity and the phosphorylation of MLC20 by MLCK (Nishikawa et al., 1984; Turbedsky et al., 1997); however, phosphorylation of the inhibitory sites in nonmuscle cells has only been observed on Ser1 and/or Ser2, but not on Thr9 in vivo (Kawamoto et al., 1989; Yamakita et al., 1994), and this is because the Ser1/Ser2 sites are resistant to dephosphorylation by myosin light chain phosphatases while phospho-Thr9 is readily dephosphorylated (Ikebe et al., 1999). Furthermore, it has been demonstrated that the phosphorylation of Ser1/Ser2 sites significantly inhibits its motor activity (Ikebe et al., 1987). Therefore, it is anticipated that the phosphorylation of Ser1/Ser2 sites has a role in regulating myosin II function in cells. The phosphorylation of MLC20 at Ser1/Ser2 but not Tht9 was observed during mitosis in mammalian cultured cells (Yamakita et al., 1994). However, the expression of unphosphorylatable MLC20 at the inhibitory sites did not affect the progression of oogenesis in Drosophila embryos (Royou et al., 2002). Therefore, it is unclear whether phosphorylation of MLC20 at the inhibitory sites is involved in down-regulation of myosin II activity during Drosophila oogenesis. Although it is anticipated that the down-regulation of myosin II activity by the phosphorylation at the inhibitory sites may be important for cell motile events, the physiological significance of this inhibitory phosphorylation of myosin II on myosin function in the cells has not been well understood.
To clarify the cellular functions of the inhibitory site phosphorylation of myosin II, we developed a site-specific anti-phosphoamino acid antibody (pSer1 Ab) that specifically recognizes the phosphorylated MLC20 at the inhibitory sites (Ser1) but not the activation sites. By using this probe, we succeeded in monitoring the spatio-temporal change in myosin II phosphorylated at the Ser1/Ser2 site(s) after external stimuli. We found that the stimulation of platelet-derived growth factor (PDGF) mediated a transient phosphorylation of MLC20 at the Ser1/Ser2 site(s). The increase in phosphorylation at the inhibitory sites coincided with the PDGF-induced disassembly of stress fibers. Expression of unphosphorylatable MLC20 at the Ser1/Ser2 sites diminished the disassembly of stress fibers and focal adhesions in 3T3 fibroblasts. These results demonstrate that the phosphorylation at the Ser1/Ser2 sites of MLC20 regulates the dynamics of actomyosin filament formation in cells.
MATERIALS AND METHODS
Materials
Smooth muscle myosin II (Ikebe and Hartshorne, 1985a), MLCK, and protein kinase C (PKC; Ikebe et al., 1987) were prepared as described previously. Actin was prepared from rabbit skeletal muscle according to the method of Spudich and Watt (1971). LY294002, PD98059, and the PKC inhibitors, GF109203X, Go6976, and Rottlerin, were purchased from EMD Chemicals, Inc. (San Diego, CA).
Antibodies
The N-terminus acetylated phosphopeptide phoshoS SKRAKTC was coupled to keyhole limpet hemocyanin at the C-terminal cysteine residue by Genmed Synthesis (South San Francisco, CA). A pSer1 antibody (Ab) was affinity-purified using the phosphopeptide and then absorbed with an unphosphopeptide. A pSer19 Ab and pTS Ab specifically recognized the phosphorylated MLC20 at Ser19 and di-phosphorylated MLC20 at Thr18 and Ser19, respectively (Komatsu et al., 2000; Komatsu and Ikebe, 2004). Anti-MLC20 antibodies were from Sigma (M4401, St. Louis, MO) and Santa Cruz Biotechnology (sc-15370, Santa Cruz, CA), and anti-Myc, -paxillin, -myosin II Abs were purchased from Sigma-Aldrich, Santa Cruz Biotechnology, Transduction Laboratories (Lexington, KY), and Covance Research Products (Madison, WI). A rabbit Ab against heavy chain of myosin IIB and MLC20 were kindly provided by Dr. R. Adelstein (National Institutes of Health, Bethesda, MD) and Dr. J. Stull (University of Texas SW Medical Center).
Cell Stimulation
NIH3T3 fibroblast cells were maintained in DME containing 10% newborn calf serum. For PDGF and 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulations, NIH3T3 or MEF/3T3 Tet-Off cells were cultured for 18 h in DME supplemented with 0.1% newborn calf serum or 0.1% fetal bovine serum (FBS). Serum-starved cells were treated with either PDGF or TPA.
Biochemical Procedures
Urea/glycerol gel electrophoresis (Perrie and Perry, 1970) and SDS-PAGE (Laemmli, 1970) were carried out as described. MLC20 was phosphorylated by MLCK and PKC as described (Ikebe and Hartshorne, 1985a; Ikebe et al., 1987). To analyze the fraction of the expressed MLC20 incorporated into myosin II, we have used an ATP-dependent actin-binding activity of myosin II as described previously (Homma et al., 2000; Wei and Adelstein, 2000) with slight modifications. MEF/3T3 cells expressing myc-tagged MLC20s were lysed in buffer I (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM MgCl2, 2 mM EGTA, 1 mM dithiothreitol, 0.2 mM N-α-p-tosyl-l-lysine chloromethl ketone [TLCK], 0.2 mM tosyl-phenylalanyl chloromethyl ketone [TPCK], 10 μg/ml leupeptin, 2 mM phenylmethylsulfonyl fluoride, and 0.01% IGEPAL CA-630) with 2 mM ATP by sonication. The lysates were centrifuged at 270,000 × g for 15 min. The supernatants were incubated with 50 mM glucose, 20 U/ml hexokinase, and 0.2 mg/ml rabbit skeletal F-actin on a rotary mixer at 4°C for 30 min to completely hydrolyze residual ATP and coprecipitate myosin II with F-actin. After the reaction solutions were centrifuged at 270,000 × g for 15 min, the pellets were resuspended with buffer I without ATP and then centrifuged at 27,000 × g for 10 min. After washing once more with buffer I, the pellets were resuspended with buffer I containing 5 mM ATP to release myosin II from F-actin. After centrifugation at 270,000 × g for 10 min, the supernatants were subjected to Western blot analysis. Immunoblotting was done as described using nitrocellulose membranes (Yano et al., 1993; Komatsu et al., 2000).
Immunofluorescence Staining and Image Processing
Immunocytochemistry was performed as described (Komatsu et al., 2000). Fluorescence images were viewed using a Leica DM IRB laser scanning confocal microscope controlled by Leica TCS SP II systems (Leica Microsystems, Heidelberg, Germany). All images were taken with same laser output to directly compare the fluorescence signal intensities. Relative fluorescence intensities of pSer1 in the whole cells areas were measured with Leica TCL SP2 software as described (Komatsu et al., 2002). Images were processed using Adobe Photoshop 5.5 software (Adobe Systems, San Jose, CA).
Quantification Analysis
For quantification of stress fiber formation, more than 100 cells in the randomly chosen fields were recorded blindly by a confocal fluorescence microscope, and the percentage of cells having stress fibers was calculated. The values shown are means ± SD from three independent experiments (more than 100 cells/experiment). For quantification of focal adhesions (FAs), the number and the size (area) of FAs were determined by “Particle analysis” function in ImageJ (Cai et al., 2006). The FAs were determined by quantifying pixels in cells stained with Ab against paxillin. We defined paxillin foci 6–30 pixels (3.22–16.05 μm2) as large FAs and 1–5 pixels (0.535–2.675 μm2) as small FAs.
