Abstract
Regulation of translation initiation plays a critical role in the control of cell growth and division in eukaryotic cells. Translation of many growth regulatory proteins including cyclins depends critically on translation initiation factors because their mRNAs are translated inefficiently. We report that clotrimazole, a potent antiproliferative agent both in vitro and in vivo, inhibits cell growth by interfering with translation initiation. In particular, clotrimazole causes a sustained depletion of intracellular Ca2+ stores, which results in activation of PKR, phosphorylation of eIF2α, and thereby in inhibition of protein synthesis at the level of translation initiation. Consequently, clotrimazole preferentially decreases the expression of the growth promoting proteins cyclin A, E and D1, resulting in inhibition of cyclin-dependent kinase activity and blockage of cell cycle in G1.
Translation initiation plays a critical role in controlling cell growth and proliferation. Indeed, the expression of most proto-oncogenes and growth regulatory proteins depends heavily on the activity of eukaryotic translation initiation factors (eIF) (1–3) because they are encoded by inefficiently translated mRNAs (4). Consistently, mitogen stimulation increases the rate of translation initiation by activating eIF2α and eIF4E, whereas mitogen starvation inhibits translation initiation by inactivating these factors (5). Furthermore, mutations of eIF4E, eIF2α or protein kinase R (PKR) that increase translation initiation lead to increased expression of growth promoting proteins and result in neoplastic transformation. In contrast, decreasing the rate of translation initiation reverses transformed phenotypes (1–3).
Clotrimazole (CLT) strongly inhibits growth of both normal and cancer cells in vitro and tumor growth in vivo (6). Our recent confirmation of the anticancer activity of CLT in syngenic animal models and of its antiproliferative effects on numerous cancer cell lines (unpublished data) prompted us to study the molecular basis of the antiproliferative activity of CLT. CLT releases Ca2+ from intracellular Ca2+ stores and inhibits restorative Ca2+ store-regulated Ca2+ influx through the plasma membrane thereby causing a sustained depletion of intracellular Ca2+ stores (6, 7). Depletion of intracellular Ca2+ stores activates PKR, resulting in phosphorylation of eIF2α on serine 51 and its concomitant inactivation (8–10). Inactivation of eIF2α inhibits formation of the ternary complex between Met-tRNA, eIF2α and GTP (Met-tRNA.eIF2α.GTP), which is usually the rate limiting step in translation initiation (11).
Based on these considerations, we reasoned that depletion of intracellular Ca2+ stores by CLT may inhibit translation initiation and thereby repress expression of growth promoting proteins. We report here that CLT inhibits cell proliferation by blocking the cell cycle specifically in G1 through Ca2+ store-mediated inhibition of translation initiation. In particular, CLT reduces synthesis and expression of G1 cyclins and thereby inhibits associated cyclin-dependent kinase (cdk) activity, which is required for progression into S phase. This mechanism of action of CLT and the fact that this small molecular weight compound can be administered safely to humans (12) underscores the potential of CLT and its derivatives as new therapeutic tools for proliferative disorders, including cancer.
MATERIALS AND METHODS
Cell Culture, Plasmids, and Transfection.
NIH 3T3 cells cultured in DMEM/10% calf serum were synchronized by reducing serum to 0.2% for 36 hr. Plasmids carrying wild-type and Ser51→Ala mutants of eIF2α (eIF2α51A), wild-type PKR, and dominant negative PKR were a gift from Monique Davies (Genetics Institute, Cambridge, MA). NIH 3T3 cells were transfected with 50 ng pBABE (which confers resistance to puromycin) and 5 μg of the respective plasmids in the presence of 20 μg of calf thymus DNA (13). Cells were transferred to new dishes 3 days later in medium containing 2.5 μg/ml puromycin. Puromycin resistant colonies were picked and grown for further analysis 10 days later. Cyclin D1 constructs were gifts from Charles Sherr (Saint Jude’s Children Hospital, Memphis, TN). Cells were transfected with cyclin D1 expression plasmid as described above, except that 50 ng of cyclin D1 plasmid was used for transfection, and transfectants were selected for by G418 (400 ng/ml).
Protein Synthesis.
Exponentially growing cells were incubated for 15 min at 37°C with or without CLT or cycloheximide (5 μg/ml), rinsed with Met-Cys free DMEM, and incubated for an additional 10 min in the presence or absence of the test drugs. Trans-35S label (50 μCi/ml) was added to the Met-Cys-free medium for 15 min at 37°C. After 3 washes with PBS, cells were harvested in lysis buffer (150 mM NaCl/50 mM Tris⋅HCl, pH 7.5/0.05% SDS/1% Nonidet P-40/1 mM benzamidine/1 mM EDTA/1 mM phenylmethylsulfonyl fluoride). Protein concentration was determined by bicinchoninic acid assay (Pierce). Equal amounts of protein were separated by SDS/PAGE or an aliquot of lysate was trichloroacetic acid-precipitated and counted in a scintillation counter.
