Abstract
Tau is a microtubule binding protein implicated in a number of human neurodegenerative disorders, including Alzheimer's disease. Phosphorylation of serine-proline/threonine-proline sites, targeted by proline-directed kinases, coincides temporally with neurodegeneration in the human diseases. Recently, we demonstrated that this unique group of serines and threonines has a critical role in controlling tau toxicity in a Drosophila model of tauopathy. Here, we use a combination of genetic and biochemical approaches to examine these sites individually and to determine which of them is primarily responsible for controlling tau neurotoxicity. Despite the importance placed on individual phosphoepitopes and their contributions to disease pathogenesis, our results indicate that no single phosphorylation residue plays a dominant role in controlling tau toxicity. These findings suggest that serine-proline/threonine-proline sites cooperate to mediate neurodegeneration in vivo.
INTRODUCTION
Phosphorylation of the microtubule-associated protein tau is elevated in neurodegenerative disease tissue, and tau-positive neurofibrillary pathology is characteristic of several disorders, including Alzheimer's disease and other tauopathies (Feany and Dickson, 1996; Lee et al., 2001). This abnormal hyperphosphorylation of tau induces an increase in the apparent molecular mass of the protein, which is observed in disease states (Lee et al., 1991). Proline-directed kinases phosphorylate tau at serine-proline (SP) or threonine-proline (TP) motifs, many of which reside in the proline-rich regions flanking the microtubule binding domains of human tau. These phosphorylation events have been linked particularly to tau-induced neurodegeneration because a group of antibodies that specifically react to phosphorylated SP and TP sites (pSP/pTP) recognize tau found in the brain tissue of Alzheimer's disease patients but not autopsy-derived tau from nondemented age-matched individuals. This group of monoclonal antibodies recognizes single or two closely spaced phosphorylated SP/TP sites, including pT181 (AT270) (Goedert et al., 1994), pS202/pT205 (AT8) (Goedert et al., 1995), pT212/pS214 (AT100) (Zheng-Fischhofer et al., 1998), pT231/pS235 (TG3) (Jicha et al., 1997a), and pS396/pS404 (PHF1) (Otvos et al., 1994). Phosphorylation at SP/TP sites is a striking feature of tau from patients with Alzheimer's disease and other tauopathies; consequently, these sites are commonly referred to as “disease-associated” sites. Aside from their development as specific diagnostic markers of tauopathies (for review, see Gong et al., 2005), monoclonal antibodies that recognize particular phosphoepitopes of tau have also been used to study the correlation between phosphorylation and disease severity (Augustinack et al., 2002) and to delineate the hierarchical pattern of tau phosphorylation (Zheng-Fischhofer et al., 1998; Shea and Cressman, 1999).
To understand how phosphorylation contributes to tau-induced neurodegeneration, many laboratories have focused on the kinases and phosphatases that regulate tau phosphorylation. Adopting a different strategy, we used a Drosophila tauopathy model (Wittmann et al., 2001) to investigate the direct targets of these enzymes, namely, the SP and TP sites of tau, and we found that they are critical regulators of toxicity in vivo. We showed that blocking phosphorylation of all 14 SP/TP sites in tau by mutating them to alanine markedly inhibited tau-induced neurodegeneration in Drosophila (Fulga et al., 2007; Steinhilb et al., 2007). Conversely, mutation of all SP/TP sites to glutamate generated the pseudophosphorylated tauE14 construct, which is significantly more toxic than tauWT (Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007).
In this report, we used our Drosophila tauopathy model to investigate how phosphorylation of individual SP/TP sites impacts tau-induced neurotoxicity. We generated phosphorylation-incompetent forms of tau in which single phosphorylation sites, or a pair of sites, were replaced by alanines. Our results revealed that although SP/TP sites are critical mediators of neurotoxicity, there is no single site whose phosphorylation is essential for neurodegeneration. These findings suggest that SP/TP sites function together to promote neurotoxicity in vivo.
MATERIALS AND METHODS
Constructs, Genetics, and Stocks
The UAS-tauWT and UAS-tauAP transgenic Drosophila lines have been described previously (Wittmann et al., 2001; Steinhilb et al., 2007). Vector pBS-htau24 (Wittmann et al., 2001) served as a template for the generation of alanine mutations at the 14 Ser/Thr-prosites of human tau by using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The codons for T111, T153, T175, T181, S199, S202, T205, T212, S214, T217, T231, S235, S396, S404, and S422 were mutated to alanine individually or in pairs, and the resulting constructs were subcloned into the pUAST vector by using the restriction enzymes KpnI and NotI. The authenticity of all constructs was confirmed by sequencing, and lines with expression level equivalent to tauWT were determined by quantitative Western blot analysis. The following transgenic strains are described in Flybase (http://flybase.bio.indiana.edu/), and were obtained from the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN) (elav-GAL4, UAS-p35), N. Dyson (Massachusetts General Hospital) (UAS-Rbf1), I. K. Hariharan (Massachusetts General Hospital) (UAS-dap), C. Lehner (University of Bayreuth) (UAS-CycE), and T. Xu (Yale University) (UAS-Tsc2ΔAKT). Crosses were maintained on standard cornmeal-based Drosophila medium at 25°C. Flies were analyzed at 1 to 3 d of age.
Western Blot
Heads from adult flies at 1 d posteclosion were homogenized in Laemmli buffer (Sigma-Aldrich, St. Louis, MO), boiled for 10 min at 100°C, and centrifuged at 13,000 × g for 2 min to remove insoluble debris. Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) by using a 10% separating gel (Cambrex, East Rutherford, NJ), transferred to nitrocellulose (Bio-Rad, Hercules, CA), blocked in 5% milk in phosphate-buffered saline with 0.1% Tween 20, and immunoblotted using one of the following antibodies: polyclonal anti-C terminal tau (Dako North America, Carpinteria, CA) at 1:106 dilution; AT8 (Innogenetics, Zwÿnderecht, Antwerp, Belgium) at 1:1000 dilution; Tau1 (Chemicon International, Temecula, CA) at 1:10,000 dilution; TG3 (P. Davies, Bronx, NY) at 1:100 dilution; PHF1 (P. Davies) at 1:500 dilution; pSer212 (BioSource International. Camarillo, CA) at 1:40,000; pSer214 (BioSource International) at 1:40,000; AT270 (Pierce-Endogen, Rockford, IL) at 1:100,000 dilution; and AT100 (Innogenetics) at 1:1000 dilution. For each blot, the same number of fly heads was used for each genotype, Ponceau S stains were performed to ensure equivalent total protein loading and even transfer, and blots were reprobed for total tau (Dako North America). Western blots were repeated a minimum of three times with different animals, and representative blots are shown. The appropriate anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibody was applied and signals were detected by chemiluminescence (Pierce-Endogen).