Statistical Analysis
All statistical analyses were performed with a Student's t test tool.
Plasmid Construction, Conditional Cell Lines, and Transfection
Mutant MLC20 in which PKC phosphorylation sites (Ser1 and Ser2) were mutated to Ala was made by site-directed mutagenesis (Yano et al., 1993). Nonmuscle human myosin IIB heavy-chain cDNA was received as a gift from Dr. R. Adelstein (National Institutes of Health) and cloned into pEYFP-C1 plasmid (Clontech, Palo Alto, CA). To delete the C-terminal amino acid residues of the human myosin IIB heavy chain, a stop codon was created at the codon 1898 for ΔC-Myosin IIB.
MEF/3T3 Tet-Off cells, which can be suppressed expression of fusion proteins in the presence of doxycycline, were grown in DMEM supplemented with 10% FBS. MLC20swere subcloned into pTRE2-Hyg (Clontech) containing a myc tag sequence. The myc tag sequence was introduced at the C-terminal end of MLC20. To obtain stably expressed MLC20-myc cell lines, colonies were maintained in 0.25 mg/ml G418, 0.25 mg/ml hygromycin, and 2 μg/ml doxycycline (Invitrogen Invitrogen, BD Biosciences, Clontech).
For transient transfection, NIH3T3 cells on a coverslip were transfected with Lipofectamine 2000 transfection reagent (Invitrogen) according to the protocol provided by the manufacturer. For transfection of stable cell lines, MEF3T3 Tet-Off cells were transfected with electroporation using a Gene Pulser II (Bio-Rad Laboratories, Richmond, CA; Komatsu et al., 2000).
RESULTS
Production of Inhibitory Site-specific Antibody
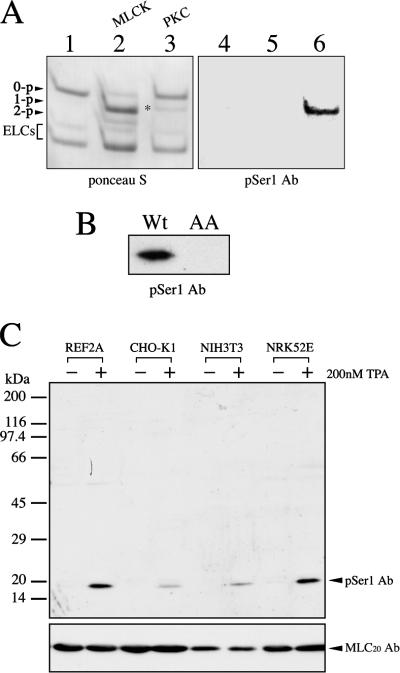
To monitor the extent and the spatial distribution of MLC20 phosphorylation at the inhibitory sites, we generated the phosphorylation site-specific antibodies. Because the phosphorylation of MLC20 at the inhibitory sites has only been observed on Ser1/2 but not on Thr9 in cells (Kawamoto et al., 1989; Yamakita et al., 1994), we produced the antibodies (pSer1 Ab) that raised against the N-terminal serine phosphorylation. The specificity of pSer1 Ab was examined by immunoblot analysis. Myosin II was phosphorylated by either MLCK or PKC, and the unphosphorylated, mono-, and diphosphorylated MLC20s were separated by urea/glycerol gel electrophoresis (Figure 1A, left), followed by immunoblotting with pSer1 Ab (Figure 1A, right). MLCK phosphorylated MLC20 to yield monophosphorylated MLC20 with minor di-phosphorylated MLC20. This represents the phosphorylation at Ser19 and Ser19/Thr18, respectively (Ikebe and Hartshorne, 1985b). On the other hand, PKC produced monophosphorylated MLC20 that is composed of MLC20 containing phospho-Thr9, phospho-Ser1, and phospho-Ser2, respectively (Ikebe et al., 1987). The pSer1 Ab only recognized the phosphorylated MLC20 of myosin II by PKC, but not phosphorylated MLC20 by MLCK and unphosphorylated MLC20. To examine whether pSer1 antibodies are specific to the N-terminal serine sites, we produced a S1A/S2A MLC20 mutant. The mutant was incubated with PKC for phosphorylation and subjected to Western blot analysis using pSer1 Ab. Although PKC phosphorylated the mutant MLC20, presumably at Thr9, the pSer1 Ab did not react with the S1A/S2A MLC20 mutant incubated with PKC (Figure 1B), indicating that pSer1 Ab recognized the phosphorylated MLC20 at the N-terminal serine sites but not Thr9 (Turbedsky et al., 1997; Varlamova et al., 2001). Figure 1C shows the Western blot of whole cell lysates of mammalian cultured cells using pSer1 Ab. Serum-starved cultured cells were stimulated with 200 nM TPA to activate PKC, and the change in the signal detected by pSer1 Ab was monitored. The pSer1 Ab recognized a band corresponding to the molecular weight of MLC20 only after TPA stimulation of the cells, indicating that the antibodies are highly specific to the phosphorylated MLC20 at the N-terminal serine.
Figure 1.
Specificity of pSer1 Ab against the phosphorylated regulatory light chain of myosin II. (A) Unphosphorylated (0-p) and phosphorylated MLC20 (1-p and 2-p) of myosin II either by MLCK or PKC were separated by alkali-urea/glycerol gel (left panel, Ponceau S staining), followed by immunoblotting with pSer1 Ab (right panel). Lane 1, unphosphorylated MLC20; lane 2, phosphorylated MLC20 by MLCK; and lane 3, phosphorylated MLC20 by PKC. ELCs, essential light chains. (B) Immunoblot analysis with pSer1 Ab was carried out for wild-type MLC20 (Wt) and S1A/S2A MLC20 (AA). Both wild-type MLC20 and S1A/S2A MLC20 were incubated with PKC in the presence of Mg2+-ATP, and the sample was analyzed by immunoblotting with pSer1 Ab. (C) Immunoblot of the whole lysates of TPA stimulated cells with pSer1 Ab. After serum starvation, REF-2A fibroblasts, CHO-K1, NIH3T3, or NRK52E epithelial cells were treated with 200 nM TPA or 0.1% DMSO (control) for 15 min, and then the whole cell lysates were subjected to immunoblotting with pSer1 Ab.
