Abstract
Metal ions are essential as well as toxic to the cell. The mechanism of metal-induced toxicity is not well established. Here, for the first time we studied two essential nutritional elements, copper and manganese, for their apoptotic effects in yeast Saccharomyces cerevisiae. Although beneficial at subtoxic levels, we demonstrated that at moderately toxic levels, both metals induce extensive apoptosis in yeast cells. At even higher concentrations, necrosis takes over. Furthermore, we investigated the molecular pathways mediating Cu- and Mn-mediated apoptotic action. Mitochondria-defective yeast exhibit a much reduced apoptotic marker expression and better survival under Cu and Mn stress, indicating mitochondria are involved in both Cu- and Mn-induced apoptosis. Reactive oxygen species (ROS) are generated in high amounts in Cu- but not in Mn-induced cell death, and Cu toxicity can be alleviated by overexpression of superoxide dismutase 2, suggesting ROS mediate Cu but not Mn toxicity. Yeast metacaspase Yca1p is not involved in Cu-induced apoptosis, although it plays an important role in the Mn-induced process. A genetic screen identified Cpr3p, a yeast cyclophilin D homologue, as mediating the Cu-induced apoptotic program. Cpr3p mutant seems to eliminate Cu-induced apoptosis without affecting ROS production, while leaving necrosis intact. These results may provide important insight into a detailed understanding at the molecular and cellular level of metal toxicity and metal accumulation diseases.
INTRODUCTION
Transition metals, as an important part of trace nutritional elements, are essential to life because of the catalytic and structural roles they play inside or outside of cells. Iron, for example, is a small but vital component of hemoglobin in billions of red blood cells, of which ∼100 million are formed every minute. Iron deficiency leads to anemia, and in serious cases, death. Copper is a component of enzymes such as superoxide dismutase (SOD)1, catalase, and dopamine hydroxylase, and it is a critical component in mitochondrial electron transport chain (ETC). Menkes syndrome, resulting from a copper transporter defect (Chelly et al., 1993; Mercer et al., 1993; Vulpe et al., 1993), is a devastating disease, and mice knockout of Cu intake gene results in early embryonic lethality (Kuo et al., 2001; Lee et al., 2001).
Aside from these beneficial effects, excessive amounts of metal ions can also be toxic to the cell. Accumulation of metal ions has been reported in all species, and it inevitably results in toxicosis. In humans, Cu toxicosis can be the result of inherited abnormalities (such as in Wilson's disease and likely some cases of Indian childhood cirrhosis) or environmental poisoning, and it often leads to hepatic cirrhosis and degeneration of the basal ganglia. Exposure to high levels of manganese, an element found in several critical enzymes within diverse locations in the cell, including the Golgi, mitochondria and cytoplasm, can lead to manganism, a Parkinson's disease-like neurological disorder with characteristic syndromes of mental difficulties and impairments in motor skills (Pal et al., 1999; Kaiser, 2003). Metal ion accumulation is even implicated in the pathogenesis of neuronal injury in Alzheimer's disease, prion-mediated encephalopathies, familial amyotrophic lateral sclerosis, and other age-related diseases (Waggoner et al., 1999). To balance out the beneficial and detrimental effects of metal ions, cells have evolved intricate and complicated systems to deal with metal deficiency and excess. Although great strides have been made toward understanding metal homeostasis in the past decade, very little is known regarding the factors that contribute to the metal toxicity.
Apoptosis is a form of programmed cell death (PCD), and it plays a central role in development and homeostasis of metazoan organisms. Apoptosis allows rapid removal of potentially threatening or undesired cells (Vaux and Korsmeyer, 1999; Beers and McDowell, 2001). Recently apoptosis is also discovered in unicellular organisms such as bacteria and yeast. In Saccharomyces cerevisiae, apoptosis-like cell death was first described for a temperature-sensitive cdc48 mutant (Madeo et al., 1997). Since then, many intra- or extracellular factors, such as the α mating-type pheromone (Severin and Hyman, 2002), low doses of hydrogen peroxide (Madeo et al., 1999), acetic acid (Ludovico et al., 2001), UV radiation (Del Carratore et al., 2002), salt (Huh et al., 2002), aspirin (Balzan et al., 2004), low sugar concentrations in the absence of additional nutrients (Granot et al., 2003), hyperosmotic stress (Silva et al., 2005), and viral killer toxins (Reiter et al., 2005) have found to be able to induce yeast apoptosis. In addition, physiological scenarios of yeast apoptosis have been described during aging process (Laun et al., 2001; Herker et al., 2004). Just as in mammalian cells (Kerr et al., 1972; Martin et al., 1995; Clifford et al., 1996), yeast apoptosis can be detected with typical markers, such as DNA fragmentation, phosphatidylserine externalization, and chromatin condensation (Madeo et al., 1997). Worth noting is that although PCD and apoptosis may not be synonymous in the mammalian field, it is standard in yeast studies that they are referred to as identical terms.
Many similarities occur between apoptotic programs in yeast and mammalian cells. A metacaspase analogous to mammalian caspases called yeast caspase-1 (Yca1p) was identified in S. cerevisiae to be required for H2O2 or aging-induced apoptosis (Madeo et al., 2002). An orthologue of key regulator such as the HtrA2-like protein (Fahrenkrog et al., 2004) has been observed in yeast. In addition, an apoptosis-inducing factor (AIF) orthologue, Aif1p, was identified in yeast that displays sequence similarity to AIF and AIF-homologous mitochondrion-associated inducer of death (AMID) and that regulates apoptosis in a similar way to that of AIF in mammalian cells (Wu et al., 2002; Wissing et al., 2004). We previously found that yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity (Li et al., 2006). Fannjiang et al. (2004) also reported that Dnm1p, the S. cerevisiae homologue of the human mitochondrial fission protein Drp1p was involved in yeast apoptosis. Similar to that in mammalian apoptosis, reactive oxygen species (ROS) play a central role in most yeast apoptotic processes. In addition, evidence has been provided for cytochrome c-associated mitochondrial involvement in yeast apoptosis (Ludovico et al., 2002; Silva et al., 2005).
Genetic tractability, combined with the remarkable conservation of gene function throughout evolution, makes yeast an ideal model to study how cells deal with metal excess and execute cell death programs. To date, there are no reports of killing mechanisms of nutritional metals in yeast. Here, we report that Cu and Mn, two representative trace nutritional metal elements, are able to trigger S. cerevisiae into an apoptosis-like PCD process, and we identified different pathways in which they induce cell death.