Histology
Heads from adult flies at 1 to 3 d posteclosion were fixed in Formalin, embedded in paraffin, and 4-μm frontal sections were prepared. Serial sections were cut through the entire brain and placed on a single glass slide, and immunostaining was performed as described previously (Khurana et al., 2006). MC1 and Alz50 were used at dilutions of 1:1, and they were detected by AlexaFluor 488-coupled secondary antibodies.
Phosphatase Treatment
Drosophila head homogenates were prepared in 1× lambda phosphatase buffer (New England Biolabs) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and 200 μg of total protein were incubated with 800 U of lambda protein phosphatase for 3 h at 37°C. Homogenates were then subjected to SDS-PAGE and immunoblot analysis as described above.
RESULTS
Generation of Individual SP/TP Transgenic Tau Mutants
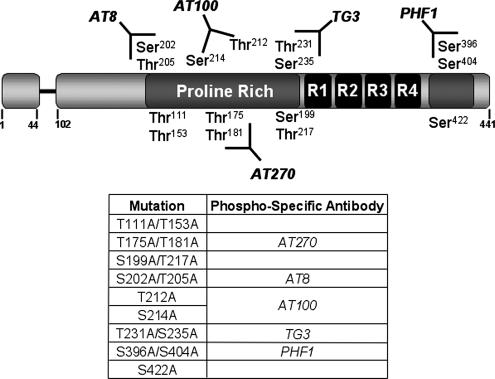
To determine the relative contribution of individual SP/TP phosphorylation sites to tau toxicity, we generated eight transgenic Drosophila lines expressing mutant forms of human tau in which the following serine and/or threonine residues were replaced by alanine: tau111/153 (T111A, T153A); tau175/181 (T175A, T181A); tau199/217 (S199A, T217A); tau202/205 (S202A, T205A); tau212 (T212A); tau231/235 (T231A, S235A); tau396/404 (S396A, S404A); and tau422 (S422A) (Figure 1). Many of these sites, when phosphorylated, are recognized by a group of monoclonal antibodies that preferentially react with tau found in patients with neurodegenerative disorders. These antibodies include AT8, AT100, AT270, TG3, and PHF1, and the phosphoepitopes recognized by each of them are schematized in Figure 1. Although not an SP site, we also generated tau214 (S214A), because Thr212 and Ser214 create the phosphoepitope recognized by the AT100 antibody, one of the most specific correlates of toxicity in human disease (Zheng-Fischhofer et al., 1998).
Figure 1.
Phosphorylation-incompetent tau mutants. Schematic representation of human microtubule-associated protein tau and the point mutations used in this study. The serine (S) and threonine (T) residues that have been replaced by alanine (A) are located in two proline-rich regions flanking the four microtubule binding domain repeats (R1–R4), and include T111A, T153A, T175A, T181A, S199A, S202A, T205A, T212A, S214A, T217A, T231A, S235A, S396A, S404A, and S422A. The phosphorylation-specific antibodies AT270 (recognizes pT175/pT181), AT8 (pS202/pT205), AT100 (pT212/pS214), TG3 (pT231/pS235), and PHF1 (pS396/pS404) are indicated in the schematic and in the summary panel below.
The Toxicity of Individual SP/TP Tau Mutants Is Similar to That of TauWT
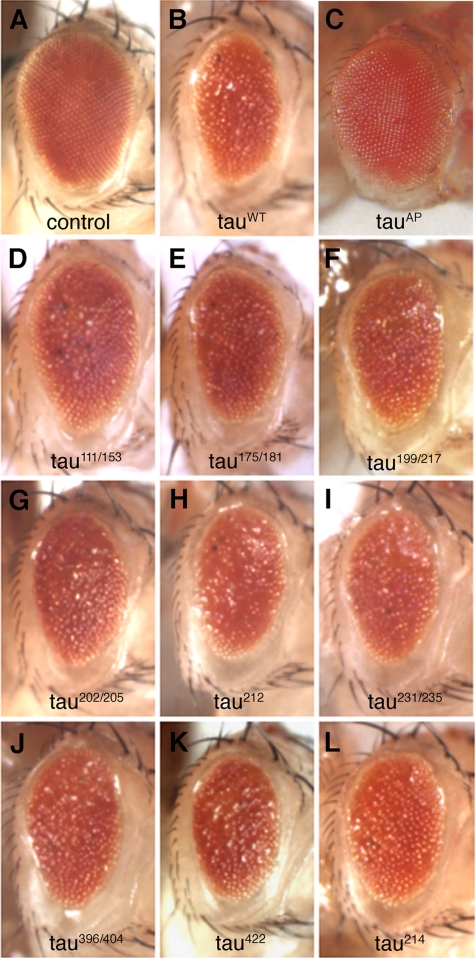
To test whether abolishing phosphorylation at individual sites would affect tau-induced neurotoxicity, wild-type and mutant forms of human tau were expressed in the Drosophila retina under the control of the GMR-GAL4 driver (Figure 2). In contrast to the normal fly eye (Figure 2A), expression of wild-type human tau (tauWT) in the retina results in toxicity that can be visualized as a moderately rough eye phenotype caused by disruption of the ordered arrangement of ommatidia (Shulman and Feany, 2003; Figure 2B). We have recently reported that expression of a phosphorylation-incompetent tau (tauAP), in which 14 disease-associated SP/TP sites have been replaced by alanine, has a marked effect on toxicity, almost completely blocking tau-induced toxicity in the eye (Fulga et al., 2007; Steinhilb et al., 2007; Figure 2C). In contrast, expression of equivalent levels of each of the individual point mutants in the retina resulted in a moderately rough eye (Figure 2, D–L) similar to that induced by tauWT (Figure 2B). These results indicate that blocking phosphorylation of individual SP/TP sites has no detectable effect on tau-induced toxicity in vivo. Because the phosphorylation state of tau depends on the cellular context within the nervous system (Grammenoudi et al., 2006), it is possible that individual site contribute differently to toxicity in other types of neurons. Unfortunately, the modest toxicity of wild-type human tau (Wittmann et al., 2001) limited our ability to assess the toxicity of our mutants in the brain.
Figure 2.