Phosphorylation of MLC20 at the Ser1/Ser2 Sites Correlates with PDGF-induced Disassembly of the Stress Fibers
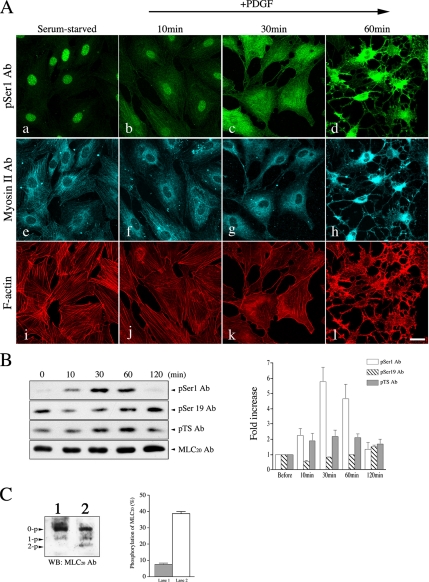
To investigate the role of phosphorylation of myosin II at the inhibitory sites on myosin function during cell motility, the serum-starved NIH3T3 cells were stimulated with PDGF, a potent stimulator of cell motility that is implicated in the activation of PKC pathway (Heldin et al., 1998). The level of MLC20 phosphorylation at Ser1 was examined by both immunostaining and immunoblotting of NIH3T3 cells with pSer1 Ab (Figure 2, A–C). Immunofluorescence images of the serum-starved NIH3T3 cells probed by pSer1 Ab showed the weak filamentous localizations of myosin II phosphorylated at Ser1/Ser2 sites of MLC20, and this was colocalized with myosin II filaments as well as actin stress fibers (Figure 2A, a, e, and i). It should be noted that the staining of the nucleus, in addition to the filamentous structures, was also observed in the serum-starved cells, suggesting that the phosphorylated MLC20 at Ser1/Ser2 seems to be present in the nucleus. This observation is similar to the previous reports that the phosphorylated MLC20 was found in the nucleus (Matsumura et al., 1998; Nagano et al., 2006). After PDGF stimulation, the disassembly of the stress fibers was observed (Figure 2A, j–l). Interestingly, the MLC20 phosphorylation at Ser1/Ser2 sites was markedly increased by PDGF and reached the maximum at 30 min after the stimulation (Figure 2B, top panel). The majority of the cells showing the disassembly of stress fibers is consistent with the time course of maximum phosphorylation of Ser1/Ser2 sites determined by Western blot analysis (Figure 2B). It should be mentioned that some cells showed the disassembly of stress-fibers at earlier times. These cells showed a relatively high level of phosphorylation of MLC20 at Ser1/Ser2 sites revealed by pSer1 Ab staining. The level of phosphorylated MLC20 at the activation sites was detected by two phospho-antibodies (pSer19 Ab and pTS Ab), which specifically recognized the phosphorylated MLC20 at Ser19 and di-phosphorylated MLC20 at Thr18 and Ser19, respectively (Komatsu et al., 2000; Komatsu and Ikebe, 2004). Phosphorylation of MLC20 at the Ser19 did not increased after PDGF stimulation. The di-phosphorylated MLC20 at the activation sites was slightly elevated upon PDGF stimulation, but it was sustained thereafter (Figure 2B, middle panels). To monitor the level of phosphorylated MLC20 at the inhibitory and activation sites, the total homogenates were subjected to alkali-urea and glycerol gel electrophoresis (Figure 2C). The amount of phosphorylated MLC20s at each site was estimated from the PDGF-induced change in the amount of phosphorylation at each site (Figure 2B) and the change in the singly and doubly phosphorylated MLC20 determined by the alkali-urea/glycerol PAGE (Figure 2C). The total amount of phosphorylated MLC20 before PDGF stimulation was ∼7.4 ± 1.6% of the total MLC20 estimated by alkali/urea gel (Figure 2C, lane 1). On the other hand, the total amount of phosphorylated MLC20 (monophosphorylated MLC20 + diphosphorylated MLC20) after PDGF stimulation was ∼38.7 ± 2.2% of the total MLC20 (Figure 2C, lane 2). Although PDGF stimulation significantly increased the phosphorylated MLC20 at Ser1, it rather decreased the phosphorylated MLC20 recognized by pSer19Ab (Figure 2B). It should be noted that the pSer19Ab recognizes both singly and doubly phosphorylated MLC20 at the activation sites (Komatsu et al., 2000); therefore, the result suggests that the total extent of phosphorylation at the activation sites was relatively decreased after PDGF stimulation. Because the amount of phosphorylation at Ser1 before PDGF stimulation was at a negligible level, the observed mono-phosphorylated MLC20 at rest should be predominantly attributed to phosphorylated MLC20 at Ser19. The amount of phosphorylated MLC20 at the activation site (Ser19) at 30 min after PDGF stimulation was ∼80% of that before PDGF stimulation (7.4 ± 1.6%; Figure 2B); therefore, it is calculated that more than 30% of the total MLC20 was phosphorylated at the Ser1/Ser2 sites after PDGF stimulation (38.7 ± 2.2 [+PDGF] − 0.8 × (7.4 ± 1.6) [Control]). The results suggest that a significant fraction of MLC20 was phosphorylated at the Ser1/Ser2 sites after PDGF stimulation.
Figure 2.
PDGF mediates the phosphorylation of MLC20 at Ser1 in cells. (A) NIH3T3 cells were treated with PDGF (20 ng/ml) as described in Materials and Methods for the indicated times. Top and bottom panels show the confocal microscopic images of cells stained with pSer1 Ab (a–d), Myosin II Ab (e–h), and Alexa Fluor546-phalloidin (i–l). The focal plane is close to the bottom of the cell. Bar, 25 μm. (B) Immunoblot of PDGF-stimulated cell lysates with pSer1 Ab, pSer19 Ab, pTS Ab, and MLC20 Ab. The whole cell lysates of PDGF-stimulated cells were subjected to SDS-PAGE followed by immunoblotting with pSer1 Ab, pSer19 Ab, pTS Ab, and MLC20 Ab. Right, the amount of phosphorylated MLC20 was determined by scanning densitometry (NIH image program). (C) Amount of phosphorylated MLC20 at the inhibitory sites and the activation sites. NIH3T3 cells were treated with 20 ng/ml PDGF for 30 min (lane 2) and then subjected to alkali-urea/glycerol gel electrophoresis, followed by immunoblotting with anti-MLC20 Ab (lane 1, control; untreated cells). Right, the fraction of phosphorylated MLC20 was determined by scanning densitometry (NIH image program). The values shown are means ± SD from three independent experiments.
Consistent with the Western blot data, the intensity of immunofluorescence signals of pSer1 Ab in the whole cells areas was significantly increased after PDGF stimulation (Figure 2A). The increase in the signal intensity was 1.4-, 3.5-, and 2.2-fold, at 10, 30, and 60 min after the stimulation (n = 10), respectively. It should be noted that the signal intensity observed in Figure 2, A–D, looks high, but this is because the cells changed their shapes and significantly decreased their cell volumes. These results suggest that the phosphorylation of the Ser1/Ser2 sites of MLC20 is involved in the PDGF-induced reorganization of actomyosin filaments.
PKCα/β Is Required for the PDGF-mediated Inhibitory Phosphorylation of MLC20
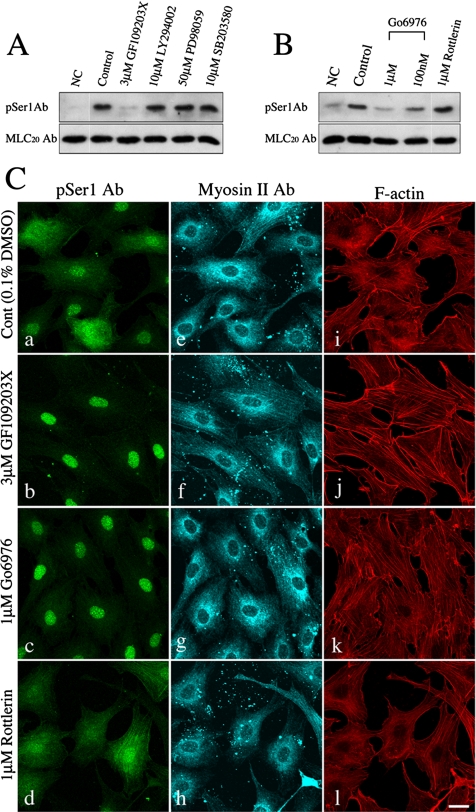
The PDGF signaling pathways have been implicated in cell growth and motility coupling with the activation of protein kinases such as phosphatidylinositol 3 kinase (PI3K), p42/p44 mitogen-activated protein kinases (MAPKs), and the PKC family (Heldin et al., 1998). To determine which of the signaling pathways triggered by PDGF stimulation are responsible for MLC20 phosphorylation at the inhibitory sites, NIH3T3 cells were pretreated with various kinase inhibitors and the effect on the MLC20 phosphorylation at the inhibitory sites was examined. Among the inhibitors tested, only the PKC inhibitor GF109203X, having a broad inhibitory spectrum against PKC isoforms, diminished the PDGF-mediated MLC20 phosphorylation at Ser1 (Figure 3A). This result indicates that PKC pathways are responsible for the PDGF-mediated phosphorylation of MLC20 at the inhibitory sites.