MATERIALS AND METHODS
Strains and Growth Conditions
Yeast strains used in this study are listed in Table 1. Yeast cells were normally grown on YPD agar plates containing glucose (2%, wt/vol), yeast extract (1%, wt/vol), peptone (2%, wt/vol), and agar (2%, wt/vol), or they were subcultured in liquid YPD medium and shaken in a mechanical shaker (160 rpm) at 30°C.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4742 | MATα his3 Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Invitrogen |
| yca1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 yca1::kanMX4 | Invitrogen |
| aif1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 aif1::kanMX4 | Invitrogen |
| cyc1Δcyc7Δ | MATαhis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 cyc1::URA3 cyc7::kanMX4 | Invitrogen |
| ndi1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ndi1::kanMX4 | Invitrogen |
| cox12Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 cox12::kanMX4 | Invitrogen |
| qcr7Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 qcr7::kanMX4 | Invitrogen |
| cpr3Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 cpr3::kanMX4 | Invitrogen |
| por1Δ | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 por1::URA3 | Invitrogen |
| ρ0 | Isogenic derivative (lacking mitochondrial DNA) | This study |
Growth and Survival Tests
Yeast cells were grown to exponential phase in liquid YPD medium. For growth assays, the initial OD600 was adjusted to 0.05 (otherwise specify) and shaken at 30°C. OD600 were measured by spectrophotometer. For survival assays, different concentrations of metal ions were added. Metal treatment time was 12 h at 30°C. CuSO4 and MnCl2 were used. Survival was assessed by colony-forming unit (cfu) on YPD agar plates divided by the OD600 measurement after 2 d of incubation at 30°C.
4,6-Diamidino-2-phenylindole (DAPI) Staining and Microscopy
The standard protocol for DAPI nuclei staining was used, as described by Streiblova (1988). Cells were collected, resuspended in 70% (vol/vol) ethanol for brief fixation and permeabilization, and then stained with DAPI solution. Cell images were recorded at room temperature from a fluorescence microscope (model ECLIPSE 80i; Nikon, Tokyo, Japan) with a digital camera (model DXM1200F; Nikon). A 100× objective lens was used. Images were processed using Adobe Photoshop7.0 software (Adobe Systems, Mountain View, CA).
Terminal Deoxynucleotidyl Transferase-mediated dUTP Nick End Labeling (TUNEL) Staining
DNA strand breaks were monitored by TUNEL with the In Situ Cell Death Detection kit, Fluorescein (Roche Applied Science, Mannheim, Germany). TUNEL labels free 3′-OH termini with fluorescein isothiocyanate (FITC)-labeled deoxyuridine triphosphate (dUTP), which can be detected under epifluorescence microscopy. Yeast cells were fixed with 3.7% (vol/vol) formaldehyde as described by Madeo et al. (1999), and the cell wall was digested with 15 U/ml lyticase (Sigma-Aldrich, St. Louis, MO) for 2 h at 28°C. The cells were then applied to polylysine-coated slides. The cell slides were rinsed with phosphate-buffered saline (PBS), incubated in permeabilization solution (0.1%, vol/vol, Triton X-100 and 0.1%, wt/vol, sodium citrate) for 2 min on ice, rinsed twice with PBS, and incubated with 10 μl of TUNEL reaction mixture (200 U ml−1 terminal deoxynucleotidyl transferase, 10 mM FITC-labeled dUTP, 25 mM Tris-HCl, 200 mM sodium cacodylate, and 5 mM cobalt chloride) for 60 min at 37°C. Finally, the slides were rinsed three times with PBS, and a coverslip was mounted. Microscope and image acquisitions were performed as described above for DAPI staining, except that a 40× objective lens was used.
ROS Detection
For detection of intracellular ROS, cells were incubated with dihydrorhodamine 123 (DHR123) (Sigma-Aldrich) for 2 h or dihydroethidium (DE) (Sigma-Aldrich) for 10 min, normally after metal treatment unless specified otherwise, and the cells were examined under fluorescent microscopy as described for TUNEL assay.
RESULTS
Effect of Cu or Mn Stress on Growth and Survival of Yeast Cells
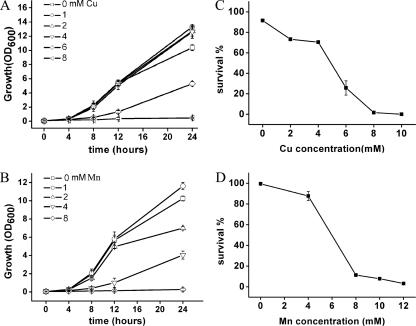
To examine the growth effect of Cu and Mn ions on yeast cells, yeast was grown under various amounts of these metals; 1 mM Cu2+ treatment seemed beneficial to cell growth, because during a 24-h incubation period cell density was higher than that in the control (Figure 1A). The 2 and 4 mM Cu2+ treatment also resulted in marginally higher cell density. The 1 mM Mn2+ treatment was found to slightly enhance cell growth after 24-h incubation (Figure 1B). Above these concentrations, however, cellular inhibition was observed, indicating high amounts of Cu and Mn ions introduced a toxic effect.
Figure 1.
Effect of Cu and Mn on yeast cell growth and survival. (A) Yeast growth under various Cu levels. Yeast strain BY4742 was incubated in liquid YPD containing a series of Cu2+ concentrations. Cell densities (OD600 values) were determined over a 24-h period. Vertical error bars represent standard deviations of the means of three independent experiments. (B) Yeast growth under various Mn levels. Effect of Mn2+ on yeast cell growth was analyzed as described in A. (C) Cu treatment decreases the percentage of surviving cells. Survival was measured by colony-forming cells on YPD agar plates, and 100% corresponds to the number of plated cells. Wild-type yeast BY4742 was incubated with different concentrations (0–10 mM) of Cu2+ for 12 h, and then cells were examined for survival. Vertical error bars represent standard deviations of the means of three independent experiments. (D) Mn treatment also decreases yeast survival ratio. For details, see in C, except Mn2+ instead of Cu2+ was used.