Mutation of individual SP/TP sites does not significantly alter tau toxicity in vivo. Compared with the organized structure of a normal eye (A), expression of tauWT in the eye (under the control of the GMR-GAL4 driver) induces retinal degeneration and a rough eye phenotype (B). TauAP, in which all 14 SP/TP sites have been mutated to alanine has no significant toxicity when expressed at levels similar to tauWT (C). By contrast, a rough eye phenotype indistinguishable from that induced by tauWT is obtained upon retinal expression of each of the following tau mutants: tau111/153 (D; T111A, T153A), tau175/181 (E; T175A, T181A), tau199/217 (F; S199A, T217A), tau202/205 (G; S202A, T205A), tau212 (H; T212A), tau231/235 (I; T231A, S235A), tau396/404 (J; S396A, S404A), tau422 (K; S422A), and tau214 (L; S214A). Control is GMR-GAL4/+.
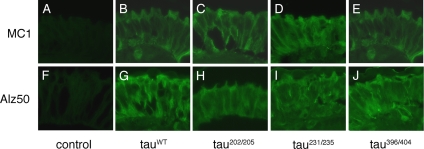
To examine whether single site phosphorylation affects the appearance of disease-specific conformations of tau, we used the antibodies MC1 and Alz50, which recognize abnormal conformations of tau associated with Alzheimer's disease and related disorders (Hyman et al., 1988; Carmel et al., 1996; Vincent et al., 1996). Immunostaining of retinal sections with MC1 yielded no signal for nontransgenic control flies (Figure 3A), but it detected equivalent levels of immunoreactivity in tauWT(3B), and flies expressing the individual points mutants. Results from tau202/205 (3C), tau231/235 (3D), and tau396/404 (3E) are shown as representative examples. We obtained similar results using the conformation-specific antibody Alz50 (Figure 3, F–J). These data suggest that blocking phosphorylation at individual sites does not preclude tau from adopting disease-associated conformations. This finding contrasts with what we observed with the phosphorylation-incompetent mutant tauAP, in which mutation of all disease-associated SP/TP sites significantly reduces the propensity of tau to assume disease-specific conformations (Steinhilb et al., 2007). Thus, although SP/TP phosphorylation of tau is required for the generation of abnormal conformations and for neurotoxicity (Steinhilb et al., 2007), there is no single phosphorylation site, or pair of sites, that accounts for these disease-related features.
Figure 3.
Individual SP/TP tau mutants adopt disease-specific conformations. Blocking phosphorylation at individual SP/TP sites does not prevent tau from adopting the abnormal conformations characteristic of neurodegenerative conditions. Retinal sections were immunostained with the conformation-specific antibodies MC1 (A–E) and Alz50 (F–J), which recognize anomalous conformations of tau found in disease states. Although there is no immunoreactivity associated with control flies (A and F; GMR-GAL4/+), equivalent levels of immunostaining were detected in transgenic flies expressing tauWT (B and G) and three representative mutants tau202/205 (C and H), tau231/235 (D and I), and tau396/404 (E and J).
Neurotoxicity Induced by SP/TP Tau Mutants Is Mediated by TOR Signaling and Cell Cycle Activation
Our model provides a relatively rapid and flexible system in which to investigate the mechanisms of tau-induced neurodegeneration, and our recent findings suggest that expression of tau in Drosophila induces the sequential activation of the target of rapamycin (TOR) signaling pathway and of the cell cycle, leading to neurodegeneration (Khurana et al., 2006). Because cell cycle activation depends upon tau phosphorylation (Khurana et al., 2006), we tested whether the mutation of single phosphoepitopes would affect the ability of TOR signaling, the cell cycle or of apoptotic pathways to mediate tau neurotoxicity.
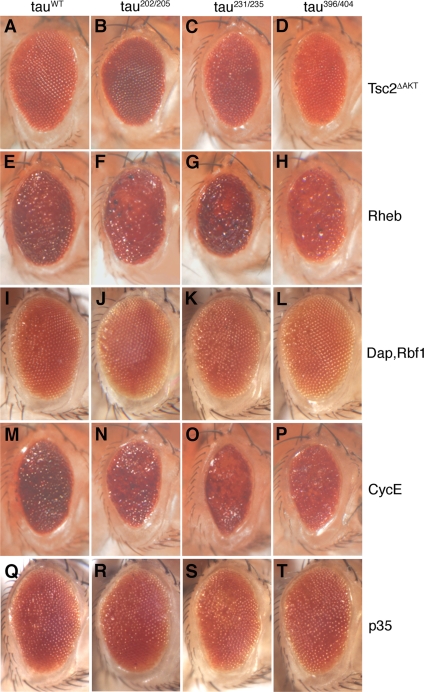
Blocking TOR signaling by coexpression of a constitutively active form of the TOR inhibitor Tsc2 (Tsc2ΔAKT) rescued tau induced toxicity in our model (Khurana et al., 2006) and suppressed to similar extents the rough eye phenotypes of tauWT (Figure 4A), tau202/205 (Figure 4B), tau231/235 (Figure 4C), and tau396/404 (Figure 4D) transgenic flies. In contrast, overexpression of Rheb, a positive modulator of the TOR pathway, enhanced tau-induced toxicity in flies expressing tauWT (Figure 4E), tau202/205 (Figure 4F), tau231/235 (Figure 4G), and tau396/404 (Figure 4H).
Figure 4.
Modulating TOR signaling, cell cycle, and apoptotic pathways modifies neurodegeneration induced by wild-type and SP/TP-mutant tau. Expression of tauWT, tau202/205, tau231/235, or tau396/404 induces similar rough eye phenotypes (Figure 2), which are affected to similar extents by manipulation of TOR signaling, the cell cycle, or apoptosis. Blocking TOR signaling (A–D), achieved by coexpression of a constitutively active form of the TOR inhibitor Tsc2 (Tsc2ΔAKT) suppressed, to equivalent degrees, the retinal degeneration of tauWT, tau202/205, tau231/235, and tau396/404 transgenic flies (A–D). Conversely, coexpression of the TOR activator Rheb enhanced to similar levels the neurotoxicity associated with expression of tauWT, tau202/205, tau231/235, or tau396/404 (E–H). Cell cycle inhibition, by coexpression of the cdk2 inhibitor Dap together with the E2F1 inhibitor Rbf1, strongly suppressed the eye toxicity induced by wild type and SP/TP mutant forms of tau (I–L). In contrast, activation of the cell cycle, by coexpression of cyclin E, enhanced tau neurodegeneration (M–P). Finally, coexpression of the apoptosis inhibitor p35 suppressed to similar degrees the rough eye phenotype of tauWT, tau202/205, tau231/235, and tau396/404 transgenic flies (Q–T). Driver is GMR-GAL4. In the absence of tau, expression of Rheb results in only minor roughness, and expression of cyclin E does not cause any significant retinal toxicity (Khurana et al., 2006).