Northern Blot.
Total RNA was separated by agarose/formaldehyde gel electrophoresis and transferred to nitrocellulose membranes. The membrane was baked, blocked by Denhart solution, and hybridized with probes prepared by random priming. The mRNA was visualized and quantified by PhosphoroImager (Molecular Dynamics).
[3H]Thymidine Incorporation.
For pulse labeling experiments, [3H]thymidine was added to cultures during the last 2 hr of incubation, the medium was decanted and cells were washed with PBS, processed, and counted (14).
Immunoprecipitation and Western Blotting.
Anti-β-actin, cyclin E, and two anticyclin D1 (R-124, HD-11) antibodies were from Santa-Cruz, and antibodies to p27KIP1 were from Transduction Laboratories (Lexington, KY). Cells were lysed in immunoprecipitation buffer, and 25–50 μg of protein was immunoprecipitated (15). For Western blotting, 25 μg of samples were separated by electrophoresis in 10–13% SDS/PAGE and immunoblotted as described (15). Antibody-antigen complexes were detected by using an ECL kit (Amersham) and horseradish peroxidase-conjugated goat secondary antibodies.
In Vitro Kinase Assays.
Cyclin E-cdk2 immuncomplexes were washed twice with kinase buffer, and 15 μl of reaction mixture (kinase buffer with 10 μCi [32P]ATP, 20 μM cold ATP, and 2 μg of either glutathione S-transferase-Rb (GST-Rb) or histone H1) was added to the immuncomplexes and incubated for 30 min at 30°C (16). The reactions were stopped by addition of 10 μl of 4X SDS-loading buffer, boiled for 5 min and centrifuged, and supernatants were separated by SDS/PAGE. Phosphorylation of substrates was quantified by PhosphoroImager (Molecular Dynamics).
Polysome Profiles.
Exponentially growing NIH 3T3 cells were treated with CLT (10 μM), thapsigargin (300 nM), or vehicle for either 30 min or 6 hr. Cycloheximide (25 μg/ml) was added for 5 min, and cells were washed and collected in ice-cold PBS/cycloheximide and lysed; equal OD at 260 nm were subjected to sucrose (15–60%) density gradient centrifugation as described by Rousseau et al. (17). The gradients were eluted while monitoring absorbency at 254 nm.
RESULTS AND DISCUSSION
CLT Inhibits Translation Initiation.
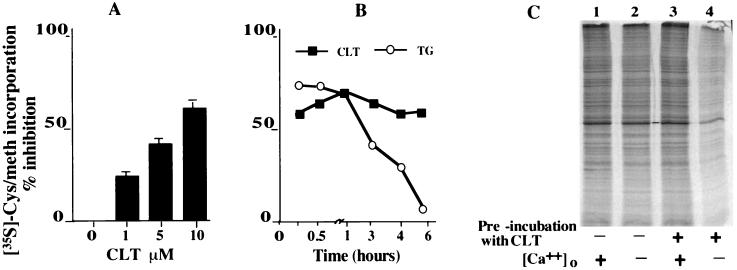
To study the effect of CLT on protein synthesis, exponentially growing 3T3 cells were pulse-labeled with 35S-Met-Cys, and incorporation of label was determined in the absence and presence of CLT. CLT inhibited protein synthesis in a dose-dependent manner (Fig. 1A). Inhibition of protein synthesis was not caused by differences in specific activity of the tracer as CLT did not affect 35S-Met-Cys uptake (data not shown). To demonstrate that inhibition of protein synthesis is the direct result of depletion of intracellular Ca2+ stores, exponentially growing cells were transiently exposed to CLT for 10 min and then washed with Ca2+-free medium containing EGTA (20 μM) to remove both CLT and Ca2+. The cells were then pulse labeled with 35S-Met-Cys in the presence or absence of external Ca2+ (2 mM) followed by SDS/PAGE and autoradiography. Cells transiently exposed to CLT reinitiated protein synthesis only when Ca2+ was added to the medium; in contrast, protein synthesis in these cells remained inhibited when they were pulse labeled in the absence of external Ca2+. Removal of external Ca2+ during the pulse-labeling period did not affect protein synthesis in cells that were not previously exposed to CLT (Fig. 1C). These results confirm that depletion of intracellular Ca2+ stores is responsible for the inhibitory effect of CLT on protein synthesis.
Figure 1.