To investigate the survival effect of Cu and Mn on yeast cells, various amounts of Cu2+ (0–10 mM) or Mn2+ (0–12 mM) were added to yeast cell cultures at the exponential growth phase in YPD medium, and then cell death ratios were analyzed. After 12-h incubation, survival was assayed by cfu counts on YPD plates. The percentage of survival of cells decreased with increasing concentrations of Cu2+ in the medium (Figure 1C). Although low concentrations of Cu2+ only had a slight effect on cell survival, when Cu2+ concentration was increased to 6 mM, there was a rapid phase of cell demise, indicating that this concentration (between 4 and 6 mM) is a turning point for yeast cells in response to extracellular Cu2+. A 12-h exposure to 6 mM Cu2+ killed >70% of yeast cells, and <2% remained viable when treated with 8 mM Cu2+. Yeast cells also exhibited decreased survival when Mn2+ concentration was increased (Figure 1D). Significant toxicity was observed starting between 4 and 8 mM, and <10% wild-type (WT) cells survived when they were exposed to 10–12 mM Mn2+. These results demonstrated that Cu and Mn ions induce cell death starting at several millimolar levels.
Cu- or Mn-induced Yeast Death Displays Characteristic Markers of Apoptosis
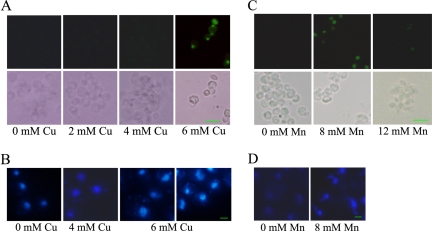
To assess whether the cell death induced by Cu and Mn ion is apoptotic, apoptotic markers were investigated. Previous study has demonstrated that yeast apoptosis is associated with the cleavage of chromosomal DNA (Madeo et al., 1997). Apoptotic DNA cleavage produces free 3′-OH termini, which can be detected by labeling with modified nucleotides (e.g., FITC-dUTP) catalyzed by terminal deoxynucleotidyl transferase (TUNEL assay). The TUNEL method is a fast and sensitive way to visualize the amount of DNA fragmentation in individual cells, and the result can be observed under fluorescent microscopy. Wild-type yeast exposed to 2 mM or 4 mM Cu2+ showed almost no TUNEL-positive cells, whereas after 12-h exposure to 6 mM Cu2+ and 8 mM Mn2+, yeast cells had an intensive fluorescent nuclear staining in the TUNEL assay. We observed 47% and 55% TUNEL-positive cells, respectively, after Cu2+ and Mn2+ treatment (Figure 2, A and C), indicating massive DNA fragmentation, whereas cells incubated in the absence of added Cu2+ and Mn2+ were unstained. Interestingly, treatment with even higher concentrations of Cu2+ resulted in death of most cells in necrosis, because DNA fragmentation could not be detected. As shown in Figure 3B, wild-type cells treated with 10 mM Cu2+ displayed negative TUNEL staining. This phenomenon also was noted for Mn2+. Although 8 mM Mn2+ was toxic to yeast and induced widespread apoptosis, at higher concentration (such as 12 mM) yeast cells died via necrosis pathway, because almost no DNA fragmentation was detected by TUNEL staining (Figure 2C).
Figure 2.
Yeast cells under Cu and Mn stress die, exhibiting typical markers of apoptosis. (A) Cells under Cu stress exhibit DNA fragmentation, as visualized by TUNEL staining. Wild-type BY4742 cells were treated with Cu2+ at indicated concentrations for 12 h. Control cells (without extra Cu2+) showed negative TUNEL staining. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. The frequency of stained cells was determined for at least 300 cells in three independent experiments. (B) DAPI staining of yeast cells under Cu stress shows abnormal chromatin condensation. Cu2+ was added at indicated concentrations. Bar, 10 μm. (C) Cells under Mn stress exhibit TUNEL staining. Wild-type cells were incubated with 8 or 12 mM Mn2+ for 12 h. TUNEL-positive cells were observed with 8 mM Mn2+ treatment, whereas hardly any were observed with 12 mM Mn2+. Control cells show negative TUNEL staining. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. The frequency of stained cells was determined for at least 300 cells in three independent experiments. (D) DAPI staining of yeast cells under 8 mM Mn2+ stress shows abnormal chromatin condensation. Bar, 10 μm.
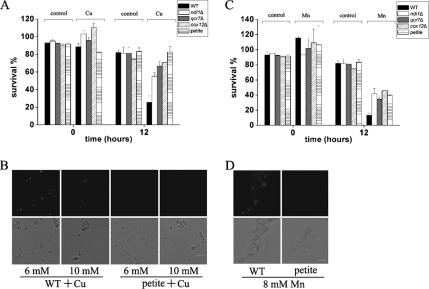
Figure 3.
Most respiratory-deficient S. cerevisiae strains are more resistant to Cu and Mn. (A) Most respiratory-deficient mutant yeast strains are more resistant to Cu2+ treatment. Survival was determined by cfu. Shown here are ndi1Δ (NADH dehydrogenase), qcr7Δ (complex III), cox12Δ (complex IV), and petite cells of S. cerevisiae. Cells were incubated for 12 h under 6 mM Cu2+, with untreated cells as control. Data for cells at time 0 are also provided. Cells at time 0 with Cu2+ treatment were only subjected to a brief Cu treatment, and they were immediately assayed for viability. So, “time 0” is only a close approximation. Vertical error bars represent standard deviations of the means of three independent experiments. (B) Petite cells exhibit insignificant TUNEL staining after 6 or 10 mM Cu2+ treatment. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. (C) Most respiratory-deficient mutant cells are more resistant to Mn2+ treatment. Mn2+ (8 mM) was used and survival was determined by cfu of cells. Other details are as described in A. (D) Petite mutant cells exhibit insignificant TUNEL staining after 8 mM Mn2+ treatment. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm.
Another familiar apoptotic feature is chromatin condensation and fragmentation, a well-established cytological hallmark of apoptosis. The nuclear alterations can be monitored by staining with DAPI. DAPI staining of cells incubated with 6 mM Cu2+ showed chromatin fragments arranged in a semicircle or distributed nuclear fragments in almost half of the cells (Figure 2B). Mn2+-treated cells also contained chromatin fragments arranged in the same way, as visualized by DAPI staining (Figure 2D). In contrast, in cultures untreated by Cu or Mn ion, chromatin appeared as a single round spot in the cells. This phenomenon also happened in cultures incubated with lower Cu concentrations, such as 4 mM. These results are consistent with the observations as described in the TUNEL assay. Together, they indicated that at moderately toxic levels Cu- and Mn-induced cell death are typically apoptotic.