Expressing the Cdk2 inhibitor Dacapo (Dap, Drosophila homologue of human p21/p27) together with the E2F1 inhibitor retinoblastoma factor-1 (Rbf1) synergistically blocks the G1/S transition of the cell cycle, and it suppresses tau-induced neurodegeneration (Khurana et al., 2006). Coexpression of Dap and Rbf1 substantially suppressed the tau-induced retinal toxicity of tauWT (Figure 4I) and of the individual SP/TP mutants. Results with tau202/205 (Figure 4J), tau231/235 (Figure 4K), and tau396/404 (Figure 4L) are shown as representative examples. Conversely, coexpression of cyclin E, which catalyzes the G1/S transition together with Cdk2, strongly enhanced to equivalent levels tau toxicity in transgenic flies expressing tauWT (Figure 4M), tau202/205 (Figure 4N), tau231/235 (Figure 4O), and tau396/404 (Figure 4P).
Finally, coexpression of the apoptosis inhibitor p35 in tau transgenic animals suppressed the rough eye phenotype of tauWT (Jackson et al., 2002; Figure 4Q), tau202/205 (Figure 4R), tau231/235 (Figure 4S), tau396/404 (Figure 4T) and of the other individual SP/TP mutants.
In summary, modulating TOR signaling, cell cycle activation, or apoptosis modified to similar extents the neurodegenerative phenotypes induced by wild-type and SP/TP-mutant forms of tau. Thus, although in our model cell cycle activation and neuronal death depend on tau phosphorylation (Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007; Steinhilb et al., 2007), our data indicate that blocking phosphorylation at restricted sites still induces neurotoxicity that is mediated by TOR signaling and cell cycle activation. These findings strongly suggest that individual SP/TP mutants induce neurodegeneration by the same mechanisms as wild-type tau.
Hyperphosphorylation of Tau Is Triggered by Largely Independent Events in Transgenic Flies
Several studies have used in vitro kinase assays to examine the process of tau phosphorylation, and they have suggested that the generation of disease-associated forms of tau requires a complex sequence of phosphorylation steps (Zheng-Fischhofer et al., 1998; Shea and Cressman, 1999). Expression of phosphorylation-incompetent tau mutants in Drosophila provided us with a system to address this question in vivo. If the steps leading to tau phosphorylation are temporally ordered and interdependent, we expect that inhibiting phosphorylation at a critical SP/TP site will prevent downstream phosphorylation events that require prior modification of that residue.
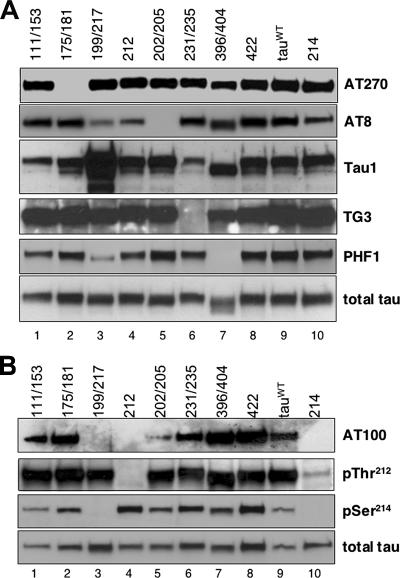
To examine the phosphorylation pattern of each tau construct, we prepared homogenates from transgenic flies expressing either tauWT or each of the individual SP/TP mutants for Western blot analysis. Nontransgenic controls showed no reactivity for any of the antibodies used (Steinhilb et al., 2007 and data not shown). As seen in Figure 5A, all extracts showed substantial reactivity with the AT270 antibody, with the exception of those derived from tau175/181-expressing flies (Figure 5A, first row, lane 2), as expected because AT270 detects phosphorylation at T175/T181 (Goedert et al., 1994). Similarly, the TG3 antibody recognized tau in all extracts with the exception of tau231/235 (Figure 5A, fourth row, lane 6), as anticipated because phosphorylation at these two sites defines the TG3 epitope (Jicha et al., 1997a). The PHF1 antibody recognizes pS396/pS404 (Otvos et al., 1994); thus, it did not react with extracts derived from tau396/404 transgenic animals (Figure 5A, fifth row, lane 7), but it identified all the other mutant forms of tau. These results suggest that blocking phosphorylation of individual SP/TP residues in vivo does not prevent phosphorylation of the remaining sites.
Figure 5.
Effect of individual SP/TP mutations on tau phosphorylation at disease-associated sites. (A) Generation of individual phosphoepitopes occurs primarily by independent phosphorylation events. Fly head extracts were subject to Western immunoblot analysis by using the following antibodies: AT270 (pT175/pT181), AT8 (pS202/pT205), Tau1 (recognizes unphosphorylated epitopes between amino acids 189 and 207), TG3 (recognizes pT231/pS235), PHF1 (PS396/pS404), and a polyclonal antibody against total tau. (B) Generation of the AT100 epitope requires phosphorylation of T212, S214, and S199/T217. Equivalent amounts of fly head extracts derived from transgenic animals expressing wild type and mutant forms of tau were analyzed by Western immunoblotting by using the following antibodies: AT100 (T212/S214), pThr212, pSer214, and a polyclonal antibody specific for total tau. Driver is GMR-GAL4.
We also examined the connection between the AT8 and Tau1 epitopes. Although the AT8 recognition site includes pS202/pT205 (Goedert et al., 1995), Tau1 preferentially recognizes unphosphorylated tau between amino acids 189 and 207, and the two antibodies have been described as complementary (Biernat et al., 1992). We anticipated that the tau202/205 mutant would not be recognized by AT8 but that it would, in turn, be highly reactive with Tau1. As predicted, tau202/205 was not recognized by AT8 (Figure 5A, second row, lane 5); however, elevated reactivity with Tau1 compared with tauWT (third row, compare lanes 5 and 9) was not seen. Instead, mutating S199 and T217 to alanine substantially increased reactivity with Tau1 (Figure 5A, third row, lane 3), suggesting that Tau1 reactivity depends more upon dephosphorylation at S199 and/or T217 than at S202 or T205. Immunoreactivity for AT8 is also partially reduced in flies unable to phosphorylate residues S199 and T217 (Supplemental Figure S1, second row, lane 3).
Collectively, the data suggest that in our Drosophila model, generation of many disease-associated phosphoepitopes does not rely on a strictly sequential or hierarchical phosphorylation cascade. Rather, many sites can be phosphorylated independently.
