Abstract
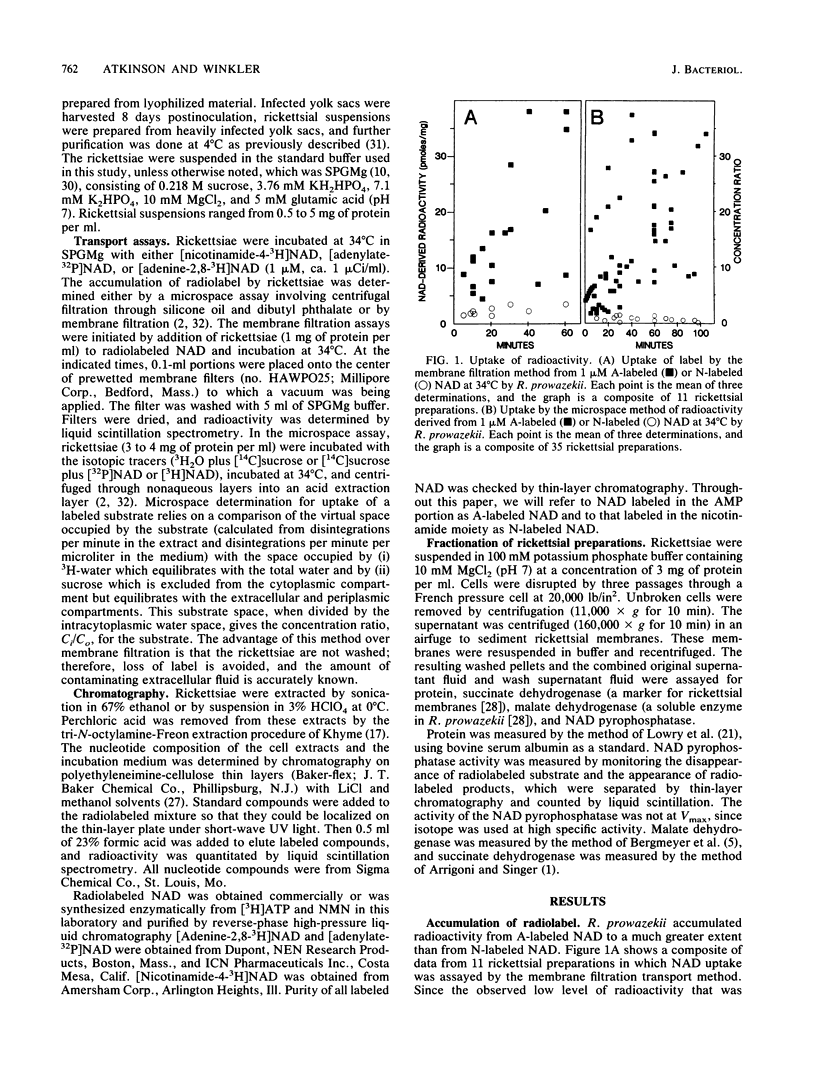
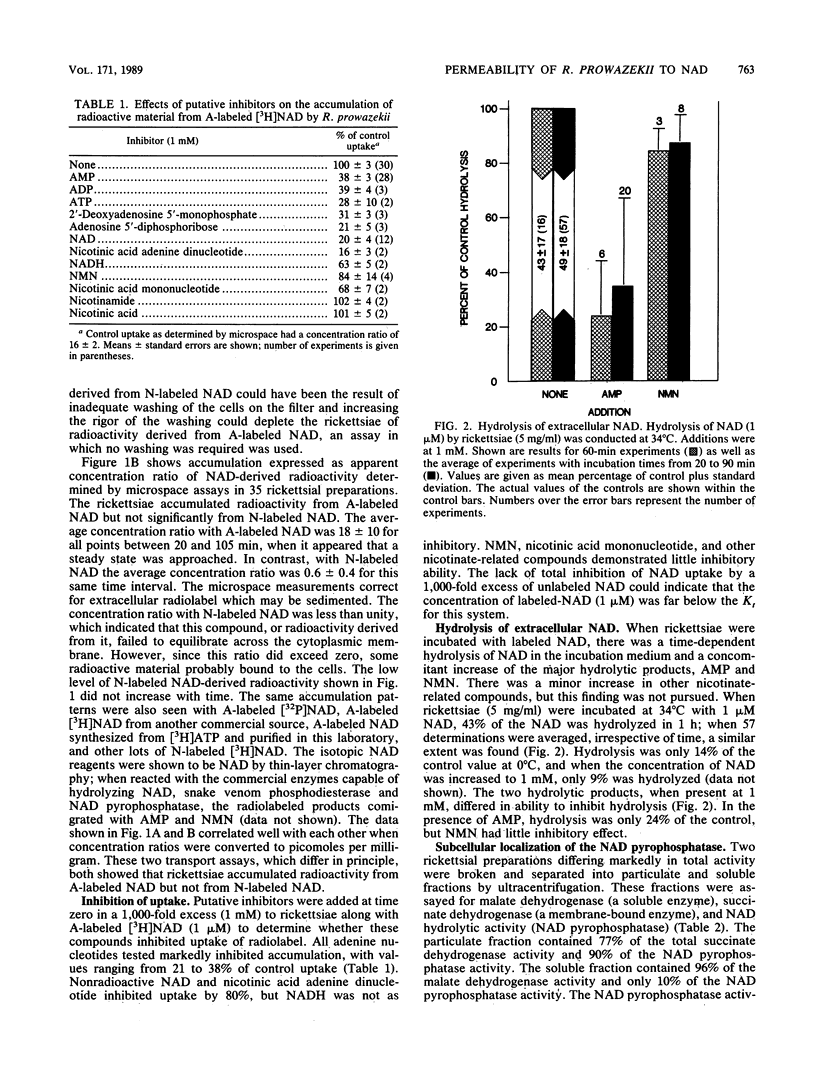
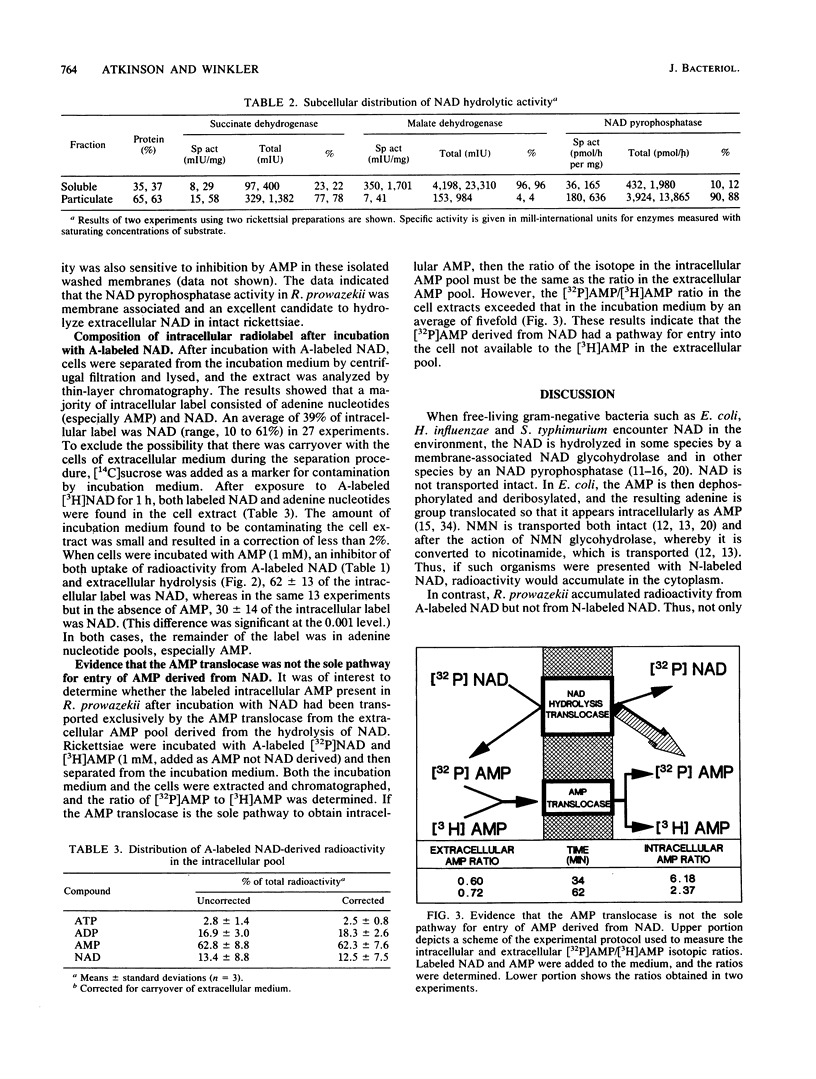
Rickettsia prowazekii accumulated radioactivity from [adenine-2,8-3H]NAD but not from [nicotinamide-4-3H]NAD, which demonstrated that NAD was not taken up intact. Extracellular NAD was hydrolyzed by rickettsiae with the products of hydrolysis, nicotinamide mononucleotide and AMP, appearing in the incubation medium in a time- and temperature-dependent manner. The particulate (membrane) fraction contained 90% of this NAD pyrophosphatase activity. Rickettsiae which had accumulated radiolabel after incubation with [adenine-2,8-3H]NAD were extracted, and the intracellular composition was analyzed by chromatography. The cells contained labeled AMP, ADP, ATP, and NAD. The NAD-derived intracellular AMP was transported via a pathway distinct from and in addition to the previously described AMP translocase. Exogenous AMP (1 mM) inhibited uptake of radioactivity from [adenine-2,8-3H]NAD and hydrolysis of extracellular NAD. AMP increased the percentage of intracellular radiolabel present as NAD. Nicotinamide mononucleotide was not taken up by the rickettsiae, did not inhibit hydrolysis of extracellular NAD, and was not a good inhibitor of the uptake of radiolabel from [adenine-2,8-3H]NAD. Neither AMP nor ATP (both of which are transported) could support the synthesis of intracellular NAD. The presence of intracellular [adenine-2,8-3H]NAD within an organism in which intact NAD could not be transported suggested the resynthesis from AMP of [adenine-2,8-3H]NAD at the locus of NAD hydrolysis and translocation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Atkinson W. H., Winkler H. H. Transport of AMP by Rickettsia prowazekii. J Bacteriol. 1985 Jan;161(1):32–38. doi: 10.1128/jb.161.1.32-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus rickettsiae at O C. J Bacteriol. 1957 Jan;73(1):56–62. doi: 10.1128/jb.73.1.56-62.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R. Phosphorylation accompanying the oxidation of glutamate by the Madrid E strain of typhus rickettsiae. J Biol Chem. 1956 May;220(1):353–361. [PubMed] [Google Scholar]