Mitochondrial ETC Is Intimately Involved in Both Cu- and Mn-induced Apoptosis
Mitochondria are essential for most types of apoptosis in animals (Newmeyer et al., 1994; Skulachev, 1999). The role of mitochondrial function was also implicated in yeast apoptosis induced by various factors, such as pheromone, hyperosmotic stress, and acetic acid (Ludovico et al., 2002; Pozniakovsky et al., 2005; Silva et al., 2005). These findings prompted us to assess the role of mitochondrial functions in programmed cell death process induced by Cu2+. Petite yeast strain, which is defective in mitochondrial DNA and respiration, was more resistant to cell death induced by Cu2+ compared with the wild-type strain (Figure 3A). In addition, the occurrence of apoptotic markers was strongly reduced (Figure 3B), clearly demonstrating the involvement of mitochondria in Cu-induced apoptosis. Mitochondria also seem to play a role in Mn-induced apoptosis, as petite strain survived better and had much less manifestation of apoptotic markers than wild-type strain when exposed to Mn2+ (Figure 3, C and D).
To test the role of ETC in Cu-induced apoptosis, several single mutants affecting ETC components in NDI1, complex III, and complex IV were analyzed. Most, but not all, of them had better survival rates under Cu stress. Results for yeast mutants ndi1Δ, qcr7Δ and cox12Δ, which are defective in NDI1, complex III, and IV respectively, were shown in Figure 3A. Although survival rates of these ETC mutants increased more than twofold after 12 h of incubation with Cu2+, worth noting is that among the mutants we analyzed, qcr10Δ, which is defective in complex III, had comparable rate of growth to that of wild type. The role of the mitochondrial respiratory chain in Mn-induced apoptosis was also examined. After incubation with Mn2+, many ETC mutants exhibited significant better survival than that of wild type (Figure 3C), suggesting that mitochondrial respiratory chain is involved in both Cu- and Mn-induced cell death.
Different Effects of Cu and Mn on Intracellular Reactive Oxygen Species Production
One of the key mechanisms by which cells trigger programmed cell death is the production of ROS. ROS has been shown to be both necessary and sufficient for inducing apoptosis in yeast (Madeo et al., 1999). DHR123 or DE were used as probes for the detection of this apoptotic marker. DHR123 can be oxidized to the fluorescent chromophore rhodamine 123 by intracellular ROS. In the presence of the superoxide anion, DE is oxidized to ethidium that intercalates within nucleic acids, staining the cell with a bright red fluorescence.
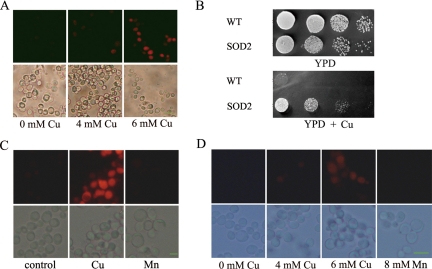
Cu2+ exposure of yeast cells led to an increase in ROS generation, which was dose dependent (Figure 4A). Exposure of wild-type yeast cells to 4 mM Cu exhibited little ROS accumulation, whereas at 6 mM a strong fluorescence signal was observed in ∼60% of treated cells. As a control, cells incubated in the absence of Cu2+ additionally displayed hardly any dihydrorhodamine staining. In contrast, ROS production was virtually not detected in yeast cells after Mn2+ treatment (Figure 4C), and this is true throughout the treatment, including 10 min, 40 min, 2 h, 4 h, 8 h, and 12 h of incubation. The same result was obtained using probe dihydroethidium (Figure 4D). To exclude the possibility of transient burst of ROS production, we additionally treated cells with Mn2+ in the presence of ROS-sensing dye (see Materials and Methods), and even under this scenario no significant ROS signal was detected (data not shown).
Figure 4.
ROS production is associated with Cu- but not Mn-induced apoptosis. (A) ROS production, as detected by dihydrorhodamin 123, in control cells and cells treated with Cu. ROS production induced by Cu2+ displayed a dose-dependent pattern. bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. (B) SOD2 overexpression reduces Cu toxicity. Five microliters of 10-fold serial dilution of yeast was spotted on YPD plates with or without added Cu2+. (C) ROS production is not detected in yeast cells treated with 8 mM Mn2+. Shown here are cells treated with 12 h of Mn2+ incubation. Similar result was observed with 10 min, 40 min, and 2, 4, or 8 h treatment. Cu-treated cells were used as positive control. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. (D) ROS production as monitored by dihydroethidium in control cells and cells treated with Cu or Mn. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm.
Intracellular ROS can be scavenged by addition of antioxidants such as ascorbic acid or vitamin E or by overexpression of oxygen stress defense genes. We found that neither ascorbate nor Vitamin E was effective in ameliorating Cu2+ toxicity on yeast cells. However, overexpression of SOD2, a Mn-containing superoxide dismutase and a ROS scavenger localized in mitochondria matrix, could significantly increase the resistance of wild-type yeast to Cu2+ (Figure 4B). We suggested that ROS is tightly associated with Cu-induced cell death in yeast and that elimination of mitochondrial ROS might confer increased resistance to Cu-induced toxicity, whereas Mn-induced toxicity is not associated with ROS production.
Different Roles of Yeast Metacaspase Yca1p in Cu- and Mn-induced Apoptosis
Yeast YCA1 gene codes for a metacaspase structurally and functionally analogous to mammalian caspases (Madeo et al., 2002). Yca1p has been previously shown to mediate apoptosis-like cell death in aged yeast cells and in yeasts treated with H2O2 or acetic acid (Madeo et al., 2002). Cytochrome c has been suggested to be important for metacaspase activation and probably acts upstream of this protease (Silva et al., 2005). Yeast Aif1p, a homologue of human AIF, was also reported to be involved in apoptosis in response to H2O2 or chronological aging (Wissing et al., 2004). AIF has been suggested to control a caspase-independent pathway of apoptosis, important for neurodegeneration and normal development (Susin et al., 1999; Cregan et al., 2002).
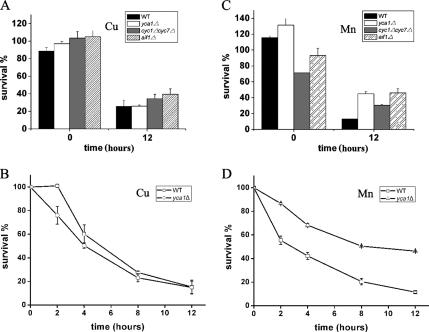
To determine whether metacaspase Yca1p and cytochrome c mediate metal-induced stress, we examined cell survival in cells exposed to Cu2+ and Mn2+. yca1Δ mutant and its isogenic WT displayed almost the same cell survival in the presence of 6 mM Cu2+ after 12 h or at different times before that (Figure 5, A and B), indicating metacaspase Yca1p did not play an important role in Cu-induced apoptosis. In addition, cyc1Δcyc7Δ (lacking cytochrome c) and aif1Δ strains did not significantly improve the viability of yeast cells when incubated with Cu2+, suggesting that none of them mediates Cu-induced apoptosis in a rather meaningful way.