Generation of the AT100 Epitope in Drosophila Requires Phosphorylation of S199/T217
SP/TP phosphorylation-specific antibodies are valuable diagnostic tools, which recognize tau phosphoepitopes that are highly enriched in Alzheimer's disease brain but that are not found in autopsy samples from healthy controls. However, low levels of SP/TP phosphorylation can also be detected in normal brain, especially in biopsy and fetal tissue (Garver et al., 1994; Matsuo et al., 1994). Thus, highly specific antibodies that only react with disease-associated tau are particularly useful. The AT100 antibody is one such example. It recognizes phosphorylated tau in Alzheimer's paired helical filaments, but it does not cross-react with phosphorylated sites found in normal fetal or adult tau (Zheng-Fischhofer et al., 1998). The physical epitope of AT100 has been mapped to include two phosphorylated residues, pT212 and pS214 (Zheng-Fischhofer et al., 1998). However, in vitro and in vivo studies provide conflicting evidence about the order of phosphorylation required to create AT100. It is also unclear whether phosphorylation of other SP/TP sites influences AT100 generation in vivo.
As anticipated, brain homogenates derived from transgenic flies expressing tau212 or tau214 were not recognized by the AT100 antibody (Figure 5B, first row, lanes 4 and 10). Phospho-specific antibodies pThr212 and pSer214 recognize each individual site, and were used to investigate the relationship between these two phosphorylation events in vivo. As shown in Figure 5B, our data suggest that phosphorylation of T212 and S214 are interdependent because mutation of S214 to alanine partially inhibited phosphorylation at T212 (second row, lane 10). However, the T212A mutation did not appreciably alter phosphorylation at S214 (third row, lane 4). Interestingly, blocking phosphorylation at S199/T217 had a significant impact on AT100 immunoreactivity. As shown in Figure 5B, extracts from tau199/217-expressing flies were not recognized by AT100 (first row, lane 3). Analyses with the pThr212 and pSer214 antibodies revealed that tau199/217 is phosphorylated at T212 (Figure 5B, second row, lane 3) but not at S214 (third row, lane 3), suggesting that in vivo phosphorylation of S199 and/or T217 is essential for S214 phosphorylation and for the appearance of the highly disease-selective AT100 epitope. In addition, our results establish that, in vivo, phosphorylation of other individual SP/TP sites is not required for AT100 generation.
Blocking Phosphorylation of a Select Group of SP/TP Sites Does Not Inhibit Tau-induced Toxicity
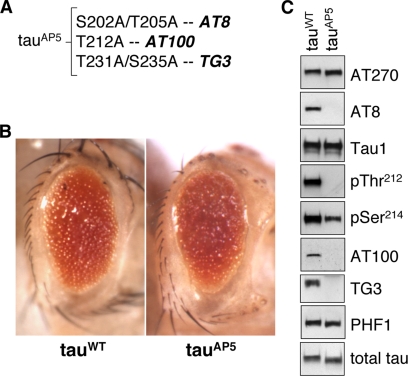
We recently showed that mutation of all 14 SP/TP sites to alanine generated the phosphorylation-incompetent tauAP construct, which has markedly reduced toxicity in Drosophila compared with tauWT (Fulga et al., 2007; Steinhilb et al., 2007). Conversely, the pseudophosphorylated construct tauE14, in which all SP/TP sites are mutated to glutamate, is much more toxic than tauWT (Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007). These observations demonstrate that SP/TP phosphorylation is critical for tau-induced neurodegeneration, but our data thus far show that neurotoxicity cannot be suppressed by blocking phosphorylation at a single site, or pair of sites (Figure 2). Based on these results, we suspected that a larger group of sites might be working together to induce toxicity. To test this hypothesis, we generated transgenic lines expressing tauAP5, a construct that has alanine mutations at three of the major disease-associated phosphoepitopes: AT8, AT100, and TG3 (Figure 6A). Selection of the mutant residues was based on published observations showing that reduced phosphorylation at AT8 and AT100 correlates with lower levels of tau-induced toxicity in Drosophila (Nishimura et al., 2004) and that the phosphorylation sites recognized by TG3 and AT100 distinguish disease-associated tau and that they are not phosphorylated in normal adult brain (Jicha et al., 1997b; Zheng-Fischhofer et al., 1998).
Figure 6.
Blocking phosphorylation at five SP/TP sites does not inhibit tau toxicity. (A) The tauAP5 mutant has the following five mutations: S202A, T205A, T212A, T231A, and S235A. This manipulation disrupts reactivity with the AT8, AT100, and TG3 antibodies. (B) TauAP5-expressing flies have the same retinal toxicity as tauWT flies. (C) Western blot analysis confirms that tauAP-expressing flies are not reactive for AT8, AT100, and TG3, whereas phosphorylation at other disease-associated sites, including T181 (AT270), S214 (pSer214), and S396/S404 (PHF1) is maintained. Reactivity with the Tau1 antibody is also observed. TauWT and tauAP5 expression levels are equivalent, as demonstrated by immunoblot by using a polyclonal total tau antibody. Driver is GMR-GAL4.
When expressed at equivalent levels in the Drosophila retina, tauAP5 (S202A, T205A, T212A, T231A, and S235A) had the same degree of retinal toxicity as tauWT (Figure 6B). Using disease-associated phospho-specific antibodies to analyze the phosphorylation status of tauAP5, we found that reactivity with AT270, PHF1, and Tau1 was unchanged compared with tauWT (Figure 6C). As expected, tauAP5 did not react with AT8, pThr212, AT100, and TG3 (Figure 6C) because the epitopes recognized by these antibodies were mutated to alanine.
Our results indicate that inhibiting phosphorylation at the five SP/TP sites recognized by AT8, AT100 and TG3 does not prevent tau-induced toxicity. Although blocking phosphorylation of the five sites we chose could not replicate the significant loss of toxicity of the tauAP mutant (Steinhilb et al., 2007), it remains possible that blocking phosphorylation at a different combination of restricted sites would be sufficient to ameliorate tau toxicity.