CLT inhibits protein synthesis. (A) Exponentially growing NIH 3T3 cells were labeled with 35S-Met-Cys with or without CLT. TCA precipitable counts were normalized for protein concentration. (B) Cells were exposed to either CLT (10 μM) or thapsigargin (300 nM), pulse-labeled with 35S-Met-Cys, and processed as in A. (C) Cells were incubated without (lanes 1 and 2) or with (lanes 3 and 4) CLT for 10 min, washed, and incubated in Met-Cys and Ca2+ free −20 μM EGTA medium without CLT. Cells were pulse labeled in the absence (lanes 2 and 4) or presence of 2 mM Ca2+ (lanes 1 and 3). Equal amount of protein was separated by SDS/PAGE and autoradiographed.
Other Ca2+ releasers such as thapsigargin inhibit protein synthesis only transiently (18). Depletion of Ca2+ stores by these agents activates Ca2+ store-regulated Ca2+ influx, which allows for the refilling of the Ca2+ stores. In contrast, CLT not only releases Ca2+ from intracellular stores but also inhibits Ca2+ store-regulated Ca2+ influx (6, 18). Therefore, CLT should have a more sustained effect on the Ca2+ stores and on protein synthesis. This prediction was tested by measuring protein synthesis in cells continuously exposed to either CLT or thapsigargin for various time intervals. Inhibition of protein synthesis by CLT remained unchanged whereas inhibition by thapsigargin completely reversed after 6 hr (Fig. 1B).
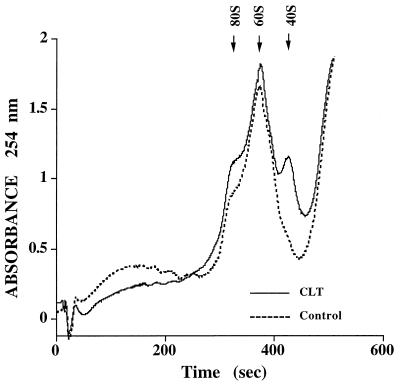
Release of Ca2+ from intracellular stores activates PKR, phosphorylates eIF2α, and inhibits translation initiation (8, 9). To test whether inhibition of protein synthesis by CLT results from inhibition of translation initiation, we determined the polysome content of sucrose density fractions derived from cells exposed to CLT. CLT treatment shifted the ribosomal profile from heavy polyribosomes to lighter polysomes, monosomes and free ribosomal subunits (Fig. 2), indicating that CLT inhibits translation initiation. To determine whether the different effect of CLT and thapsigargin on protein synthesis shown in Fig. 1B is caused by a differential effect on translation initiation, we determined the ribosomal profile in cells exposed to either CLT or thapsigargin for 30 min or 6 hr. In three experiments, the heavy polysome fraction averaged 42% of total ribosomal material in control cells and 23% and 21% in cells exposed for 30 min to CLT or thapsigargin, respectively. After 6-hr exposure the polysome fraction represented 36% of total ribosomal material for control, 24% for CLT and 34% for thapsigargin. These results show that CLT causes sustained inhibition of translation initiation whereas the effect of thapsigargin is only transient. They are consistent with the differential effect of CLT and thapsigargin on protein synthesis shown in Fig. 1B, and indicate that inhibition of cell growth by thapsigargin is not the result of sustained inhibition of protein synthesis.
Figure 2.
CLT inhibits formation of polyribosomes. Exponentially growing 3T3 cells were challenged with CLT (10 μM, solid line) or vehicle (broken line) for 30 min. Extracts were prepared and equal OD at 260-nm units were separated by sucrose density gradient centrifugation and polysome profile of the gradients were obtained. The position of 80S, 60S, and 40S material is indicated by arrows.