Figure 3.
Effect of the protein kinase inhibitors on the PDGF-mediated Ser1 phosphorylation. (A) Immunoblotting of PDGF-stimulated NIH3T3 cells in the presence of various kinase inhibitors. Serum-starved NIH3T3 cells were preincubated with the protein kinase inhibitors for 10 min (3 μM GF109203X, PKC inhibitor; 10 μM LY294002, PI3K inhibitor; 50 μM PD98059, MEK inhibitor; and SB203580, p38 MAP kinase inhibitor; respectively) before PDGF (20 ng/ml) stimulation. The reaction was stopped at 30 min after stimulation, and the whole cell lysates were subjected to immunoblot analysis with pSer1 Ab (top panel) and MLC20 Ab (bottom panel), respectively. NC, no treatment with PDGF; control, 0.1% DMSO. (B) Immunoblotting of PDGF-stimulated NIH3T3 cells in the presence of PKC inhibitors. Serum-starved NIH3T3 cells were treated with 20 ng/ml PDGF for 30 min in the presence of 0.1% DMSO (control), 3 μM GF109203X, 1 μM or 100 nM Go6976, or 1 μM Rottlerin. Cells were preincubated with the PKC inhibitors for 10 min before cell stimulation. The whole cell lysates were subjected to Western blotting with pSer1 Ab (top panel) and MLC20 Ab (bottom panel), respectively. NC, no treatment with PDGF. (C) Immunostaining of PDGF-stimulated NIH3T3 cells in the presence of PKC inhibitors. Serum-starved NIH3T3 cells were treated with 20 ng/ml PDGF for 30 min under the same condition as in B. The cells were stained with pSer1 Ab (a–d), Myosin II Ab (e–h) and Alexa Fluor546-phalloidin (i–l). Bar, 25 μm.
To verify which PKC isoforms are responsible for the PDGF-mediated phosphorylation of MLC20 at the inhibitory sites, NIH3T3 cells were pretreated with various PKC inhibitors having distinct isoenzyme specificities. The cells were examined either by Western blot or under a fluorescent microscope. The decrease in MLC20 phosphorylation at the inhibitory sites by PKCα/β specific inhibitor (Go6976) as well as GF109203X was found by Western blot analysis (Figure 3B). Consistent with this result, the decrease in the stress fibers after 30 min of PDGF stimulation was rescued by these inhibitors (Figure 3C). In contrast, Rottlerin, a PKCδ preferential inhibitor, failed to decrease the MLC20 phosphorylation (Figure 3B) and did not rescue the stress fiber disassembly induced by PDGF (Figure 3Cl). These results strongly suggest that Ca2+-dependent PKC isoforms (PKCα/β) play a critical role in the PDGF-induced disassembly of stress fibers via myosin II phosphorylation at the inhibitory sites.
Phosphorylation of MLC20 at Ser1/Ser2 Sites Is Involved in the Regulation of the Disassembly of Stress Fibers Induced by PDGF Receptor Stimulation
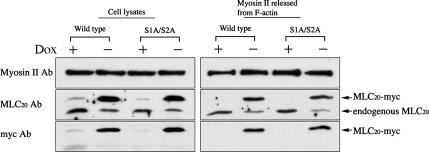
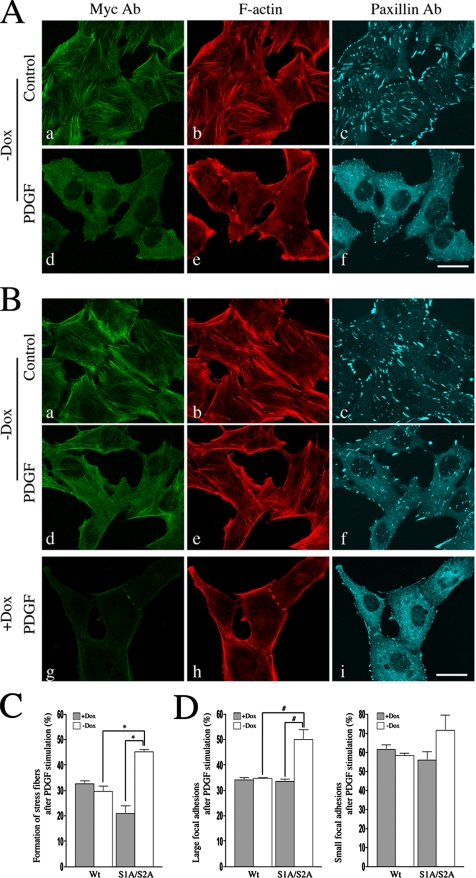
To study the role of phosphorylation of MLC20 at the inhibitory sites on the disassembly of stress fibers, we produced stably transfected MEF/3T3 Tet-Off cell lines expressing myc-tagged MLC20s. The reconstitution of myc-tagged MLC20 to myosin II was analyzed by using an ATP-dependent actin-binding property of myosin II (see Materials and Methods for details). As shown in Figure 4, both wild-type and S1A/S2A MLC20 stable cell lines were cultured in the presence or absence of doxycycline (Dox) and were subjected to an actin-binding assay. The expression level of myc-tagged MLC20 in each clone was ∼80% of the total MLC20, respectively (Figure 4, left panel: Cell lysates). The amount of myc-tagged MLC20 incorporated into myosin II was ∼3.5 times higher than that of endogenous MLC20 (Figure 4, right panel). Furthermore, the localization of the myc-tagged MLC20 signal showed filamentous localization that coincides with the localization of F-actin (Figure 5, A and B). The result indicates that myc-tagged MLC20 was effectively incorporated into myosin II in the stress fibers.
Figure 4.
Inducible expression of myc-tagged wild-type or S1A/S2A MLC20 in the stable transfectants. MEF/3T3 Tet-Off cells were cultured with (+) or without (−) doxycycline (Dox) to suppress or induce the expression of myc-tagged wild-type or S1A/S2A MLC20. Myosin II having endogenous and/or expressed MLC20s in cell lysates were coprecipitated with F-actin (see Materials and Methods for details). After releasing myosin II from F-actin by ATP, the supernatants were subjected to immunoblot, and the signals were detected with Myosin II Ab, MLC20 Ab, and myc Ab.
Figure 5.