Phosphorylation at S396/S404 Has a Substantial Effect on the Electrophoretic Mobility of Tau
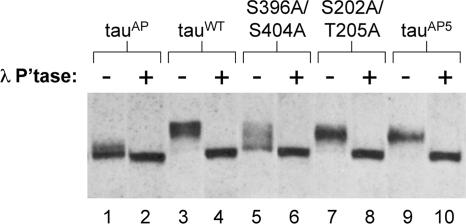
It is well established that aberrantly phosphorylated tau, characteristic of disease states, exhibits a shift toward lower mobility on SDS-PAGE (Baudier and Cole, 1987; Flament and Delacourte, 1989; Greenberg and Davies, 1990). Phosphatase treatment studies revealed that the decreased electrophoretic mobility of disease-associated tau is mainly due to phosphorylation (Lee et al., 1991; Ksiezak-Reding et al., 1992). Consistent with these reports, we found that treatment of fly brain homogenates with λ-phosphatase increased the mobility of tauWT ((Steinhilb et al., 2007) and Figure 7, compare lanes 3 and 4). Moreover, mutation of all SP/TP sites to alanine in tauAP increased its electrophoretic mobility compared with tauWT (Steinhilb et al., 2007; Figure 7, lanes 1 and 3). Phosphatase treatment increased the migration of tauAP modestly (Steinhilb et al., 2007; Figure 7, lanes 1 and 2), indicating that the small shift in molecular weight observed in tauAP before treatment is due to phosphorylation at non-SP/TP sites.
Figure 7.
Phosphorylation of S396/S404 significantly alters the electrophoretic mobility of tau. Drosophila head homogenates derived from transgenic flies expressing wild type and mutant forms of tau were incubated in the presence or absence of lambda phosphatase, followed by prolonged electrophoretic separation (to more easily detect differences in migration by SDS-PAGE) and by Western immunoblotting with a polyclonal tau antibody. Driver is GMR-GAL4.
To determine whether single SP/TP phosphorylation events substantially affect tau mobility in vivo, we tested all individual SP/TP mutants and found that tau396/404 had an increased electrophoretic mobility compared with tauWT (Figure 7, compare lanes 3 and 5). We confirmed that this difference in mobility was a consequence of phosphorylation, by treating with lambda phosphatase (lane 6). In contrast to tau396/404, the electrophoretic mobility of all other individual SP/TP point mutants was equivalent to that of tauWT, as can be appreciated for tau202/205, included in Figure 7 (lanes 7 and 8) as a representative of all other mutants. We also determined that blocking phosphorylation at the five SP/TP sites in tauAP5 had only a modest effect on tau migration compared with the effect of S396A/S404A mutations (compare lanes 3, 5 and 9). As shown in Figure 7, phosphatase treatment caused all of the tau proteins to migrate at the same molecular weight (lanes 2, 4, 6, 8, and 10), suggesting that phosphorylation is responsible for the molecular weight differences observed by SDS-PAGE.
Thus, in contrast to other individual SP/TP sites, blocking phosphorylation at S396/S404 resulted in a significant increase in the electrophoretic mobility of tau, which suggests that phosphorylation of S396 and/or S404 in vivo may contribute substantially to the mobility shift of hyperphosphorylated tau.
DISCUSSION
Hyperphosphorylation of tau has been linked extensively to neurodegeneration (Lee et al., 2001), and we and others have previously shown that SP/TP phosphorylation is critical for toxicity in a Drosophila model of human neurodegenerative disorders, including Alzheimer's (Jackson et al., 2002; Nishimura et al., 2004; Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007; Steinhilb et al., 2007). However, despite the extensive attention devoted to single phosphorylation sites throughout the literature, we now show that in our in vivo model phosphorylation of no single SP/TP site is essential for tau-induced neurotoxicity.
The significance of individual phosphorylation sites and the temporal and spatial appearance of specific phosphoepitopes of tau have been primarily investigated using purified proteins in in vitro kinase reactions or in tissue culture models using transient overexpression (Zheng-Fischhofer et al., 1998; Shea and Cressman, 1999). A major advantage of our Drosophila model is that, in a time- and cost-effective manner, we can ask biologically relevant questions in the context of a whole nervous system that mimics aspects of human disease pathogenesis. We tested whether inhibiting tau phosphorylation at each of the major disease-associated sites affects neurotoxicity in an animal model, and whether tau phosphorylation in vivo relies on a hierarchical sequence of events.
Mutating all SP/TP sites to alanine significantly ameliorates tau toxicity (Fulga et al., 2007; Steinhilb et al., 2007) and replacing them by phospho-mimetic glutamates exacerbates tau-induced neurodegeneration (Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007). Although these findings support the hypothesis that SP/TP phosphorylation plays a fundamental role in neurotoxicity, we now show that mutating individual SP/TP sites to alanine has no clear effect on tau-induced degeneration in vivo. As represented in Figure 2, when expressed in the Drosophila retina, each of the individual SP/TP point mutants induces the same moderately rough eye as tauWT. Furthermore, blocking phosphorylation at five SP/TP sites abolishes reactivity with three important disease-specific antibodies without affecting tau-induced toxicity (Figure 6). Thus, we have established that inhibiting tau phosphorylation at restricted SP/TP sites in vivo does not reduce toxicity, suggesting that multiple sites work together to promote neurodegeneration.
Abnormal conformations of tau, recognized by specific antibodies such as Alz50 and MC1, have been associated with degeneration and can be detected before the appearance of neurofibrillary pathology (Hyman et al., 1988; Jicha et al., 1999a). Notably, tau phosphorylation may help stabilize these aberrant conformations (Carmel et al., 1996). In agreement with this hypothesis, we showed that inhibiting phosphorylation of all SP/TP sites significantly decreases the generation of disease-associated conformations of tau (Steinhilb et al., 2007). In contrast, transgenic flies expressing individual SP/TP tau mutants have similar levels of abnormally folded protein as tauWT-expressing flies (Figure 3), further underscoring the correlation between hyperphosphorylation, conformational changes, and tau-induced neurotoxicity.
Markers consistent with aberrant activation of the cell cycle and alterations in TOR signaling have been described in Alzheimer's disease tissue (An et al., 2003; Li et al., 2004; Griffin et al., 2005), and these two pathways are critical mediators of tau-induced toxicity in our model (Khurana et al., 2006). Here, we show that the neurodegenerative phenotype of individual SP/TP mutants also relies on the activation of TOR signaling and the cell cycle (Figure 4), which indicates that blocking individual phosphorylation sites does not alter the mechanisms of tau-induced neurotoxicity.
Several studies suggest that tau phosphorylation at specific sites relies on a sequential and interdependent order of events (Kimura et al., 1996; Zheng-Fischhofer et al., 1998; Shea and Cressman, 1999; Augustinack et al., 2002). However, we show that individual SP/TP mutations do not completely prevent phosphorylation from occurring at other sites, showing that in vivo phosphorylation of individual SP/TP sites do not absolutely require phosphorylation at other SP/TP (Figure 5). However, a degree of interdependence is shown by partial reduction in AT8 immunoreactivity in flies expressing tau199/217 (Figure 5A and Supplemental Figure S1). In addition, full phosphorylation at serine 212 requires phosphorylation of the non-SP/TP site serine 214 (Figure 5B).