Figure 5.
Yeast metacaspase Yca1p plays different roles in Cu- and Mn-induced apoptosis. (A) Cell survival of wild type and three mutant strains lacking metacaspase Yca1p (yca1Δ), cytochrome c (cyc1Δcyc7Δ), or yeast Aif1p (aif1Δ) after 12 h of Cu stress. Cells were incubated with 6 mM Cu2+ for 12 h, and survival was evaluated by the percentage of cfu of plated cells. All of these mutants did not exhibit significantly better survival than the control under Cu treatment. Vertical error bars represent standard deviations of the means of three independent experiments. (B) Cell survival of yeast yca1Δ mutant and its isogenic wild type (BY4742) versus time in the presence of 6 mM Cu2+. yca1Δ mutant cells did not exhibit better survival than the control during the entire incubation time. Vertical error bars represent standard deviations of the means of three independent experiments. (C) Cell survival of wild type, yca1Δ, cyc1Δcyc7Δ, and aif1Δ strains incubated with 8 mM Mn2+ for 12 h. Evaluation was performed as described in A. All of these mutants displayed significantly better survival than the control under Mn treatment. (D) Cell survival of yeast yca1Δ mutant versus time in the presence of 8 mM Mn2+. yca1Δ mutant displayed better survival than the control (BY4742) during the incubation time. Vertical error bars represent standard deviations of the means of three independent experiments.
In contrast to the Cu2+ findings, yca1Δ and cyc1Δcyc7Δ strains manifested better survival than wild-type strains after Mn2+ treatment (Figure 5C). The time course of cell survival was performed in the presence of 8 mM Mn2+. yca1Δ mutant displayed a significantly better survival compared with the wild type (Figure 5D), indicating metacaspase Yca1p is involved in Mn-induced yeast apoptosis program. The role of AIF1 in Mn-induced apoptosis was also examined. Loss of AIF1 seemed to improve the viability of cells exposed to Mn2+. Altogether, these results demonstrated that contrasting pathways are adopted by Cu2+ and Mn2+ in inducing apoptosis.
Yeast Cyclophilin D Homologue Cpr3p Mediates Cu-induced Apoptosis
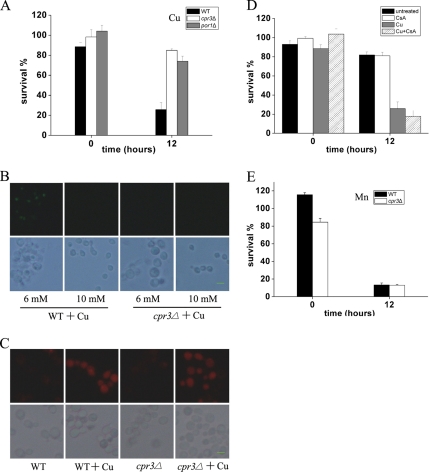
Because Cu-induced apoptosis, in contrast to that of Mn, seems to be independent of caspase, cytochrome c, or AIF pathway, we proceeded to investigate which gene(s) or pathway might mediate Cu-induced apoptosis. We performed a genetic screen to search for gene(s) that may be involved in Cu-induced cell death cascade. A yeast deletion pool was plated on Cu2+-containing plates and enriched for Cu2+-resistant clones. These clones were then individually tested for their Cu2+ resistance. Among the clones identified and characterized, most of them (8 of 11) turned out to be from a single mutant, cpr3Δ. CPR3 is a homologue of mammalian cyclophilin D, and it encodes for the mitochondrial peptidyl-prolyl cis-trans isomerase. It has been shown before that cyclophilin D and the mitochondrial permeability transition are required for mediating Ca2+- and oxidative damage-induced cell death (Halestrap, 2005). As shown in Figure 6A, cpr3Δ displayed increased resistance to Cu-induced cell death compared with the wild-type control. Furthermore, TUNEL-positive cells could not be detected in cpr3Δ mutant (Figure 6B) under Cu2+ stress, indicating deletion of CPR3 may abolish apoptosis induced by Cu2+. To analyze whether Cpr3p mediates ROS generation by Cu or not, we examined the ROS production in cpr3Δ cells, and we found ROS production was largely intact (Figure 6C). That resistance of cpr3Δ cells to Cu was comparable with that of petite cells suggests necrosis was unaffected in cpr3Δ cells. Finally, we checked CPR3 involvement in Mn-induced apoptosis. Deletion of CPR3 was not able to promote cell survival after Mn2+ treatment (Figure 6E). This means CPR3 probably does not participate in the process of Mn-mediated apoptotic program.
Figure 6.
cpr3Δ mediates Cu- but not Mn-induced apoptosis. (A) cpr3Δ and por1Δ mutant cells are more resistant to Cu2+ treatment. Survival was determined by cfu. Cells were incubated with 6 mM Cu2+ for 12 h. Vertical error bars represent standard deviations of the means of three independent experiments. (B) cpr3Δ cells display little TUNEL staining after 6 or 10 mM Cu2+ treatment. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. (C) ROS generation by Cu seem not to be affected in cpr3Δ cells. ROS as shown was detected by dihydroethidium after 12 h of 6 mM Cu2+ treatment. Bottom, phase contrast microscopy; top, fluorescence microscopy of the same cells. Bar, 10 μm. (D) Cyclosporine A does not significantly alleviate Cu toxicity to yeast cells. Cyclosporine A (20 μg/ml) was added to WT (BY4742) cell cultures with or without 6 mM Cu2+ and incubated for 12 h. Survival was determined by cfu. Vertical error bars represent standard deviations of the means of three independent experiments. (E) cpr3Δ mutant cells are not resistant to Mn2+ treatment. Survival was determined by cfu. Cells were incubated with 8 mM Mn2+ for 12 h. Vertical error bars represent standard deviations of the means of three independent experiments.