Effect of the phosphorylation of MLC20 at the inhibitory sites on the disassembly of stress fibers in MEF/3T3 Tet-Off cells. MEF/3T3 Tet-Off cells were cultured in the absent of doxycycline (Dox) to induce the expression of myc-tagged wild-type or S1A/S2A MLC20. After 3 d, the cells were serum-starved for 18 h and then treated with PDGF for 40 min, followed by immunostaining with myc Ab, paxillin Ab, and Alexa Fluor546-phalloidin. Bar, 30 μm. (A) Immunostainig of MEF/3T3 Tet-Off cells expressed with wild-type MLC20. (B) Immunostaining of S1A/S2A MLC20 stable cells in the absent or presence of Dox. myc Ab (green), Alexa Fluor546-phalloidin (red), and paxillin (blue). Bar, 25 μm. (C) Quantification of PDGF-induced the disassembly of stress fibers in MEF/3T3 Tet-Off cells. *p < 0.003 compared with controls (+Dox) and wild-type MLC20s. (D) Quantitative comparisons of the focal adhesions in MEF/3T3 Tet-Off cells. #p < 0.02 compared with controls (+Dox) and wild-type MLC20s. The values shown are means ± SD from three independent experiments (>300 cells; 100 cells/experiment). The focal adhesion area were defined as follows: paxillin foci: large focal adhesions, 3.22–16.05 μm2 (6–30 pixels); and small focal adhesions, 0.535–2.675 μm2 (1–5 pixels).
To evaluate the role of the phosphorylation of MLC20 at the inhibitory sites on the disassembly of the stress fibers, the MEF/3T3 Tet-Off cells, expressing either wild-type or S1A/S2A MLC20, were cultured for 3 d without Dox to induce the expression of myc-tagged MLC20s. As shown in Figure 5, the cells expressing S1A/S2A MLC20 (Figure 5B, a–c) as well as those expressing wild-type MLC20 (Figure 5A, a–c) showed filamentous localization of MLC20. This coincides with the localization of F-actin, indicating that the mutation at Ser1/Ser2 sites of MLC20 does not change the myosin II localization before PDGF stimulation. After 40 min of PDGF stimulation, the cells expressing the wild-type MLC20 showed the disassembly of stress fibers, accompanied by the decrease in the number of focal adhesion (Figure 5A, d–f). In contrast, the PDGF-induced disassembly of stress fibers was attenuated in the cells expressing the unphosphorylatable S1A/S2A MLC20 (Figure 5B, d–f). On the other hand, the majority of the cells in the presence of Dox, thus inhibiting the expression of S1A/S2A MLC20 showed the disassembly of stress fibers (Figure 5B, g–i). The fraction of the cells forming the stress fibers in the control cells was 32.7 ± 2.0 and 29.6 ± 3.6% in the presence and absence of Dox, respectively, after PDGF stimulation (Figure 5C).
On the other hand, a significant increase in the stress fiber formation was observed for the cells expressing S1A/S2A MLC20 at 40 min after PDGF stimulation. The fraction of the cells forming the stress-fibers was 21.0 ± 5.3% before the expression of S1A/S2A MLC20, and the value was significantly increased to 45.3 ± 1.5% (p < 0.003, t test; Figure 5C). We also quantified focal adhesions in controls versus S1A/S2A MLC20-expressing cells and found that the number of large focal adhesions was significantly different between the controls and S1A/S2A MLC20-expressing cells after PDGF stimulation. PDGF stimulation decreased the number of the large focal adhesions (3.22–16.05 μm2), but the decrease in the number was reduced by the expression of S1A/S2A MLC20 (p < 0.02, t test; Figure 5D).
On the other hand, the decrease in the small focal adhesions (0.535–2.675 μm2) after the stimulation was not significantly different between controls and S1A/S2A MLC20-expressing cells (p = 0.2870, 0.168, and 0.150 [Wt (Dox +/−) vs. S1A/S2A and S1A/S2A; Dox (+) versus (−)], t test; Figure 5D). Similar results were obtained when NIH3T3 cells were transiently transfected with S1A/S2A MLC20-expressing vector. At 40 min after PDGF stimulation, the wild-type MLC20-transfected cells showed the disassembly of stress fibers accompanied with the decrease in the number of focal adhesion (not shown). In contrast, the expression of S1A/S2A MLC20 significantly attenuated the PDGF-induced disassembly of the stress fiber compared with the cells transfected with the wild-type MLC20 (not shown). The fraction of the cells forming the stress fibers in the wild-type transfected cells was 3.4 ± 1.5% (n = 150; 40–60 transfected cells analyzed in three independent experiments) at 40 min after PDGF stimulation. The value was the same as that of the nontransfected cells (4.0 ± 2.5%, n = 174). On the other hand, a significant increase in the stress fiber formation was observed for the cells transfected with S1A/S2A MLC20 (17.4 ± 0.7%; n = 150, p < 0.008, t test). These results further support the idea that the phosphorylation of MLC20 at Ser1/Ser2 sites affects the stability of stress fibers, thus contributing to the morphological changes induced by PDGF.
Effect of Heavy-Chain Phosphorylation on the Disassembly of Stress Fibers
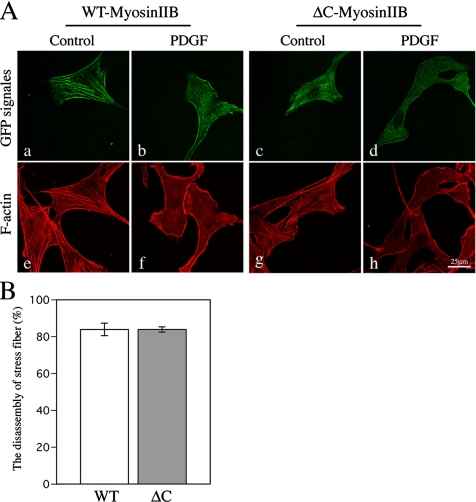
Previous studies have reported that PKC phosphorylates the heavy chains of nonmuscle myosin IIB in the nonhelical tailpiece, resulting in the inhibition of the assembly of myosin IIB into filaments in vitro (Murakami et al., 1998; Bresnick, 1999). Although it is not known if PDGF stimulation induces the phosphorylation of myosin IIB heavy chain, we examined whether the heavy-chain phosphorylation is involved in the regulation of the disassembly of stress fibers in cells. NIH3T3 cells were transfected with yellow fluorescent protein (YFP)-tagged ΔC-Myosin IIB in which the nonhelical tail sequence containing multiple phosphorylation sites is deleted (Murakami et al., 1990, 1998). The expression level of wild-type and ΔC-Myosin IIB in cells was estimated by measuring the fluorescence intensity of YFP signals, and the cells that expressed a similar level of the wild-type or ΔC-Myoisn IIB were used for the experiment. The amount of myosin II filaments in the cell expressing exogenous wild-type myosin IIB was estimated by staining with anti-myosin heavy-chain IIB antibodies. The signal intensities were about three times higher than that of endogenous myosin IIB in the untransfected cells, suggesting that the expressed wild-type myosin IIB was incorporated into the myosin filaments. We could not use the myosin IIB–specific antibodies to detect the incorporation of ΔC-Myoisn IIB into filaments, because the antibodies recognize the nonhelical tail domain (Phillips et al., 1995). However, because YFP-myosin IIB with and without the nonhelical tail were incorporated into the filaments with similar extent, it is expected that ΔC-Myoisn IIB is also incorporated into the filaments. Before the PDGF stimulation, both the wild-type and ΔC-Myosin IIB–transfected cells showed filamentous localization that coincided with the stress fibers (Figure 6A, a, e, and c, g). After 30 min of PDGF stimulation, the filamentous localization of myosin IIB was diminished that correlated with the disassembly of the stress fibers (Figure 6A, b, f, and d, h). The number of cells showing PDGF-induced disassembly of stress fibers was not significantly different between wild-type and ΔC-Myosin IIB–expressing cells (p > 0.98, t test; Figure 6B). The role of the phosphorylation at the inhibitory sites of MLC20 and the heavy-chain phosphorylation at the tail nonhelical-piece on the formation of the myosin filament structures is discussed in the Discussion.
