Abstract
B-1 B-cells constitute a distinctive population cells that are enriched for self-reactive B cell receptors (BCRs). These BCRs are encoded by a restricted set of heavy and light chains, including heavy chains that lack nontemplated nucleotide additions at the V-D and D-j joining regions. One prototype natural autoantibody produced by B-1 B cells binds to a cryptic determinant exposed on senescent red blood cells that includes the phosphatidylcholine (PtC) moiety. The VH11Vκ9 BCR that accounts for a large fraction of the anti-PtC specificity is underrepresented in other B-cell populations, including newly-formed B cells in bone marrow, and the transitional B cells, follicular B cells, and marginal zone B cells in spleen. Previous work has shown that VH11 heavy chains pair ineffectively with surrogate light chain (SLC) and so do not promote development in bone marrow, but instead allow fetal liver maturation because of a fetal preferences for weaker pre-BCR signaling. Such inefficient SLC pairing constitutes one constraint on maturation of B cells containing VH11 rearrangements that biases their generation to fetal development. Here, we examine another possible bottleneck to the B1 cell repertoire: light chain pairing with VH11 heavy chain, finding very significant preferences.
Keywords: anti-PtC, B-1 B cell, B cell receptor, natural autoantibody, VH11
Introduction
B-1 cells, characterized by expression of the CD5 cell surface glycoprotein, are notable for production of natural autoantibodies [1–5]. In particular, B cell receptors (BCRs) reactive to a determinant on senescent red blood cells that includes the phosphatidylcholine (PtC) moiety are abundant in the B-1 population, where they represent 5–10% of the total [6, 7]. Anti-PtC B cells predominantly utilize VH11 or VH12 VH genes, each paired with a prototypic light chain, Vκ9 or Vk4 respectively [8–12].
Whereas B-1 cells represent a small portion (5%) of the total adult mouse B cell pool, they are enriched in certain anatomical locations, including the peritoneal and pleural cavities [13]. B-1 B cells are detected at birth in spleen and shortly thereafter in the peritoneal cavity, before appearance of follicular or marginal zone B cells. Cell transfer experiment showed that Ig+ B cells in the peritoneal cavity could reconstitute the B-1 B cell population in irradiated recipients [14], but that Ig− precursors in bone marrow were much less efficient. Interestingly, Ig− precursors from the liver of fetal/newborn animals could effectively produce B-1 B cells, suggesting that development of these cells was relatively divergent from the conventional bone marrow pathway operating in adult animals [15]. Later transfer experiments comparing the cell surface phenotype of B cells produced in immunodeficient SCID mice injected with DJ-rearranged pro-B cells [16] showed that those from fetal liver produced predominantly B-1 type B cells, whereas those from bone produced a more typical follicular B cell population (sometimes referred to as “B-2”).
Considering their predominant origin during fetal/neonatal development, many B cells in this population have heavy chains with decreased or absent non-templated nucleotide addition at the V-D and D-J junctions [17]. This is the result of absent or low level expression of terminal deoxynucleotidyl transferase (TdT), the enzyme that mediates this process [18], when Ig heavy chain rearrangements take place in fetal liver B-cell development [19]. Studies of Ig heavy and light chain V gene diversity in the B-1 population have noted the frequent occurrence of particular VH-VL association [20]. Furthermore, unlike follicular B cells, B-1 cells exhibit an increased frequency of lambda light chains [13], again suggesting selection for particular BCRs in the B-1 population.
The recurrence of BCRs with particular VH-VL combinations and the enrichment of lambda light chain has been considered by some to result from strong antigen-dependent selection of the B-1 B cell repertoire [21]. However, in the case of VH11, an additional force mediating a bias to fetal development is weak association of VH11 heavy chains with surrogate light chain [22]. A critical checkpoint in bone marrow B cell development is the assembly of the newly-formed heavy chain with pre-existing B-cell restricted peptides, λ5 and Vpre-B, that together constitute surrogate light chain [23]. This complex, together with two other pre-existing B-cell restricted adapter molecules, Igα and Igβ, forms the pre-BCR, analogous to the BCR expressed in mature B cells. Failure to effectively assemble this complex results in continued recombinase activating gene (RAG1/2) expression and a failure to suppress ongoing heavy chain rearrangement. Also, the proliferative burst that normally occurs with heavy chain expression and activation of the kappa light chain locus, a prerequisite for light chain rearrangement, do not occur in the absence of pre-BCR signaling. Thus, B lineage cells that rearrange VH11 in bone marrow are severely handicapped relative to those with rearrangements of different VH genes that do associate with SLC and thereby generate an effective pre-BCR signal.