Historically, the AT8 and Tau1 antibodies were considered to be complementary in that AT8 reacted with phosphorylated serines 199 and/or 202 and 205, whereas Tau1 recognized this same stretch of amino acids in an unphosphorylated form (Biernat et al., 1992; Goedert et al., 1995). Because the time of the original mapping, the relationship between these antibodies has been shown to be less coincident (Szendrei et al., 1993; Shea and Cressman, 1999). Our results are in agreement with these latter observations. Although the tau202/205 mutant is not recognized by AT8, as expected, its reactivity with Tau1 is not elevated compared with that of tauWT (Figure 5). Instead, we show that the tau199/217 mutant has substantially increased reactivity with Tau1, suggesting that Tau1 is more specific for the unphosphorylated residues S199 and/or T217 than S202/T205.
The AT100 antibody is one of the most specific correlates of neurodegeneration, because it recognizes tau present in the brains of patients with Alzheimer's disease, but it does not tend to cross-react significantly with normal tissue (Zheng-Fischhofer et al., 1998). Thus, in an attempt to understand disease pathogenesis, much attention has been devoted to elucidating the steps required for the formation of this epitope. AT100 specifically reacts with two phosphorylated residues, pT212 and pS214 (Zheng-Fischhofer et al., 1998). Although some studies suggest that the process of AT100 generation first requires phosphorylation of T212 by glycogen synthase kinase (GSK)3b followed by phosphorylation at S214 by protein kinase A (PKA) (Scott et al., 1993; Zheng-Fischhofer et al., 1998; Jicha et al., 1999b), other reports suggest that prephosphorylation of tau at Ser214 by nonproline-directed kinases, including PKA, greatly enhances the ability of GSK to phosphorylate tau at T212 (Singh et al., 1995; Gong et al., 2005). Using our Drosophila model, in which kinases and phosphatases are endogenously regulated, we find that mutating either T212 or S214 to alanine does not prevent phosphorylation at S214 or T212, respectively (Figure 5), strongly suggesting that, in vivo, phosphorylation of T212 does not require prior phosphorylation of S214, and vice versa.
Interestingly, we show that inhibiting phosphorylation at S199/T217 prevents phosphorylation at the non-SP/TP site S214, which completely blocks formation of the AT100 epitope (Figure 5). Although S199 and T217 lie outside the recognition motif of AT100, our finding is consistent with other reports suggesting that phosphorylation of these sites is important for AT100 reactivity. Interestingly, previous in vitro studies found that a recombinant tau construct containing the T217A mutation could not form the AT100 epitope when treated with purified kinases (Zheng-Fischhofer et al., 1998; Yoshida and Goedert, 2006). Also, in autopsy-derived brain tissue from Alzheimer's disease patients, AT100 immunostaining is associated with phosphorylation of S199 (Maurage et al., 2003). Together, our results suggest that, in addition to the phosphorylation of its recognition motif (pT212/pS214), tau phosphorylation at S199 and/or T217 is a prerequisite for AT100 generation in vivo.
Finally, we demonstrate that the decrease in electrophoretic mobility characteristic of hyperphosphorylated tau depends on phosphorylation at S396/S404 (Figure 7). We also show that blocking phosphorylation at five SP/TP sites in the tauAP5 mutant does not as significantly affect tau mobility, consistent with the notion that a few site-specific phosphorylation events may contribute disproportionately to altered tau migration. Interestingly, previous in vitro studies had argued that the reduced electrophoretic mobility of disease-associated tau might not due to the particularly high degree of phosphorylation, but rather to phosphorylation at specific residues. Similar observations from other groups support a significant effect of C-terminal phosphorylation on the electrophoretic mobility of tau (Steiner et al., 1990; Scott et al., 1993).
In summary, our data suggest that there is no single SP/TP phosphorylation site in tau that preferentially endows the protein with neurotoxicity, suggesting that the sites work together to mediate tau toxicity in vivo. These observations are important because, according to in vitro data, the two proline-directed protein kinases that have been most convincingly implicated in tau pathology, GSK-3β and cdk5, can target multiple SP/TP sites in tau (for review, see Gong et al., 2005). Moreover, blocking GSK-3β and cdk5 activities has been shown to be protective in cell culture systems and animal models of neurodegeneration (Zheng et al., 2002; Mudher et al., 2004; Noble et al., 2005). Thus, together with our previous studies demonstrating the critical role of SP/TP phosphorylation in tau neurotoxicity (Khurana et al., 2006; Dias-Santagata et al., 2007; Fulga et al., 2007; Steinhilb et al., 2007), our current findings underscore the therapeutic potential of kinase inhibitors that can target multiple proline-directed phosphorylation sites for the treatment of Alzheimer's disease and other tauopathies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Doug Rennie of the CBRC Transgenic Fly Core for injection services. We are grateful to the Bloomington Stock Center and individual investigators who generously sent stocks and antibodies (see Materials and Methods). This work was supported by National Institutes of Health grants AG-19790 and AG-05134 (to M.B.F.).