Another component involved in the formation of mitochondrial permeability transition pore (mPTP) complex is yeast POR1 (the voltage-dependent anion channel [VDAC]). To test whether POR1 is involved in Cu-induced cell death, we measured survival of por1Δ cells exposed to Cu2+. The result in Figure 6A showed por1Δ cells exhibited significantly reduced Cu-induced cell death, supporting the key role of mPTP played in Cu-induced apoptosis. Cyclosporine A is known to block mitochondrial permeability transition pore opening by binding to cyclophilin D in mammalian cells. However, the addition of cyclosporine A did not alleviate Cu2+ toxicity (Figure 6D). We explain this by that yeast mitochondria may not be sensitive to the action of cyclosporine A, as reported in the work by Jung et al. (1997).
DISCUSSION
Unlike that of other environmental toxins, such as H2O2 or acetic acid, effects of Cu2+ and Mn2+ on cells are highly dosage sensitive. Within a relatively wide range harmful toxins such as H2O2 may induce various amounts of apoptosis. For example, according to Madeo et al. (1999), 0.3–15 mM H2O2 can induce various amounts of apoptosis and inhibit cellular growth to different extents. But for Cu2+, 2 mM does not induce apoptosis, and it does not even significantly inhibit yeast growth. In fact, we often observed that 1–3 mM Cu2+ might be beneficial for cell growth, because yeast with this subtoxic level of Cu2+ grow slightly better than without Cu2+ after 24 h of incubation, although the dead/living cell ratio is increased. At 6 mM Cu2+, extensive apoptosis occurs, resulting poor cell growth, whereas at 10 mM apoptosis is largely replaced by necrosis. This level of dosage sensitivity may reflect the Janus-faced nature of metal ions, and it differs them from other environmental toxins. It also implies that an extremely intricate system is involved in metal utilization and response, of which disruption can have dire consequences.
Cu- and Mn-induced apoptosis is mitochondria dependent, as demonstrated by increased resistance toward Cu2+ or Mn2+ in mitochondria-defective strains. However, petite or ETC mutant strains are still vulnerable to the action of metal ions, albeit higher levels of metal ions are needed. We found that 10 mM Cu2+ or 12 mM Mn2+ can dramatically inhibit petite yeast. This level is consistent with what we observed for necrosis to occur. Thus, Cu and Mn kill normal yeast through apoptosis or necrosis, depending on the dosage, but in mitochondria-defective yeast strains, cells can only adopt the necrotic pathway to die.
ROS play a central role in inducing apoptotic markers and mediating cell death in yeast (Madeo et al., 1999; Laun et al., 2001; Ludovico et al., 2001; Mazzoni et al., 2003; Weinberger et al., 2003). Transition metal Cu is one of the most potent elements catalyzing Fenton's reaction. ROS such as the superoxide anion radical (O2⨪) are generated by all aerobic cells as byproducts of a number of metabolic reactions or in response to various stimuli. And mitochondria ETC is though to be a major site of ROS production according to an endogenous and continuous physiological process under aerobic conditions. Much of the O2⨪ generated by mitochondria is thought to be from electron leakage in the components of the mitochondrial electron transport chain. It is thus not surprising that ETC and ROS play important roles in Cu-induced apoptosis. Our results, however, indicate that Mn2+ induces apoptosis without significant ROS production. In the absence of ROS generation, it is somewhat difficult to understand why ETC is involved in Mn-induced apoptotic program in yeast. Previously, Ludovico et al. (2002) found that mitochondrial respiration is essential for S. cerevisiae to undergo a programmed cell death induced by acetic acid. Functional electron transport was also reported to be required for oxygen deprivation-induced cell death by HT1080 human fibrosarcoma cells (McClintock et al., 2002). Therefore, it is possible that Mn2+ needs a functional ETC to execute its PCD, whereas Cu2+ needs a functional ETC to generate ROS and possibly needs it to execute its PCD as well. From this perspective, ETC may play a dual role in the process of Cu-induced apoptosis.
Although overexpression of SOD2 in yeast leads to significantly increased resistance to Cu-induced cell death compared with the vector control, it is not known why ascorbate and vitamin E, two antioxidants that can protect against oxidative damage, are ineffective against the damaging effects of Cu2+. One hypothetical explanation is that vitamin E, like ascorbate, can catalyze the reduction of Cu2+ to Cu+ (Yoshida et al., 1994; Albertini and Abuja, 1999), and the rapid reaction of Cu+ with basal levels of H2O2 produced by mitochondria could lead to the generation of more, instead of less, reactive radicals.
YCA1 gene in S. cerevisiae encodes a metacaspase that is involved in yeast apoptosis in response to different stimuli (Madeo et al., 2002; Herker et al., 2004; Wadskog et al., 2004; Wissing et al., 2004). The present study showed that metacaspase Yca1p deficiency strain yca1Δ has increased cell survival compared with wild-type strain in the presence of Mn2+. Moreover, during the entire time course yca1Δ mutant manifested better cell survival than that of wild type. These results demonstrate that yeast metacaspase Yca1p plays an important role in Mn-induced yeast apoptosis. On the contrary, loss of metacaspase Yca1p activity did not promote cell survival in the presence of Cu2+, suggesting that yeast metacaspase Yca1p is not involved in the PCD process induced by Cu2+. Hauptmann et al. (2006) also reported that defects in N-glycosylation-induced apoptosis is independent of metacaspase Yca1p. Both these findings indicate the existence of a metacaspase Yca1p-independent apoptotic pathway in yeast.
Our genetic screen identified Cpr3p, a yeast homologue of cyclophilin D (Dolinski et al., 1997), to be involved in Cu-induced apoptosis. Cyclophilin D, a prolyl isomerase located within the mitochondrial matrix, together with adenine nucleotide translocator, a VDAC, consist the mPTP. Mitochondrial permeability transition is associated with mitochondrial swelling, outer membrane rupture, and the release of apoptotic mediators (Halestrap, 2005). Previously, cyclophilin D has been implicated in both necrosis and apoptosis programs (Halestrap, 2005; Schneider, 2005). In our study, cpr3Δ mutant was resistant to 6 mM Cu-induced cell death but still vulnerable to 10 mM Cu, and it died without appearance of DNA fragmentation, exactly as petite strain behaves. Therefore our investigation indicated that Cpr3p mediates apoptosis process induced by Cu2+ in yeast. This is consistent with the observation that ROS is associated with Cu-induced apoptosis, because it has been shown that mPTP can mediate ROS apoptotic activity (Baines et al., 2005).