- BOVARNICK N. R., ALLEN E. C., PAGAN G. The influence of diphosphopyridine nucleotide on the stability of typhus rickettsiae. J Bacteriol. 1953 Dec;66(6):671–675. doi: 10.1128/jb.66.6.671-675.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Kinney D. M., Moat A. G. Pyridine nucleotide cycle of Salmonella typhimurium: isolation and characterization of pncA, pncB, and pncC mutants and utilization of exogenous nicotinamide adenine dinucleotide. J Bacteriol. 1979 Mar;137(3):1165–1175. doi: 10.1128/jb.137.3.1165-1175.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard D., Rechsteiner M., Manlapaz-Ramos P., Imperial J. S., Cruz L. J., Olivera B. M. The pyridine nucleotide cycle. Studies in Escherichia coli and the human cell line D98/AH2. J Biol Chem. 1981 Aug 25;256(16):8491–8497. [PubMed] [Google Scholar]
- Hochstadt-Ozer J. The regulation of purine utilization in bacteria. IV. Roles of membrane-localized and pericytoplasmic enzymes in the mechanism of purine nucleoside transport across isolated Escherichia coli membranes. J Biol Chem. 1972 Apr 25;247(8):2419–2426. [PubMed] [Google Scholar]
- KOHNO S. Studies on metabolism of rickettsiae. I. Studies on dehydrogenases of Rickettsia mooseri. Jpn J Med Sci Biol. 1959 Oct;12:375–385. doi: 10.7883/yoken1952.12.375. [DOI] [PubMed] [Google Scholar]
- Kahn D. W., Anderson B. M. Characterization of Haemophilus influenzae nucleotide pyrophosphatase. An enzyme of critical importance for growth of the organism. J Biol Chem. 1986 May 5;261(13):6016–6025. [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehman I. R. DNA ligase: structure, mechanism, and function. Science. 1974 Nov 29;186(4166):790–797. doi: 10.1126/science.186.4166.790. [DOI] [PubMed] [Google Scholar]
- Liu G., Foster J., Manlapaz-Ramos P., Olivera B. M. Nucleoside salvage pathway for NAD biosynthesis in Salmonella typhimurium. J Bacteriol. 1982 Dec;152(3):1111–1116. doi: 10.1128/jb.152.3.1111-1116.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist R., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. I. Exponential growth. J Biol Chem. 1971 Feb 25;246(4):1107–1116. [PubMed] [Google Scholar]