Abbreviations used:
- SP/TP
serine-proline/threonine-proline
- TOR
target of rapamycin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-05-0428) on October 10, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- An W. L., Cowburn R. F., Li L., Braak H., Alafuzoff I., Iqbal K., Iqbal I. G., Winblad B., Pei J. J. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer's disease. Am. J. Pathol. 2003;163:591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack J. C., Schneider A., Mandelkow E. M., Hyman B. T. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- Baudier J., Cole R. D. Phosphorylation of tau proteins to a state like that in Alzheimer's brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J. Biol. Chem. 1987;262:17577–17583. [PubMed] [Google Scholar]
- Biernat J., et al. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992;11:1593–1597. doi: 10.1002/j.1460-2075.1992.tb05204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G., Mager E. M., Binder L. I., Kuret J. The structural basis of monoclonal antibody Alz50's selectivity for Alzheimer's disease pathology. J. Biol. Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Dias-Santagata D., Fulga T. A., Duttaroy A., Feany M. B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 2007;117:236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M. B., Dickson D. W. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann. Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- Flament S., Delacourte A. Abnormal tau species are produced during Alzheimer's disease neurodegenerating process. FEBS Lett. 1989;247:213–216. doi: 10.1016/0014-5793(89)81337-7. [DOI] [PubMed] [Google Scholar]
- Fulga T. A., Elson-Schwab I., Khurana V., Steinhilb M. L., Spires T. L., Hyman B. T., Feany M. B. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat. Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- Garver T. D., Harris K. A., Lehman R. A., Lee V. M., Trojanowski J. Q., Billingsley M. L. Tau phosphorylation in human, primate, and rat brain: evidence that a pool of tau is highly phosphorylated in vivo and is rapidly dephosphorylated in vitro. J. Neurochem. 1994;63:2279–2287. doi: 10.1046/j.1471-4159.1994.63062279.x. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Cohen P., Vanmechelen E., Vandermeeren M., Cras P. Epitope mapping of monoclonal antibodies to the paired helical filaments of Alzheimer's disease: identification of phosphorylation sites in tau protein. Biochem. J. 1994;301:871–877. doi: 10.1042/bj3010871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Vanmechelen E. Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 1995;189:167–169. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- Gong C. X., Liu F., Grundke-Iqbal I., Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J. Neural. Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- Grammenoudi S., Kosmidis S., Skoulakis E. M. Cell type-specific processing of human Tau proteins in Drosophila. FEBS Lett. 2006;580:4602–4606. doi: 10.1016/j.febslet.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA. 1990;87:5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R. J., Moloney A., Kelliher M., Johnston J. A., Ravid R., Dockery P., O'Connor R., O'Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer's disease pathology. J. Neurochem. 2005;93:105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Wolozin B. L., Davies P., Kromer L. J., Damasio A. R. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann. Neurol. 1988;23:371–379. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- Jackson G. R., Wiedau-Pazos M., Sang T. K., Wagle N., Brown C. A., Massachi S., Geschwind D. H. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34:509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jicha G. A., Berenfeld B., Davies P. Sequence requirements for formation of conformational variants of tau similar to those found in Alzheimer's disease. J. Neurosci. Res. 1999a;55:713–723. doi: 10.1002/(SICI)1097-4547(19990315)55:6<713::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Jicha G. A., Bowser R., Kazam I. G., Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J. Neurosci. Res. 1997a;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Jicha G. A., Lane E., Vincent I., Otvos L., Jr, Hoffmann R., Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer's disease. J. Neurochem. 1997b;69:2087–2095. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- Jicha G. A., Weaver C., Lane E., Vianna C., Kress Y., Rockwood J., Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J. Neurosci. 1999b;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana V., Lu Y., Steinhilb M. L., Oldham S., Shulman J. M., Feany M. B. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr. Biol. 2006;16:230–241. doi: 10.1016/j.cub.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Kimura T., Ono T., Takamatsu J., Yamamoto H., Ikegami K., Kondo A., Hasegawa M., Ihara Y., Miyamoto E., Miyakawa T. Sequential changes of tau-site-specific phosphorylation during development of paired helical filaments. Dementia. 1996;7:177–181. doi: 10.1159/000106875. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Liu W. K., Yen S. H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992;597:209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A 68, a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Goedert M., Trojanowski J. Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Li X., An W. L., Alafuzoff I., Soininen H., Winblad B., Pei J. J. Phosphorylated eukaryotic translation factor 4E is elevated in Alzheimer brain. Neuroreport. 2004;15:2237–2240. doi: 10.1097/00001756-200410050-00019. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Maurage C. A., Sergeant N., Ruchoux M. M., Hauw J. J., Delacourte A. Phosphorylated serine 199 of microtubule-associated protein tau is a neuronal epitope abundantly expressed in youth and an early marker of tau pathology. Acta Neuropathol. 2003;105:89–97. doi: 10.1007/s00401-002-0608-7. [DOI] [PubMed] [Google Scholar]
- Mudher A., et al. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry. 2004;9:522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- Nishimura I., Yang Y., Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- Noble W., et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc. Natl. Acad. Sci. USA. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos L., Jr, Feiner L., Lang E., Szendrei G. I., Goedert M., Lee V. M. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J. Neurosci. Res. 1994;39:669–673. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- Scott C. W., Spreen R. C., Herman J. L., Chow F. P., Davison M. D., Young J., Caputo C. B. Phosphorylation of recombinant tau by cAMP-dependent protein kinase. Identification of phosphorylation sites and effect on microtubule assembly. J. Biol. Chem. 1993;268:1166–1173. [PubMed] [Google Scholar]
- Shea T. B., Cressman C. M. The order of exposure of tau to signal transduction kinases alters the generation of “AD-like” phosphoepitopes. Cell Mol. Neurobiol. 1999;19:223–233. doi: 10.1023/A:1006977127422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman J. M., Feany M. B. Genetic modifiers of tauopathy in Drosophila. Genetics. 2003;165:1233–1242. doi: 10.1093/genetics/165.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. J., Haque N., Grundke-Iqbal I., Iqbal K. Rapid Alzheimer-like phosphorylation of tau by the synergistic actions of non-proline-dependent protein kinases and GSK-3. FEBS Lett. 1995;358:267–272. doi: 10.1016/0014-5793(94)01445-7. [DOI] [PubMed] [Google Scholar]
- Steiner B., et al. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990;9:3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilb M. L., Dias-Santagata D., Mulkearns E. E., Shulman J. M., Biernat J., Mandelkow E. M., Feany M. B. S/P and T/P phosphorylation is critical for tau neurotoxicity in Drosophila. J. Neurosci. Res. 2007;1:1271–1278. doi: 10.1002/jnr.21232. [DOI] [PubMed] [Google Scholar]
- Szendrei G. I., Lee V. M., Otvos L., Jr Recognition of the minimal epitope of monoclonal antibody Tau-1 depends upon the presence of a phosphate group but not its location. J. Neurosci. Res. 1993;34:243–249. doi: 10.1002/jnr.490340212. [DOI] [PubMed] [Google Scholar]
- Vincent I., Rosado M., Davies P. Mitotic mechanisms in Alzheimer's disease? J. Cell Biol. 1996;132:413–425. doi: 10.1083/jcb.132.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C. W., Wszolek M. F., Shulman J. M., Salvaterra P. M., Lewis J., Hutton M., Feany M. B. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Goedert M. Sequential phosphorylation of tau protein by cAMP-dependent protein kinase and SAPK4/p38delta or JNK2 in the presence of heparin generates the AT100 epitope. J. Neurochem. 2006;99:154–164. doi: 10.1111/j.1471-4159.2006.04052.x. [DOI] [PubMed] [Google Scholar]
- Zheng-Fischhofer Q., Biernat J., Mandelkow E. M., Illenberger S., Godemann R., Mandelkow E. Sequential phosphorylation of Tau by glycogen synthase kinase-3beta and protein kinase A at Thr212 and Ser214 generates the Alzheimer-specific epitope of antibody AT100 and requires a paired-helical-filament-like conformation. Eur. J. Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C. A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of tau protein in transfected cells. Eur. J. Biochem. 2002;269:4427–4434. doi: 10.1046/j.1432-1033.2002.03133.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.