Cu-induced apoptosis has been reported in mammalian epithelial breast cancer MCF7 cells (Ostrakhovitch and Cherian, 2005) and p53 seems to mediate the ROS generation. Cu-induced apoptosis in MCF7 cells is associated with AIF release and its translocation into the nucleus. However, there is no p53 homologue in yeast, and aif1Δ mutant does not promote survival of yeast cells exposed to Cu2+, implying yeast cells adopt different apoptotic program under Cu2+ stress. In our study, ETC may play a role in the ROS generation of Cu-induced yeast apoptosis, and cyclophilin D homologue Cpr3p, perhaps as well as the other components of mPTP, mediates Cu-induced apoptosis. A similar kind of apoptotic program may be used by cadmium-treated mammalian cells. Shih et al. (2004) found that heavy metal cadmium, an environmental toxin, can induce apoptosis in MRC-5 fibroblasts, and this process is independent of caspase. In that study, mitochondrial ETC and mPTP seem to be early targets of Cd, which in turn causes the mitochondrial ROS to leak out, eventually leading cells to apoptosis. Similar to our findings, Mn2+ has also been reported to induce apoptosis in mammalian cells, such as PC12 and human B cells (Schrantz et al., 1999; Hirata, 2002), and activation of caspase family proteases is required for the apoptotic process. Mn-induced apoptosis in HeLa cells was shown to be accompanied by production of ROS (Oubrahim et al., 2001). Our results indicated that in addition to caspase activation, mitochondria also play a role in Mn-induced apoptosis. But we did not detect significant ROS production in yeast cells exposed to Mn2+.
Yeast has evolved sophisticated mechanisms to deal with extreme metal deficiency and excess due to its highly variable living environments in the wild. As a whole, our results demonstrated that two essential nutritional elements, Cu and Mn, induce yeast apoptosis in an extremely dosage-sensitive way via different pathways. ROS play different roles in these two processes, which are generated greatly in Cu-induced cell death but not in Mn-induced cell death. Furthermore, overexpression of SOD2 can increase Cu resistance in wild-type yeast cells. Yeast caspase and cytochrome c seem not to be involved in Cu-induced process, but they play an important role in Mn-induced process. Further evidence is provided that Cpr3p is central to the programmed cell death process induced by Cu ion in S. cerevisiae. Our finding about the different roles of mitochondrial ETC played in Cu- and Mn-induced apoptosis may provide interesting insight into the mitochondrial involvement in the process of different apoptotic programs. We hope this study would help further understanding of the death mechanisms, and the unique aspects of them, as used by the cell to deal with possible toxic effects impacted by nutritional metal ions.
ACKNOWLEDGMENTS
This research was supported by National Basic Research Program of China grant 2005CB522503 and by National Science Foundation of China grants 30528004, 30470973, and 30330340.
Abbreviations used:
- DHR123
dihydrorhodamine 123
- ETC
electron transport chain
- mPTP
mitochondrial permeability transition pore
- PCD
programmed cell death
- ROS
reactive oxygen species.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0431) on September 19, 2007.
REFERENCES
- Albertini R., Abuja P. M. Prooxidant and antioxidant properties of Trolox C, analogue of vitamin E, in oxidation of low-density lipoprotein. Free Radic. Res. 1999;30:181–188. doi: 10.1080/10715769900300201. [DOI] [PubMed] [Google Scholar]
- Baines C. P., et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Balzan R., Sapienza K., Galea D. R., Vassallo N., Frey H., Bannister W. H. Aspirin commits yeast cells to apoptosis depending on carbon source. Microbiology. 2004;150:109–115. doi: 10.1099/mic.0.26578-0. [DOI] [PubMed] [Google Scholar]
- Beers E. P., McDowell J. M. Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr. Opin. Plant Biol. 2001;4:561–567. doi: 10.1016/s1369-5266(00)00216-8. [DOI] [PubMed] [Google Scholar]
- Chelly J., Tumer Z., Tonnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A. P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- Clifford J., Chiba H., Sobieszczuk D., Metzger D., Chambon P. RXRalpha-null F9 embryonal carcinoma cells are resistant to the differentiation, anti-proliferative and apoptotic effects of retinoids. EMBO J. 1996;15:4142–4155. [PMC free article] [PubMed] [Google Scholar]
- Cregan S. P., et al. Apoptosis-inducing factor is involved in the regulation of caspase-independent neuronal cell death. J. Cell Biol. 2002;158:507–517. doi: 10.1083/jcb.200202130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Carratore R., Della Croce C., Simili M., Taccini E., Scavuzzo M., Sbrana S. Cell cycle and morphological alterations as indicative of apoptosis promoted by UV irradiation in S. cerevisiae. Mutat. Res. 2002;513:183–191. doi: 10.1016/s1383-5718(01)00310-2. [DOI] [PubMed] [Google Scholar]
- Dolinski K., Muir S., Cardenas M., Heitman J. All cyclophilins and FK506 binding proteins are, individually and collectively, dispensable for viability in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1997;94:13093–13098. doi: 10.1073/pnas.94.24.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrog B., Sauder U., Aebi U. The S. cerevisiae HtrA-like protein Nma111p is a nuclear serine protease that mediates yeast apoptosis. J. Cell Sci. 2004;117:115–126. doi: 10.1242/jcs.00848. [DOI] [PubMed] [Google Scholar]
- Fannjiang Y., Cheng W. C., Lee S. J., Qi B., Pevsner J., McCaffery J. M., Hill R. B., Basanez G., Hardwick J. M. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot D., Levine A., Dor-Hefetz E. Sugar-induced apoptosis in yeast cells. FEMS Yeast Res. 2003;4:7–13. doi: 10.1016/S1567-1356(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- Hauptmann P., Riel C., Kunz-Schughart L. A., Frohlich K. U., Madeo F., Lehle L. Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 2006;59:765–778. doi: 10.1111/j.1365-2958.2005.04981.x. [DOI] [PubMed] [Google Scholar]
- Herker E., Jungwirth H., Lehmann K. A., Maldener C., Frohlich K. U., Wissing S., Buttner S., Fehr M., Sigrist S., Madeo F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y. Manganese-induced apoptosis in PC12 cells. Neurotoxicol. Teratol. 2002;24:639–653. doi: 10.1016/s0892-0362(02)00215-5. [DOI] [PubMed] [Google Scholar]