Yet B cells with VH11 heavy chain are abundant in the B-1 population and so must be able to progress to the mature B cell stage. The explanation appears to lie in a difference between pre-B cells in fetal liver and bone marrow: while the latter require a strong pre-BCR signal, the former proliferate well and progress to light chain rearrangement with the weaker signal that VH11 does deliver [22]. In fact, it appears that strong pre-BCR signals, characteristic of heavy chains rearranged in bone marrow actually induce fetal liver pre-B cells to cease proliferating, thereby turning the bone marrow handicap of pre-B cells with VH11 heavy chains into an asset.
Considering that the association of SLC with heavy chain is thought to constitute a kind of screen for VDJ structures that will likely pair with a broad assortment of light chains, it is not unreasonable to hypothesize that VDJ associating weakly with SLC may only associate with relatively distinctive light chains [24]. Thus we proposed to test the capacity of a prototypic VH11 heavy chain to associate with several different light chains. We observed very significant preferences for certain light chains during the course of this work. Unexpectedly, we found that Vκ9, the prototypic light chain normally found in association with VH11, was intermediate in capacity to mediate BCR assembly, relative to other light chain tested. We discuss the significance of this finding in the context of BCR-mediated selection in the B-1 B cell population. Other issues related to B cells, natural autoantibodies, and autoimmunity have been discussed previously in the Journal of Autoimmunity 25–34.
Methods
Animals
BALB/c (ICR) female mice, 6–12 wks old, bred in our animal facility, were used in most experiments. All experiments done with mice were conducted under an approved animal protocol.
Cell lines/growth conditions
The R1 and N38 pro-B cell lines were maintained in RPMI 1640 supplemented with 5% FCS, L-glutamine, gentamycin, HEPES, and 2-ME at 37° C in a humidified, 6.5% CO2 incubator. Cells were split approximately every 48 hours to avoid overgrowth.
Fluorescent staining reagents
Monoclonal anti-VH11 anti-PtC idiotype antibody (anti-VH11id, P18 -3H7) and anti-Vk9 anti-PtC idiotype antibody (anti-Vk9id, P18-13B5) were made by immunizing rats with purified IgM anti-PtC (VH11/DH/JH1, Vk9/Jk2) 1–10E8 hybridoma antibody, similar to the method described previously for generating other anti-idiotype antibodies35. The Vk9 anti-PtC gene is identical to M14840 8 and MUSIGKBP (Genbank). Reactivity of these antibodies to normal mouse spleen B cells were <1% by flow cytometry staining analysis. Their reactivity to VH11id μ or Vk9idκ does not require VH/VL combined idiotype, as assessed by staining Ig transgenic mice and from analysis of Ig transfectant antibody, expressing either VH11id μ, Vk9id κ, or both. P18-3H7 is a rat IgG1/κ and P18-13B5 is a rat IgG2a/κ. These anti-idiotype antibodies and monoclonal antibodies to B220 (RA3-6B2), IgM (331.12), CD5 (53-7.3), CD19 (1D3), and AA4 (AA4.1) were produced, purified, and conjugated with fluorochromes (fluorescein, FL; phycoerythrin, PE; Cy7-phycoerythrin, Cy7-PE; allophycocyanin, APC; Cascade Blue, CB) or with biotin, in our laboratory. The following staining reagents were purchased: QDot605 avidin (Invitrogen, Carlsbad, CA), PE anti-CD21 (7G6) (BD Biosciences, San Jose, CA).