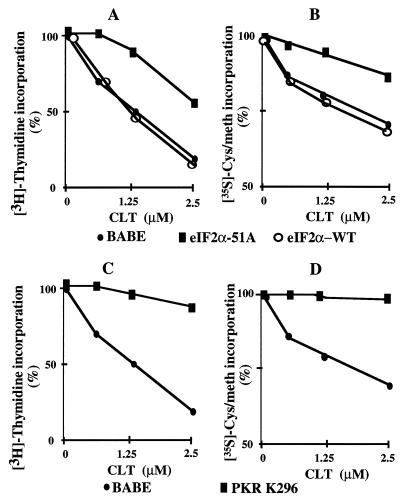
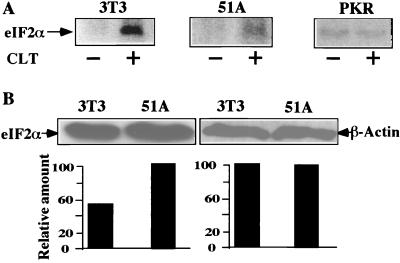
To test whether inhibition of translation initiation by CLT is caused by activation of PKR and phosphorylation of eIF2α, cell lines expressing either the nonphosphorylatable eIF2α (eIF2α51A) or dominant negative PKR (PKR-K296) were constructed and tested for resistance to CLT. Both eIF2α51A and PKR-K296 cells were resistant to inhibition of cell growth and protein synthesis by CLT (Fig. 3). These results suggest that activation of PKR and phosphorylation of eIF2α are responsible for the inhibitory effect of CLT on protein synthesis and cell growth. To confirm this interpretation, we measured phosphorylation of eIF2α. Exponentially growing NIH 3T3- and eIF2α51A- or PKR-K296-transfected cells were labeled with [32P]orthophosphate for 3.5 hr and challenged with CLT or vehicle for 30 min before immunoprecipitation of eIF2α. Treatment with CLT caused a 10-fold increase in phosphorylation of eIF2α in NIH 3T3 cells (Fig. 4A). In contrast, CLT did not cause any phosphorylation of eIF2α in dominant negative PKR transfectants (Fig. 4A) whereas it induced an intermediate level of phosphorylation (≈3-fold) in eIF2α-51A transfectants (Fig. 4A). These differences were not caused by different specific activity of the ATP pools (data not shown), measured as described (19). Phosphorylation of eIF2α increases its affinity for eIF2B and interferes with eIF2B-catalyzed GDP–GTP exchange necessary to initiate a new round of translation. Because the molar ratio of eIF2B/eIF2 is low, phosphorylation of 25–30% of eIF2α is sufficient to abrogate almost completely both activity of eIF2B and initiation of protein synthesis (11). Because eIF2α51A-expressing cells contain endogenous phosphorylatable eIF2α (Fig. 4B), CLT treatment of these cells results in some phosphorylation of endogenous eIF2α (Fig. 4A). This observation may explain the residual sensitivity of eIF2α51A transfectants to inhibition of cell growth and protein synthesis by CLT (Fig. 3 A and B).
Figure 3.
Dominant negative PKR and eIF2α-51A confer resistance to CLT. NIH 3T3 cell were transfected with pBABE, wild type (WT) or eIF2α-51A (A and B), or dominant negative PKR (C and D). Selected clones were tested for resistance to CLT by [3H]thymidine incorporation (A and C) or by 35S-Met-Cys incorporation (B and D).
Figure 4.
CLT causes activation of PKR and phosphorylation of eIF2α. (A) Exponentially growing NIH 3T3-, PKR-K296-, or eIF2α-51A-transfected cells were labeled with [32P]orthophosphate (200 μCi/ml) for 3.5 hr. One-half of the cells were challenged with CLT (10 μM) for 30 min, lysed in IP buffer, and RNase–DNase treated. TCA precipitable counts were determined, and equal number of counts were immunoprecipitated with anti-eIF2α antibody and separated by SDS/PAGE, and phosphorylation of eIF2α was quantified by PhosphoroImager. (B) Maternal NIH 3T3 or eIF2α-51A transfectants were lysed and 20 μg of protein was separated by SDS/PAGE and immunoblotted with anti-eIF2α or β-actin specific antibodies.
CLT Inhibits Cell Cycle Progression Specifically in G1.
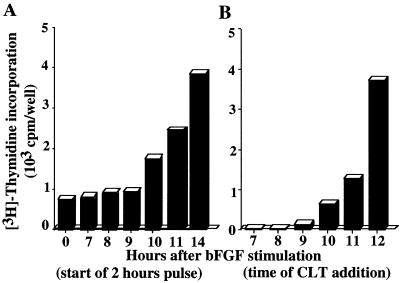
Synthesis of new proteins drives the cell cycle through the restriction point in late G1 (20). If CLT inhibits cell growth solely by interfering with initiation of protein synthesis, it should inhibit cell cycle progression without interfering with initial growth factor signaling. Exposure of quiescent 3T3 cells to basic fibroblast growth factor (bFGF) in the presence of CLT did not inhibit mitogen-activated protein kinase activity (data not shown) demonstrating that CLT does not interfere with early mitogenic signaling. To test whether CLT blocks cell cycle progression before the restriction point, quiescent NIH 3T3 cells were stimulated with bFGF and the effect of adding CLT at hourly intervals on entry into S phase was monitored. Quiescent cells entered S phase 12–13 hr after bFGF stimulation (Fig. 5A). CLT addition to these cells until late G1 (10–11 hr after bFGF), prevented progression into S phase. However, addition of CLT at later times failed to inhibit G1-S transition (Fig. 5B), indicating that CLT blocks cell proliferation specifically in G1, most probably before the restriction point (20). These results were confirmed by cell cycle analysis using laser scanning cytometry (ref. 21 and data not shown).