Figure 6.
Deletion of C-terminal heavy-chain phosphorylation sites in the nonhelical tailpiece. (A) NIH3T3 cells were transfected with either YFP-tagged wild-type or ΔC-Myosin IIB. Twelve hours after transfection, the cells were serum-starved for 18 h and then the starved transfected cells were treated with 20 ng/ml PDGF for 30 min, followed by staining with Alexa Fluor546-phalloidin for visualizing F-actin. Bar, 25 μm. (B) The bar graphs show quantification of PDGF-induced disassembly of stress fibers in NIH3T3 cells transfected with YFP-wild-type Myosin IIB or ΔC-Myosin IIB. Transfected cells (n = 40–65) were analyzed in three independent experiments (wild type, n = 165; ΔC, n = 137; p > 0.98, t tests). The values shown are means ± SD from three independent experiments.
DISCUSSION
Actomyosin contractility in nonmuscle cells plays a fundamental role in various types of cellular motility including cell migration and cell division (Lauffenburger and Horwitz, 1996; Geiger and Bershadsky, 2001; Matsumura, 2005). Despite the abundant research of myosin II functions coupled with phosphorylation of MLC20 at the activation sites, little is known about the phosphorylation of MLC20 at the inhibitory sites. The present study provides the first evidence that the phosphorylation of MLC20 at Ser1/Ser2 sites plays an important role in the normal reorganization of actomyosin structures induced by the stimulation of PDGF signaling.
Because PDGF stimulation could activate various protein kinase pathways (Heldin et al., 1998), we attempted to identify the kinase responsible for the PDGF-mediated phosphorylation of MLC20 at the inhibitory sites in cells. Using the various types of kinase-specific inhibitors, we found that PKC but not PI3 kinase and MAPKs is responsible for the phosphorylation. Furthermore, our results suggest that the phosphorylation of MLC20 at the inhibitory sites is mediated by conventional PKC isoforms α/β (Figure 3). Supporting this observation, as previously reported, conventional PKC is the major kinase responsible for the inhibitory phosphorylation of MLC20 in mitotic extracts (Varlamova et al., 2001). It was originally reported that the cdc2 kinase is responsible for the phosphorylation of MLC20 at the inhibitory sites in mitosis (Satterwhite et al., 1992); however, it was subsequently reported that there are kinases other than cdc2 kinase responsible for the phosphorylation of MLC20 at the inhibitory sites in mitotic cells from mammalian cultured cells (Yamakita et al., 1994) and sea urchin eggs (Komatsu et al., 1997). An in vitro biochemical study has shown that PKCα has approximately threefold greater catalytic activity than PKCβ for MLC20 as a substrate and that PKCα phosphorylates Ser1/2 and Thr9 of MLC20, whereas PKCβ predominantly phosphorylated Thr9 of MLC20 (Varlamova et al., 2001). Furthermore, PKCα is the most abundant conventional PKC isoforms in the NIH3T3 cells (Goodnight et al., 1995). Therefore, we concluded that the phosphorylation of MLC20 at the inhibitory sites upon PDGF stimulation is predominantly catalyzed by conventional PKCα.
It was previously reported that expression of the charge reversal form of the MLC20 mutant at the activation sites (substitution of Thr18 and Ser19 by Asp) promotes stable stress fiber formation in NIH3T3 cells (Amano et al., 1998) and that the increase in MLC20 phosphorylation at the activation sites, via inhibition of myosin phosphatase, induces both formation of stress fibers and focal adhesions (Totsukawa et al., 2000). In addition, the mutation of the activation sites of MLC20 to unphosphorylatable residues (S19A/Thr18/A) results in the inhibition of the myosin II contractile activity (Komatsu et al., 2000) and the reduction of the number of actomyosin filaments (Komatsu and Ikebe, unpublished observations). These results suggest that the activation of the myosin activity is critical to induce the formation of myosin filaments and necessary to maintain the structure of stress fibers and focal adhesions (Figure 7A).
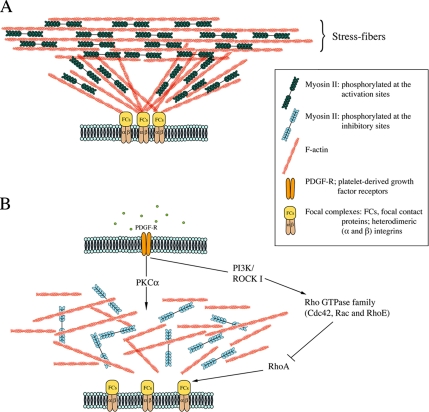
Figure 7.
Model for the role of myosin II phosphorylation in a negative and positive manner. (A) The generation of force by the complex of actin and active form of myosin II stabilizes the actomyosin filaments and focal adhesions. (B) The effects of stimulation of the cells with PDGF. PDGF transiently activates PKCα and Rho GTPase family (RhoE, Rac, and Cdc42) resulting in the down-regulation of myosin II and RhoA activities, which contributes to the reorganization of actin cytoskeleton.
Recently it was reported that PDGF stimulation triggers the transient phosphorylation of Rho GTPase family member RhoE in NIH3T3 cells (Riento et al., 2005) and proposed that RhoE phosphorylation increases the stability of RhoE protein resulting in the disruption of stress fibers through the inhibition of signaling downstream of RhoA (Guasch et al., 1998; Riento et al., 2005). Other Rho family members Rac and Cdc42 have also been shown to be transiently activated by PDGF stimulation that lead to the down-regulation of RhoA activity thus attenuating the stress fiber formation (Sander et al., 1999; Jimenez et al., 2000). On the basis of these observations together with our present data, we propose that the PKC-dependent down-regulation of myosin motor activity and the down-regulation of RhoA concertedly control the change in actin cytoskeletal structure upon PDGF stimulation (Figure 7B).
How does the phosphorylation at the inhibitory sites induce disassembly of stress fiber and the decrease in the focal adhesion? It was previously reported that the phosphorylation at the inhibitory sites inhibits the motor activity of myosin II phosphorylated at the activation sties, but not the myosin filament formation (Nishikawa et al., 1984; Ikebe et al., 1987; Ikebe and Reardon, 1990). Therefore, we think that the inhibition of myosin II motor activity by the phosphorylation of MLC20 at the inhibitory sites is in part responsible for the PDGF-induced disassembly of stress fibers and the decrease in the focal adhesion. Supporting this view, blebbistatin, a specific inhibitor of actin-activated myosin II ATPase activity but not the filament formation, blocked the formation of actomyosin stress fibers and focal adhesions (Straight et al., 2003; Kovacs et al., 2004; Hotulainen and Lappalainen, 2006). This result suggests that the myosin motor activity is critical for the formation of stress fibers and focal adhesions. In favor of this view, Burridge and coworkers suggested that the activation of myosin II activity produces the force driving the formation of stress fibers and focal adhesions (Chrzanowska-Wodnicka and Burridge, 1996). Because stress fibers are thought to attach to the focal adhesions that provide the force required for anchoring them to the cell matrix (Geiger and Bershadsky, 2001; Geiger et al., 2001), it is therefore likely that the decrease in the myosin II–driven force by the phosphorylation of MLC20 at the inhibitory sites influences the formation of focal adhesions as well as the stress fibers.