Flow cytometry
For flow cytometry analysis, cells were peleted by centrifugation and resuspended in deficient RPMI-1640, supplemented with 3% newborn calf serum and 0.05% NaN3 (staining buffer). For determination of surface IgM levels, antibody used was APC-anti-mouse IgM(331.12). Samples were incubated on ice for 20 min. and then cells were washed twice with staining buffer. Each sample was resuspended in a final volume of 200 μL, including propidium iodide (PI) for dead cell exclusion. Flow analysis and sorting of transduced cells was done using a BD Biosciences FACS-VantageSE/DiVa, equipped with three laser excitation lines (407nm, 488nm, 600nm) and 12-color detection. Some flow analysis was done on a BD Biosciences LSR-II flow cytometer (3 laser, 10 detectors). Analysis of each data set was performed using FlowJo software (Treestar, Ashland, OR). For determination of surface Ig express, samples were normalized for variation in GFP expression by dividing the median GFP fluorescence values by the highest GFP median fluorescence value for the set. This value was then multiplied by the median IgM fluorescence value.
Production of heavy chain/light chain vectors
RNA obtained from cell lines previously transfected with light chain transgenic vectors was converted into cDNA using Superscript II (Invitrogen), amplified by the Tgo proofreading DNA polymerase, and cloned using the Topo-TA kit (Invitrogen). Each kappa chain insert utilized for retroviral plasmid construction was verified by sequence analysis. Kappa chain insert was obtained by restriction enzyme digention, gel purified and ligated into appropriately digested retroviral vector plasmid (pMIG/GFP). A VH11-μ retrovirus was prepared similarly, but in a CFP vector. Stocks of retroviral plasmid were prepared by maxi-prep (Qiagen, Valencia, CA).
Retroviral supernatant production
Retroviral pseudoparticles were produced using a protocol from G. Nolan (Stanford University). In brief, Phoenix cells in Iscove’s medium supplemented with 10% FCS, 2-ME, and Pen/Strep were cotransfected using calcium-phosphate precipitation with 20 μg of vector plasmid DNA and 5 μg pCL-Eco plasmid. For transfection, equal volumes of CaCl2/plasmid solution and phosphate-HBS solution were mixed thoroughly. Phoenix cells were pretreated with a 1:1000 dilution of 30 mM chloroquine 10 minutes prior to transfection. The DNA mixture was then added dropwise to the wells of Phoenix cells. Plates were returned to the incubator for 24 hours and then medium was replaced with fresh RPMI 1640 supplemented with Pen/Strep, L-glutamine, nonessential amino acids, 2-ME and sodium pyruvate. Virus-laden supernatant was harvested at 24 and 48 hours following the medium replacement. At harvest, supernatant was chilled on ice for 10 minutes, debris pelleted by brief high speed centrifugation, and finally 1 mL aliquots (to which 100 μL of FCS was added) were frozen at −80° C.
Ttransduction of cell lines
Cells were harvested from cultures growing at a density of 2–7×105/mL, and >90% viable, as assessed by trypan blue exclusion. 1×105 cells were used for each transduction in a final volume of 500 μL in a 24-well tissue culture plate. In some cases, retroviral supernatants for both heavy chain/CFP and light chain/GFP were added simultaneously. 0.5 μL of Polybrene (4 mg/mL) was added to each well prior to “spin-fection”, where viral particles were pelleted onto cells by centrifugation for 1 hour at 2250 RPM, 37° C. Then transduced cells were returned to the incubator for 2 hours before addition of 1.5 mL fresh medium per well, bringing the final volume to 2 mL. Transduced cells were allowed to proliferate 48 hours before further analysis.
Q-PCR
Total RNA was prepared by sorting cells into “Solution D” lysis/denaturing solution, followed by acid-phenol extraction and isopropanol precipitation, as described previously [36]. cDNA was synthesized by adding 1 μL oligo-(dT)12–18 primer (0.5 μg/μL, Invitrogen) to 20 μL total RNA; heating at 70C for 10 min; cooling on ice for 2 min.; adding 8 μL 5X first-strand buffer (Invitrogen), 4 μL 0.1 M DTT (Invitrogen), 4 μL dNTPs (Promega; each dNTP at 10 mM), 1 μL random hexamer primers (20 U/mL, Pharmacia), 2 μL RNAsin (40 U/mL, Promega), 2 μL Superscript II (200 U/mL, Invitrogen) and then incubating at 42C for 2 hr. Gene expression was quantitated by real-time PCR: analyses performed in triplicate in 25 μL volumes using an ABI7500 thermal cycler. For each tube, 12.5 μL ABI TaqMan 2x Mastermix (polymerase and dNTPs), 1.25 μL probe mix (ABI), 9.25 μL DEPC-H20, and 2 μL template (typically diluted 1:3 from cDNA synthesis volume) were added. TdT and β-actin primers were designed using ABI Primer Express software and synthesized using a probe containing FAM6 and BHQ1. ABI software was used to quantify/calculate Ct values and determine relative gene expression levels, standardizing using β-actin values.