Figure 5.
CLT inhibits G1/S transition. (A) Quiescent 3T3 cells were stimulated with bFGF (5 ng/ml). Progression into S phase was monitored by measuring [3H]thymidine incorporation at the indicated times after bFGF stimulation. (B) To determine the CLT-sensitive period, bFGF-stimulated cells were challenged with 10 μM CLT at the indicated times after bFGF addition and pulsed for 2 hr with [3H]thymidine added 14 h after bFGF stimulation.
CLT Abrogates Expression of Cyclins and cdk Activity.
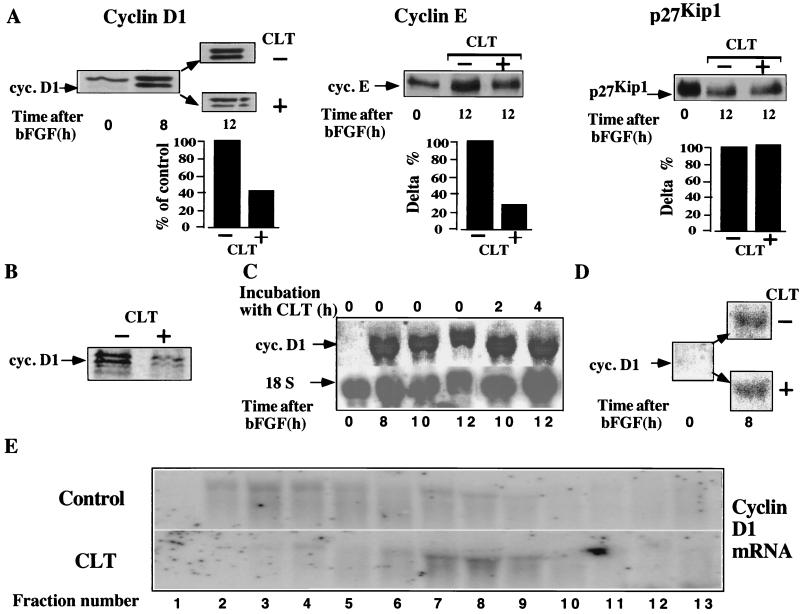
The major transitions of the eukaryotic cell cycle are governed by cdks. Cdk activity is tightly controlled by cyclin levels, formation of cyclin cdk complexes, post-translational modifications of cdks, and interactions with cdk inhibitors (22–28). Both cyclin D and E are rate limiting for S phase entry (29–32); consistently, partial inhibition of protein synthesis by low concentrations of cycloheximide blocks cell cycle progression in late G1 if added before the restriction point. To determine whether inhibition of cell cycle progression by CLT in late G1 was caused by modulation of the cell cycle regulatory proteins, we analyzed the effect of CLT on the levels of cyclins A, D1, and E, cdk2, cdk4, and of the cdk inhibitor p27KIP1, as well as of β actin. CLT significantly down-regulated expression of cyclin D, E (Fig. 6A), and A (not shown) but not of β actin (Fig. 8), p27KIP1 (Fig. 5A), or cdk2 and 4 (data not shown). These results suggest that CLT blocks cell cycle progression by preventing cyclin accumulation. To determine whether CLT inhibits expression of cyclin D1 by interfering with its synthesis, exponentially growing NIH 3T3 cells were pulse labeled with 35S-Met-Cys with or without CLT and equal amounts of protein were immunoprecipitated with anti cyclin D1 antibody. CLT inhibited cyclin D1 synthesis significantly (Fig. 6B) indicating that the effect of CLT on cyclin D1 expression is mediated, at least in part, by inhibition of its synthesis.
Figure 6.
CLT abrogates expression of cyclins. (A) Quiescent NIH 3T3 cells were stimulated with bFGF and challenged with CLT (10 μM) after 8 hr. Cells were lysed 4 hr later, and 25 μg protein was immunoblotted with antibodies to cyclin D1, cyclin E, or p27Kip1. Note that the upper band in cyclin D1 immunoblot is a different immunoreactive protein because it is not recognized by another anticyclin D1 antibody (see Fig. 8B) and its intensity remains unchanged in serum-starved cells, which do not express cyclin D1. (B) NIH 3T3 cells growing exponentially in bFGF were labeled with 35S-Met-Cys (100 μCi/ml) for 1 hr with or without CLT (10 μM). One hundred migrograms of protein was immunoprecipitated with anti cyclin D1 antibody. Immuncomplexes were separated by SDS/PAGE and visualized by PhosphoroImager. (C) Quiescent cells were stimulated with bFGF for 8 hr, then CLT (10 μM) was added, and cells were harvested either 2 or 4 hr later for Northern blot analysis of cyclin D1 or 18S mRNA. (D) Quiescent NIH 3T3 cells were stimulated with bFGF and simultaneously challenged with or without CLT (10 μM); expression of cyclin D1 mRNA was determined by Northern blotting after 8 hr. (E) RNA extracted from the fractionated sucrose gradients shown in Fig. 2, was separated by formaldhyde-agarose gel electrophoresis and hybridized to cyclin D1 specific probe.