- Huh G. H., Damsz B., Matsumoto T. K., Reddy M. P., Rus A. M., Ibeas J. I., Narasimhan M. L., Bressan R. A., Hasegawa P. M. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002;29:649–659. doi: 10.1046/j.0960-7412.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- Jung D. W., Bradshaw P. C., Pfeiffer D. R. Properties of a cyclosporin-insensitive permeability transition pore in yeast mitochondria. J. Biol. Chem. 1997;272:21104–21112. doi: 10.1074/jbc.272.34.21104. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Manganese: a high-octane dispute. Science. 2003;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y. M., Zhou B., Cosco D., Gitschier J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA. 2001;98:6836–6841. doi: 10.1073/pnas.111057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun P., Pichova A., Madeo F., Fuchs J., Ellinger A., Kohlwein S., Dawes I., Frohlich K. U., Breitenbach M. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 2001;39:1166–1173. [PubMed] [Google Scholar]
- Lee J., Prohaska J. R., Thiele D. J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA. 2001;98:6842–6847. doi: 10.1073/pnas.111058698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Sun L., Liang Q., Wang J., Mo W., Zhou B. Yeast AMID homologue Ndi1p displays respiration-restricted apoptotic activity and is involved in chronological aging. Mol. Biol. Cell. 2006;17:1802–1811. doi: 10.1091/mbc.E05-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico P., Rodrigues F., Almeida A., Silva M. T., Barrientos A., Corte-Real M. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol. Biol. Cell. 2002;13:2598–2606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludovico P., Sousa M. J., Silva M. T., Leao C., Corte-Real M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology. 2001;147:2409–2415. doi: 10.1099/00221287-147-9-2409. [DOI] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Frohlich K. U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Ligr M., Grey M., Sigrist S. J., Wolf D. H., Frohlich K. U., et al. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., et al. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 2002;9:911–917. doi: 10.1016/s1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni C., Mancini P., Verdone L., Madeo F., Serafini A., Herker E., Falcone C. A truncated form of KlLsm4p and the absence of factors involved in mRNA decapping trigger apoptosis in yeast. Mol. Biol. Cell. 2003;14:721–729. doi: 10.1091/mbc.E02-05-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D. S., Santore M. T., Lee V. Y., Brunelle J., Budinger G. R., Zong W. X., Thompson C. B., Hay N., Chandel N. S. Bcl-2 family members and functional electron transport chain regulate oxygen deprivation-induced cell death. Mol. Cell Biol. 2002;22:94–104. doi: 10.1128/MCB.22.1.94-104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., et al. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Farschon D. M., Reed J. C. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell. 1994;79:353–364. doi: 10.1016/0092-8674(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch E. A., Cherian M. G. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–121. doi: 10.1007/s10495-005-6066-7. [DOI] [PubMed] [Google Scholar]
- Oubrahim H., Stadtman E. R., Chock P. B. Mitochondria play no roles in Mn(II)-induced apoptosis in HeLa cells. Proc. Natl. Acad. Sci. USA. 2001;98:9505–9510. doi: 10.1073/pnas.181319898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal P. K., Samii A., Calne D. B. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Pozniakovsky A. I., Knorre D. A., Markova O. V., Hyman A. A., Skulachev V. P., Severin F. F. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J. Cell Biol. 2005;168:257–269. doi: 10.1083/jcb.200408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter J., Herker E., Madeo F., Schmitt M. J. Viral killer toxins induce caspase-mediated apoptosis in yeast. J. Cell Biol. 2005;168:353–358. doi: 10.1083/jcb.200408071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. D. Cyclophilin D: knocking on death's door. Sci STKE. 2005;2005 doi: 10.1126/stke.2872005pe26. pe26. [DOI] [PubMed] [Google Scholar]
- Schrantz N., Blanchard D. A., Mitenne F., Auffredou M. T., Vazquez A., Leca G. Manganese induces apoptosis of human B cells: caspase-dependent cell death blocked by bcl-2. Cell Death Differ. 1999;6:445–453. doi: 10.1038/sj.cdd.4400508. [DOI] [PubMed] [Google Scholar]
- Severin F. F., Hyman A. A. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 2002;12:R233–R235. doi: 10.1016/s0960-9822(02)00776-5. [DOI] [PubMed] [Google Scholar]
- Shih C. M., Ko W. C., Wu J. S., Wei Y. H., Wang L. F., Chang E. E., Lo T. Y., Cheng H. H., Chen C. T. Mediating of caspase-independent apoptosis by cadmium through the mitochondria-ROS pathway in MRC-5 fibroblasts. J. Cell Biochem. 2004;91:384–397. doi: 10.1002/jcb.10761. [DOI] [PubMed] [Google Scholar]
- Silva R. D., Sotoca R., Johansson B., Ludovico P., Sansonetty F., Silva M. T., Peinado J. M., Corte-Real M. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol. Microbiol. 2005;58:824–834. doi: 10.1111/j.1365-2958.2005.04868.x. [DOI] [PubMed] [Google Scholar]
- Skulachev V. P. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol. Aspects Med. 1999;20:139–184. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Streiblova E. Cytological methods. In: Campbell J., Buffers J. M., editors. Yeast–A Practical Approach. Oxford, United Kingdom: IRL Press; 1988. pp. 9–49. [Google Scholar]
- Susin S. A., et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Korsmeyer S. J. Cell death in development. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Wadskog I., Maldener C., Proksch A., Madeo F., Adler L. Yeast lacking the SRO7/SOP1-encoded tumor suppressor homologue show increased susceptibility to apoptosis-like cell death on exposure to NaCl stress. Mol. Biol. Cell. 2004;15:1436–1444. doi: 10.1091/mbc.E03-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner D. J., Bartnikas T. B., Gitlin J. D. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- Weinberger M., Ramachandran L., Burhans W. C. Apoptosis in yeasts. IUBMB Life. 2003;55:467–472. doi: 10.1080/15216540310001612336. [DOI] [PubMed] [Google Scholar]
- Wissing S., et al. An AIF orthologue regulates apoptosis in yeast. J. Cell Biol. 2004;166:969–974. doi: 10.1083/jcb.200404138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Xu L. G., Li X., Zhai Z., Shu H. B. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem. 2002;277:25617–25623. doi: 10.1074/jbc.M202285200. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Tsuchiya J., Niki E. Interaction of alpha-tocopherol with copper and its effect on lipid peroxidation. Biochim. Biophys. Acta. 1994;1200:85–92. doi: 10.1016/0304-4165(94)90121-x. [DOI] [PubMed] [Google Scholar]