Results
VH11Vκ9+ B cells are enriched in B cell populations not derived from the bone marrow
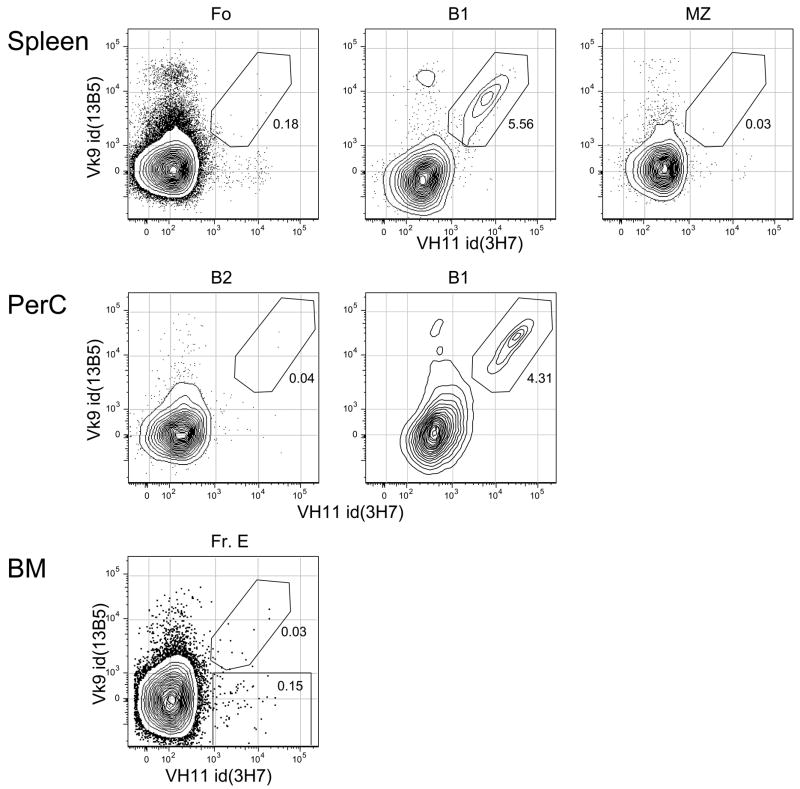
We analyzed the expression of VH11 and Vκ9 in different populations of B cells by staining with anti-idiotype antibodies mice and performing flow cytometry, as shown in Figure 1. Cells simultaneously expressing both VH11 and Vκ9 were found only with B-1 type B cells, both in spleen and peritoneal cavity. VH11+Vκ9− cells were rare in any B cell population in either of these locations. Bone marrow newly-formed B cells (labeled “Fr. E” in the figure) contained some VH11+Vκ9− B cells, but 10-fold fewer than might be expected, based on the genomic representation of 1–2 VH11 genes [9].
Figure 1.
Single cell suspensions were stained with a combination of fluorescent reagents, FL-anti-VH11id(3H7), PE-anti-CD21/35(7G6), Cy5PE-anti-Vκ9id(13B5), Cy55PE-anti-CD93(AA4.1), APC-anti-CD5(53-7), Cy55APC-anti-CD19(1D3), Cy7APC-anti-CD23(B3B4), CasB-anti-IgM(331.12), QDot605-anti-CD43(S7). Cells were stained with propidium iodide to allow elimination of dead cells (PI+). Forward light scatter height versus area gating was used to eliminate aggregates and forward versus side scatter gating was used as a “lymphoid cell” gate to eliminate small debris and large granular non-lymphoid cells. In spleen, Fo cells were gated CD19+IgM+CD93−CD23+; B1 cells were gated CD19+IgM+CD93−CD23−CD43+CD5+; MZ cells were gated CD19+IgM++CD93−CD23−CD21/35++. In peritoneal cavity (PerC), B-2 cells were gated CD19+IgM+CD43−CD5−; B-1 cells were gated CD19+IgM+CD43+CD5+. In bone marrow, newly-formed B cells (Fr. E) were gated CD19+IgM+CD93+.