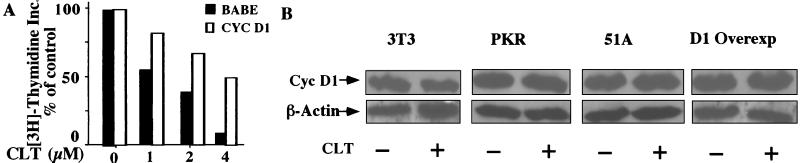
Figure 8.
Forced expression of cyclin D1 confers resistance to CLT (A); and CLT does not inhibit expression of cyclin D1 in cyclin D1-, PKR-K296-, or eIF2α-51A-transfected cells. Exponentially growing cells were challenged with or without CLT (2.5 μM) for 8 h. Cells were harvested and expression of cyclin D1 and β-actin was determined by immunoblotting.
To investigate whether inhibition of cyclin D1 synthesis occurs at the level of translation or mRNA accumulation, we studied the effect of CLT on cyclin D1 mRNA levels. Quiescent NIH 3T3 cells were stimulated with bFGF, challenged with CLT at different time intervals, and expression of cyclin D1 mRNA was determined by Northern blotting. To normalize for loading, the same membrane was stripped and hybridized with an 18S rRNA-specific probe. Levels of cyclin D1 mRNA were not affected by CLT (Fig. 6 C and D), indicating that CLT inhibits cyclin D1 synthesis at the level of translation. Importantly, these results demonstrate that CLT does not interfere with the mitogenic signaling upstream of cyclin D1 transcription. They also indicate that the reduced expression of G1 cyclins and the consequent complete abrogation of cdk activity (see below) are likely to be responsible for and not the consequence of the antiproliferative effects of CLT.
To demonstrate directly that CLT inhibits synthesis of cyclin D1 at the level of translation initiation, RNA was extracted from polysome fractions derived from the experiment shown in Fig. 2A and hybridized with a cyclin D1-specific probe. Treatment with CLT caused a shift of cyclin D1 mRNA from heavier to lighter polysomal fractions. These data confirm that CLT inhibits translation initiation of cyclin D1 mRNA.
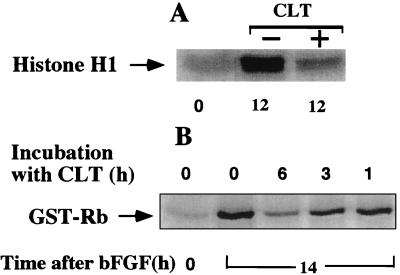
The functional significance of interfering with bFGF-stimulated cyclin expression was studied by measuring the activity of cyclin E-cdk2 complexes immunoprecipitated with anticyclin E antibody from the cell extracts used in the experiment depicted in Fig. 5A. Exposure to CLT for 6 hr in late G1 completely abrogated cyclin E-associated kinase activity (Fig. 7A). This result was not a direct effect of CLT on the activity of cyclin E-dependent kinase because the addition of CLT directly to the kinase reaction had no effect (data not shown). Furthermore, CLT did not inhibit cdk2 activity after exposure for up to 3 hr (Fig. 7B), and the decline of cdk2 activity in the presence of CLT paralleled the progressive reduction in cyclin E levels (data not shown). These results indicate that CLT inhibits the activation of cyclin E-dependent kinase primarily by decreasing the levels of cyclin E. If CLT inhibited cdk activity by inhibiting cdk or cdc25 activity directly or by causing activation of a Wee1-like kinase, the kinetics of inhibition would have been faster and independent of the cyclin E levels (33).
Figure 7.
CLT abrogates cdk2 activity. (A) Extracts prepared from the experiment depicted in Fig. 5A were immunoprecipitated with anticyclin E antibodies, and the activity of associated kinase was determined by using Histone H1 as substrate. (B) Activity of cyclin E-cdk2 complex after CLT addition was determined in bFGF-stimulated cells exposed to CLT (10 μM) for 1, 3, and 6 hr (CLT was added 8, 11, or 13 hr after bFGF). Cells were harvested 14 hr after initial bFGF stimulation; 25 μg of protein was immunoprecipitated with anticyclin E antibodies, and kinase activity was determined by using glutathione S-transferase-Rb as substrate.