Previous in vitro biochemical studies have suggested that the phosphorylation of myosin II at the nonhelical tail plays a role in the disassembly of myosin filamentous structures (Murakami et al., 1998, 2000; Dulyaninova et al., 2005). In the present study, the expression of S1A/S2A MLC20 attenuated the PDGF-induced disassembly of stress fibers (Figure 5), whereas the expression of ΔC-Myosin IIB did not significantly change the disassembly of stress fibers (Figure 6). It was recently reported that phosphorylation of nonmuscle myosin heavy-chain (MHC)-II-B at the tail by atypical PKC zeta (aPKCζ) destabilizes the myosin IIB filaments in an epidermal growth factor (EGF)-dependent manner (Even-Faitelson and Ravid, 2006). However, aPKCζ did not phosphorylate the MLC20 (Varlamova et al., 2001). It is therefore likely that the phosphorylation of MLC20 and MHC by PKC isoforms are differentially involved in the reorganization of actomyosin filaments during the cell motile events modulated based on the types of extracellular stimuli. Interestingly, it was reported that bradykinin and EGF-mediated MHC-IIA and -IIB phosphorylation is associated with a loss of cortical myosin II (van Leeuwen et al., 1999; Straussman et al., 2001). These previous works also suggest that MHC phosphorylation is involved in the regulation of microfilament disassembly in the cell cortex. Taking these findings together, it is plausible that the phosphorylation of MLC20 at the inhibitory sites and MHC phosphorylation at the nonhelical tail play distinct roles in the actomyosin dynamics in different spatial domains in cells. This view is supported by the recent report showing that the expressed MHC-IIB, in which the PKC phosphorylation sites were converted to Asp residues, was able to localize at the stress fibers, but this mutation attenuated the localization of the mutant MHC-IIB to the cell cortex (Rosenberg and Ravid, 2006). Moreover, it was recently reported that myosin II isoforms (IIA and IIB) differentially contributes to the cell motility (Even-Ram et al., 2007; Vicente-Manzanares et al., 2007), suggesting that myosin isoforms are regulated by distinct mechanisms in motile cells. Supporting this notion, biochemical studies revealed that phosphate incorporation of MHC-IIB by PKC is much faster than that of myosin IIA and, in contrast, that filament assembly of myosin IIA is regulated by its binding protein (Mts1) but not that of myosin IIB (Murakami et al., 1998, 2000; Dulyaninova et al., 2005). To clarify the roles of phosphorylation of MLC20 and MHC on actomyosin dynamics in distinct cellular domains will be the subjects of future work.
On the basis of our findings, together with the results of previous studies, we propose a model for reorganization of actomyosin structure upon PDGF stimulation in the cells (Figure 7). The initial stage of the PDGF receptor stimulation is the dynamic cytoskeletal rearrangement involving a decrease in the stress fiber and a reduction in the focal adhesion complexes (Bockus and Stiles, 1984; Heldin et al., 1998). Present results suggest that the down-regulation of myosin II activity via the phosphorylation of MLC20 at the Ser1/Ser2 sites is the factor determining reorganization of actomyosin structure in this process. It has been thought that the mechanical force linking the focal adhesion and the cytoskeleton influences the stress fiber formation, and we think that the down-regulation of the contractile activity of myosin II by PDGF-mediated phosphorylation at the Ser1/Ser2 sites facilitates the normal disassembly of the stress fibers and the actin cytoskeletal reorganization. The later stage of PDGF receptor stimulation is the promotion of cell motility. The force generated by the activation of myosin II motor activity is thought to be essential for this motile process. Recently, we found that the disruption of zipper-interacting protein (ZIP) kinase by siRNA decreases the myosin II phosphorylation at the activation sites and leads to the inhibition of PDGF-induced cell migration as well as wound healing of NIH3T3 cells (Komatsu and Ikebe, 2004). Taken together with the present study, we conclude that the regulation of myosin II, in both negative and positive manner, controls the reorganization of the actomyosin filament and the generation of motile force via modulating the motor activity and myosin filament stability.
ACKNOWLEDGMENTS
We thank Dr. R. Adelstein and Dr. J. Stull for gifts of nonmuscle myosin IIB cDNA and antibodies. We thank Dr. S. Watanabe (University of Massachusetts) for providing rabbit skeletal F-actin. This work was supported by National Institutes of Health Grants AR41653, HL073050, AR048526, AR048898, and DC006103 (M.I.) and the American Heart Association Grant 0535419T (S.K.).
Abbreviations used:
- PDGF
platelet-derived growth factor
- MLCK
myosin light-chain kinase
- MLC20
myosin regulatory light chain, 20 kDa
- PKC
protein kinase C
- PI3K
phosphatidylinositol 3 kinase
- MAPK
p42/p44 mitogen-activated protein kinase
- Dox
doxycycline
- TPA
12-O-tetradecanoylphorbol-13-acetate
- MHC
myosin heavy chain.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1076) on October 10, 2007.
REFERENCES
- Amano M., Chihara K., Nakamura N., Fukata Y., Yano T., Shibata M., Ikebe M., Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Bengur A. R., Robinson E. A., Appella E., Sellers J. R. Sequence of the sites phosphorylated by protein kinase C in the smooth muscle myosin light chain. J. Biol. Chem. 1987;262:7613–7617. [PubMed] [Google Scholar]
- Bockus B. J., Stiles C. D. Regulation of cytoskeletal architecture by platelet-derived growth factor, insulin and epidermal growth factor. Exp. Cell Res. 1984;153:186–197. doi: 10.1016/0014-4827(84)90460-9. [DOI] [PubMed] [Google Scholar]
- Bresnick A. R. Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 1999;11:26–33. doi: 10.1016/s0955-0674(99)80004-0. [DOI] [PubMed] [Google Scholar]
- Cai Y., et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys. J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulyaninova N. G., Malashkevich V. N., Almo S. C., Bresnick A. R. Regulation of myosin-IIA assembly and Mts1 binding by heavy chain phosphorylation. Biochemistry. 2005;44:6867–6876. doi: 10.1021/bi0500776. [DOI] [PubMed] [Google Scholar]
- Even-Faitelson L., Ravid S. PAK1 and aPKCzeta regulate myosin II-B phosphorylation: a novel signaling pathway regulating filament assembly. Mol. Biol. Cell. 2006;17:2869–2881. doi: 10.1091/mbc.E05-11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Ram S., Doyle A. D., Conti M. A., Matsumoto K., Adelstein R. S., Yamada K. M. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat. Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Geiger B., Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 2001;13:584–592. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Geiger B., Bershadsky A., Pankov R., Yamada K. M. Transmembrane crosstalk between the extracellular matrix–cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Goodnight J. A., Mischak H., Kolch W., Mushinski J. F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J. Biol. Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- Guasch R. M., Scambler P., Jones G. E., Ridley A. J. RhoE regulates actin cytoskeleton organization and cell migration. Mol. Cell. Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Ostman A., Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Homma K., Saito J., Ikebe R., Ikebe M. Ca(2+)-dependent regulation of the motor activity of myosin V. J. Biol. Chem. 2000;275:34766–34771. doi: 10.1074/jbc.M003132200. [DOI] [PubMed] [Google Scholar]