VH11Vκ9+ B1 cells are enriched in the peritoneal cavity, compared to the spleen
The analysis described above also allowed us to determine the absolute numbers of VH11Vκ9 B cells in spleen and peritoneal cavity, revealing that there were 3–4 fold more VH11 anti-PtC B-1 cells in the peritoneal cavity (Table 1). This finding reinforces the idea that distinct microenvironments may favor the selection or persistence of B-cell populations with particular antigenic specificities.
Table 1.
Absolute numbers of V11+ B cells detected in various B cell subpopulations.
| B cell Subpopulation | Cell number | Std. Err. |
|---|---|---|
| BM Fr. E VH11+ | 967 | 238 |
| Spl Fo VH11+Vκ9+ | 2105 | 593 |
| Spl MZ VH11+Vκ9+ | 176 | 42 |
| Spl B-1 VH11+Vκ9+ | 51,455 | 4,856 |
| PerC B-1 VH11+Vκ9+ | 177,635 | 20,375 |
| PerC B-2 VH11+Vκ9+ | 85 | 34 |
Cell numbers determined by flow cytometry analysis of 4 adult (2–3 month old) BALB/c mice. Cells analyzed as described in Figure 1.
Production of VH11+ B-cells declines with age in a VH11 transgenic mouse
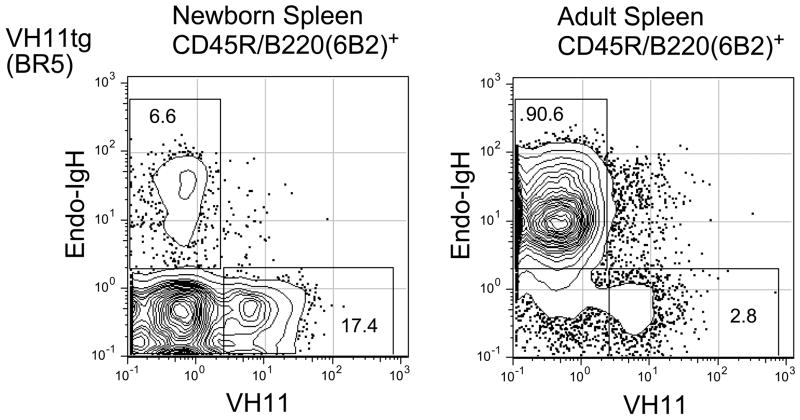
Flow cytometric analysis of surface heavy chain expression in spleen cells from newborn and adult VH11Tg mice (BR5, low copy number VH11 line) is shown in Figure 2. This analysis compares expression of transgenic (VH11+) and non-transgenic (endo-IgH+) BCRs on B lineage cells and shows that, whereas the majority (>70%) of B cells express VH11 in spleen of newborn animals, very few VH11+ B cells (3%) are found in the B cell pool in spleen of adult animals. This is a consequence of transgene expressing B cells failing to suppress rearrangement of the endogenous IgH locus during bone marrow B cell development, and then selection of such cells by pre-BCR signaling. An important point is that most B cells expressing endogenous IgH do not also co-express VH11, even though the transgene is not deleted by endogenous rearrangement. This might indicate the failure of VH11 to associate with the light chain rearranged in these B cells, relative to the heavy chain produced by rearrangement of the endogenous IgH locus.
Figure 2.
B lineage cells in VH11 Transgenic (BR5 line) spleen from newborn and adult animals. Single cell suspensions were stained with fluorescent antibodies specific for CD45R/B220(6B2), VH11id, and endogenous IgM (b allotype). Panels show B220+ gated cells.