To confirm that down regulation of G1 cyclin expression plays a critical role in inhibition of cell growth by CLT, NIH 3T3 cells were transfected with cyclin D1 cDNA and tested for resistance to CLT. Cells overexpressing cyclin D1 were sensitive to the inhibitory effect of CLT on protein synthesis in a manner comparable with maternal cells (data not shown). In contrast, they were markedly resistant to the inhibitory effect of CLT on cell growth (Fig. 8A). To test whether resistance of eIF2α-51A, PKR-K296, and cyclin D1 transfectants correlated with expression of cyclin D1, transfected cells and maternal 3T3 cells were incubated with or without CLT for 8 hr and expression of β-actin and cyclin D1 was determined by immunoblotting. The bands were quantified and the ratio of cyclin D1 to β-actin obtained. CLT abrogated expression of cyclin D1 in maternal NIH 3T3 cells by 49% but had no effect on expression of cyclin D1 in cyclin D1, PKR-K296, or eIF2α-51A transfectants (Fig. 8B). These results confirm that abrogation of cyclin accumulation plays a significant role in the inhibition of cell growth by CLT.
The data presented here demonstrate that CLT exerts its antiproliferative activity by releasing Ca2+ from intracellular stores while inhibiting their refilling via Ca2+ stores-regulated Ca2+ channels in the plasma membrane. In this manner, CLT empties the intracellular Ca2+ stores and thereby induces activation of PKR causing phosphorylation of eIF2α and sustained inhibition of protein synthesis at the level of translation initiation. Consequently, cell cycle progression is blocked in G1 at least in part because of reduction of cyclin expression and abrogation of associated cdk activity. This interpretation is confirmed by our finding that CLT abrogated expression of cyclin D1, E, and A without any apparent effect on expression of either β actin or p27KIP1 (Fig. 6 A and B and Fig. 8B). This apparent preferential effect of CLT on the expression of cyclins may explain why in experimental animal models of cancer, CLT affects the growth rate and metastatic potential of tumors with no apparent toxicity, even after 10 weeks of continuous daily administration (6). Whether CLT also inhibits translation initiation of other cell regulatory proteins, and whether it preferentially affects translation initiation of a subset of proteins is currently under investigation.
The models of ribosomal protein synthesis proposed by Lodish and Rapoport (34, 35) predict that reducing the rate of translation initiation would preferentially affect translation of inefficiently translated mRNAs. mRNAs with a highly structured 5′-untranslated region tend to be translated inefficiently. Interestingly, a large proportion of growth regulatory proteins and oncogenes are encoded by mRNAs that contain complex and highly structured 5′-untranslated region and are therefore inefficiently translated. Furthermore, it has been proposed recently that the relative translation inefficiency of growth promoting proteins plays a role in the maintenance of appropriate restrains on cell growth (11, 36, 37). It is tempting to propose that by depleting Ca2+ stores, activating PKR, and phosphorylating eIF2α, CLT preferentially affects proteins coded by mRNAs that posses complex and highly structured mRNAs.
The uncovering of the antiproliferative mechanism of action of CLT reported here identifies translation initiation as a target for cancer therapy. Our recent identification of the pharmacophore in the CLT molecule responsible for its Ca2+ depleting and antiproliferative action (unpublished results) opens new avenues for the development of more potent and selective modulators of translation initiation for cancer therapy.
Acknowledgments
The authors are grateful to Dr. Daniel C. Tosteson and Dr. Magdalena T. Tosteson for continuous support and to Dr. Victor Dzau for helpful comments. The authors are also grateful to Dr. M. Davis for kindly providing the PKR and eIF2 plasmids, to Dr. C. Sheer for the cyclin D1 plasmid, and to Dr. N. Sonnenberg, Dr. A. Craig, and Professor A. Alcazar for the anti-eIF2 antibodies. This article is dedicated to the memory of Dr. T. W. Smith (1937–1997), Chief of Cardiovascular Medicine at Brigham and Women’s Hospital, a remarkable gentleman and scholar and an enthusiastic supporter of this work.
Note Added in Proof:
Experiments completed after submission of this manuscript show that CLT does not inhibit protein synthesis in cell-free reticulocyte lysates, confirming that CLT is not a direct inhibitor of translation initiation. Furthermore, we have documented that CLT (10 μM) releases Ca2+ from purified microsomes (bovine adrenal) and does not inhibit sarco(endo)plasmic reticulum Ca2+ (SERCA)-ATPase.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CLT, clotrimazole; PKR, protein kinase R; cdk, cyclin-dependent kinase; bFGF, basic fibroblast growth factor; eIF, eukaryotic translation initiation factors.