- Manlapaz-Fernandez P., Olivera B. M. Pyridine nucleotide metabolism in Escherichia coli. IV. Turnover. J Biol Chem. 1973 Jul 25;248(14):5150–5155. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Lehman I. R. Diphosphopyridine nucleotide: a cofactor for the polynucleotide-joining enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1700–1704. doi: 10.1073/pnas.57.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. K., Winkler H. H. Separation of inner and outer membranes of Rickettsia prowazeki and characterization of their polypeptide compositions. J Bacteriol. 1979 Feb;137(2):963–971. doi: 10.1128/jb.137.2.963-971.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Daugherty R. M. Acquisition of glucose by Rickettsia prowazekii through the nucleotide intermediate uridine 5'-diphosphoglucose. J Bacteriol. 1986 Sep;167(3):805–808. doi: 10.1128/jb.167.3.805-808.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Inhibitory and restorative effects of adenine nucleotides on rickettsial adsorption and hemolysis. Infect Immun. 1974 Jan;9(1):119–126. doi: 10.1128/iai.9.1.119-126.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Membrane transport in rickettsiae. Methods Enzymol. 1986;125:253–259. doi: 10.1016/s0076-6879(86)25021-1. [DOI] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Yagil E., Beacham I. R. Uptake of adenosine 5'-monophosphate by Escherichia coli. J Bacteriol. 1975 Feb;121(2):401–405. doi: 10.1128/jb.121.2.401-405.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZATMAN L. J., KAPLAN N. O., COLOWICK S. P., CIOTTI M. M. The isolation and properties of the isonicotinic acid hydrazide analogue of diphosphopyridine nucleotide. J Biol Chem. 1954 Aug;209(2):467–484. [PubMed] [Google Scholar]
- ZATMAN L. J., KAPLAN N. O., COLOWICK S. P. Inhibition of spleen diphosphopyridine nucleotidase by nicotinamide, an exchange reaction. J Biol Chem. 1953 Jan;200(1):197–212. [PubMed] [Google Scholar]