- Hotulainen P., Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J. Cell Biol. 2006;173:383–394. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Effects of Ca2+ on the conformation and enzymatic activity of smooth muscle myosin. J. Biol. Chem. 1985a;260:13146–13153. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J. Biol. Chem. 1985b;260:10027–10031. [PubMed] [Google Scholar]
- Ikebe M., Hartshorne D. J., Elzinga M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J. Biol. Chem. 1987;262:9569–9573. [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Phosphorylation of bovine platelet myosin by protein kinase C. Biochemistry. 1990;29:2713–2720. doi: 10.1021/bi00463a014. [DOI] [PubMed] [Google Scholar]
- Ikebe R., Reardon S., Mitsui T., Ikebe M. Role of the N-terminal region of the regulatory light chain in the dephosphorylation of myosin by myosin light chain phosphatase. J. Biol. Chem. 1999;274:30122–30126. doi: 10.1074/jbc.274.42.30122. [DOI] [PubMed] [Google Scholar]
- Jimenez C., Portela R. A., Mellado M., Rodriguez-Frade J. M., Collard J., Serrano A., Martinez A. C., Avila J., Carrera A. C. Role of the PI3K regulatory subunit in the control of actin organization and cell migration. J. Cell Biol. 2000;151:249–262. doi: 10.1083/jcb.151.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Bengur A. R., Sellers J. R., Adelstein R. S. In situ phosphorylation of human platelet myosin heavy and light chains by protein kinase C. J. Biol. Chem. 1989;264:2258–2265. [PubMed] [Google Scholar]
- Komatsu S., Ikebe M. ZIP kinase is responsible for the phosphorylation of myosin II and necessary for cell motility in mammalian fibroblasts. J. Cell Biol. 2004;165:243–254. doi: 10.1083/jcb.200309056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S., Miyazaki K., Tuft R. A., Ikebe M. Translocation of telokin by cGMP signaling in smooth muscle cells. Am. J. Physiol. Cell Physiol. 2002;283:C752–C761. doi: 10.1152/ajpcell.00501.2001. [DOI] [PubMed] [Google Scholar]
- Komatsu S., Murata-Hori M., Totsukawa G., Murai N., Fujimoto H., Mabuchi I., Hosoya H. Identification of p34cdc2 kinase from sea urchin Hemicentrotus pulcherrimus and its involvement in the phosphorylation of myosin II regulatory light chain in the metaphase extract. Gene. 1997;198:359–365. doi: 10.1016/s0378-1119(97)00338-7. [DOI] [PubMed] [Google Scholar]
- Komatsu S., Yano T., Shibata M., Tuft R. A., Ikebe M. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J. Biol. Chem. 2000;275:34512–34520. doi: 10.1074/jbc.M003019200. [DOI] [PubMed] [Google Scholar]
- Kovacs M., Toth J., Hetenyi C., Malnasi-Csizmadia A., Sellers J. R. Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A., Horwitz A. F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Matsumura F. Regulation of myosin II during cytokinesis in higher eukaryotes. Trends Cell Biol. 2005;15:371–377. doi: 10.1016/j.tcb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Ono S., Yamakita Y., Totsukawa G., Yamashiro S. Specific localization of serine 19 phosphorylated myosin II during cell locomotion and mitosis of cultured cells. J. Cell Biol. 1998;140:119–129. doi: 10.1083/jcb.140.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami N., Chauhan V. P., Elzinga M. Two nonmuscle myosin II heavy chain isoforms expressed in rabbit brains: filament forming properties, the effects of phosphorylation by protein kinase C and casein kinase II, and location of the phosphorylation sites. Biochemistry. 1998;37:1989–2003. doi: 10.1021/bi971959a. [DOI] [PubMed] [Google Scholar]
- Murakami N., Healy-Louie G., Elzinga M. Amino acid sequence around the serine phosphorylated by casein kinase II in brain myosin heavy chain. J. Biol. Chem. 1990;265:1041–1047. [PubMed] [Google Scholar]
- Murakami N., Kotula L., Hwang Y. W. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry. 2000;39:11441–11451. doi: 10.1021/bi000347e. [DOI] [PubMed] [Google Scholar]
- Nagano K., Bornhauser B. C., Warnasuriya G., Entwistle A., Cramer R., Lindholm D., Naaby-Hansen S. PDGF regulates the actin cytoskeleton through hnRNP-K-mediated activation of the ubiquitin E(3)-ligase MIR. EMBO J. 2006;25:1871–1882. doi: 10.1038/sj.emboj.7601059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Sellers J. R., Adelstein R. S., Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J. Biol. Chem. 1984;259:8808–8814. [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem. J. 1970;119:31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. L., Yamakawa K., Adelstein R. S. Cloning of the cDNA encoding human nonmuscle myosin heavy chain-B and analysis of human tissues with isoform-specific antibodies. J. Muscle Res. Cell Motil. 1995;16:379–389. doi: 10.1007/BF00114503. [DOI] [PubMed] [Google Scholar]
- Riento K., Totty N., Villalonga P., Garg R., Guasch R., Ridley A. J. RhoE function is regulated by ROCK I-mediated phosphorylation. EMBO J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Ravid S. Protein kinase Cγ regulates myosin IIB phosphorylation, cellular localization, and filament assembly. Mol. Biol. Cell. 2006;17:1364–1374. doi: 10.1091/mbc.E05-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou A., Sullivan W., Karess R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 2002;158:127–137. doi: 10.1083/jcb.200203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander E. E., ten Klooster J. P., van Delft S., van der Kammen R. A., Collard J. G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite L. L., Lohka M. J., Wilson K. L., Scherson T. Y., Cisek L. J., Corden J. L., Pollard T. D. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc 2, a mechanism for the timing of cytokinesis. J. Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr. Opin. Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Straussman R., Even L., Ravid S. Myosin II heavy chain isoforms are phosphorylated in an EGF-dependent manner: involvement of protein kinase C. J. Cell Sci. 2001;114:3047–3057. doi: 10.1242/jcs.114.16.3047. [DOI] [PubMed] [Google Scholar]
- Tan J. L., Ravid S., Spudich J. A. Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbedsky K., Pollard T. D., Bresnick A. R. A subset of protein kinase C phosphorylation sites on the myosin II regulatory light chain inhibits phosphorylation by myosin light chain kinase. Biochemistry. 1997;36:2063–2067. doi: 10.1021/bi9624651. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. N., van Delft S., Kain H. E., van der Kammen R. A., Collard J. G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;1:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- Varlamova O., Spektor A., Bresnick A. R. Protein kinase C mediates phosphorylation of the regulatory light chain of myosin-II during mitosis. J. Muscle Res. Cell Motil. 2001;22:243–250. doi: 10.1023/a:1012289905754. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Zareno J., Whitmore L., Choi C. K., Horwitz A. F. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J. Cell Biol. 2007;176:573–580. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q., Adelstein R. S. Conditional expression of a truncated fragment of nonmuscle myosin II-A alters cell shape but not cytokinesis in HeLa cells. Mol. Biol. Cell. 2000;11:3617–3627. doi: 10.1091/mbc.11.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J. Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., Araki Y., Hales S. J., Tanaka M., Ikebe M. Boundary of the autoinhibitory region of smooth muscle myosin light-chain kinase. Biochemistry. 1993;32:12054–12061. doi: 10.1021/bi00096a016. [DOI] [PubMed] [Google Scholar]