References
- 1.Lazaris-Karatzas A, Montine K S, Sonenberg N. Nature (London) 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 2.Rousseau D, Gingras A C, Pause A, Sonenberg N. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 3.Koromilas A E, Roy S, Barber G N, Katze M G, Sonenberg Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 4.Kozak M. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 5.Montine K S, Henshaw E C. Biochim Biophys Acta. 1989;1014:282–288. doi: 10.1016/0167-4889(89)90224-3. [DOI] [PubMed] [Google Scholar]
- 6.Benzaquen L R, Brugnara C, Byers H R, Gattoni-Celli S, Halperin J A. Nat Med. 1995;1:534–540. doi: 10.1038/nm0695-534. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez J, Montero M, Garcia-Sancho J. J Biol Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- 8.Hinnebusch A G. Semin Cell Biol. 1994;5:417–426. doi: 10.1006/scel.1994.1049. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez de Aldana C R, Dever T E, Hinnebusch A G. Proc Natl Acad Sci USA. 1993;90:7215–7219. doi: 10.1073/pnas.90.15.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brostrom C O, Chin K V, Wong W L, Cade C, Brostrom M A. J Biol Chem. 1989;264:1644–1649. [PubMed] [Google Scholar]
- 11.Pain V M. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 12.Brugnara C, Gee B, Armsby C C, Kurth S, Sakamoto M, Rifai N, Alper S L, Platt O S. J Clin Invest. 1996;97:1227–1234. doi: 10.1172/JCI118537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigh L A, Cooper G M. Mol Cell Biol. 1988;8:3235–3243. doi: 10.1128/mcb.8.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halperin J A, Taratuska A, Nicholson-Weller A. J Clin Invest. 1993;91:1974–1978. doi: 10.1172/JCI116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aktas H, Hong C, Cooper G M. Mol Cell Biol. 1997;17:3850–3857. doi: 10.1128/mcb.17.7.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Proc Natl Acad Sci USA. 1996;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prostko C R, Dholakia J N, Brostrom M A, Brostrom C O. J Biol Chem. 1995;270:6211–6215. doi: 10.1074/jbc.270.11.6211. [DOI] [PubMed] [Google Scholar]
- 19.Childs K F, Ning X-H, Bolling S F. J Chromatogr B: Biomed Appl. 1996;678:181–186. doi: 10.1016/0378-4347(95)00474-2. [DOI] [PubMed] [Google Scholar]
- 20.Pardee A B. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 21.Luther E, Kamentsky L A. Cytometry. 1995;23:272–278. doi: 10.1002/(SICI)1097-0320(19960401)23:4<272::AID-CYTO2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Pines J. Biochem Soc Trans. 1996;24:15–33. doi: 10.1042/bst0240015. [DOI] [PubMed] [Google Scholar]
- 23.Elledge S J, Richman R, Hall F L, Williams R T, Lodgson N, Harper J W. Proc Natl Acad Sci USA. 1992;89:2907–2911. doi: 10.1073/pnas.89.7.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–125. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 25.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 26.Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts J M, Tempst P, Massagué Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 27.King R W, Jackson P K, Kirschner M W. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 28.Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 29.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 30.Matsushime H, Roussel M F, Ashmun R A, Sherr C J. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 31.Knoblich J A, Sauer K, Jones L, Richardson H, Saint R, Lehner C F. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 32.Rosenblatt J, Gu Y, Morgan D O. Proc Natl Acad Sci USA. 1992;89:2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs T. Dev Biol. 1992;153:1–15. doi: 10.1016/0012-1606(92)90087-w. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich R, Rapoport T A. J Theor Biol. 1980;86:279–313. doi: 10.1016/0022-5193(80)90008-9. [DOI] [PubMed] [Google Scholar]
- 35.Lodish H F. Nature (London) 1974;251:385–388. doi: 10.1038/251385a0. [DOI] [PubMed] [Google Scholar]
- 36.Rosenwald I B, Kaspar R, Rousseau D, Gehrke L, Leboulch P, Chen J-J, Schmidt E V, Sonenberg N, London I M. J Biol Chem. 1995;270:21176–21180. doi: 10.1074/jbc.270.36.21176. [DOI] [PubMed] [Google Scholar]
- 37.Rosenwald I B, Lazaris-Karatzas A, Sonenberg N, Schmidt E V. Mol Cell Biol. 1993;13:7358–7363. doi: 10.1128/mcb.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]