Changes in TdT expression in transduced pro-B cells indicates weak pre-BCR signaling by VH11-μ and stronger signaling by VH11-μ complexed with Vκ9 light chain
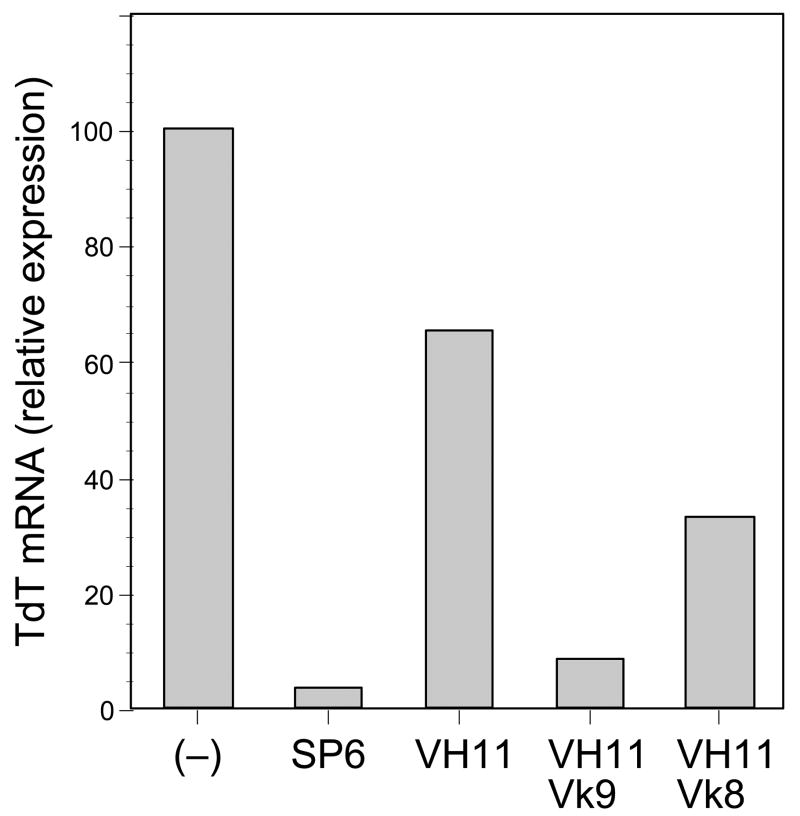
Quantitative real-time PCR analysis was performed with mRNA prepared from a pro-B (IgH−, IgL−) cell line, Ret02, transduced with the VH11-μ transgene construct, following retroviral provision of light chains (Figure 3). This cell line showed no cytoplasmic μ expression by staining analysis (data not shown). A control heavy chain, SP6, known to associate effectively with SLC, was transfected into the pro-B line as a pre-BCR positive control and the parental Ret02 served as a pre-BCR negative control, which we arbitrarily set to 100% TdT mRNA expression in this analysis [37]. The VH11 heavy chain transfected cell line showed only a modest decrease in TdT expression compared to the parental line (approximately 35%). However, introduction of the Vκ9 light chain induced a substantial drop in TdT expression. This result suggests that VH11 pairs more effectively with Vκ9 than with SLC, as signaling by either a pre-BCR or a BCR can lead to down-regulation of TdT mRNA. Interestingly, when a different, B2-derived Vκ8 light chain was introduced into the VH11 cell line, TdT mRNA expression was approximately 4-fold higher than with the VH11Vκ9 pairing. This may indicate that Vκ9 is a better “fit” for VH11 than Vκ8, such that Vκ8 lacks the capacity to associate effectively with VH11 to produce a stable BCR. That is, without effective pairing and effective transduction of pre-BCR/BCR signal, these cells cannot effectively down-regulate and extinguish this early B-cell developmental marker.
Figure 3.
Levels of TdT mRNA in transduced cell lines, assayed by quantitative PCR. Transduced cells were purified by sorting GFP+ cells, then RNA was prepared and analyzed as described in the Methods. Maximum levels, detected in the parental R1 line were arbitrarily set to 100%.
Variable efficiency of VH11 heavy chain association with diverse light chain
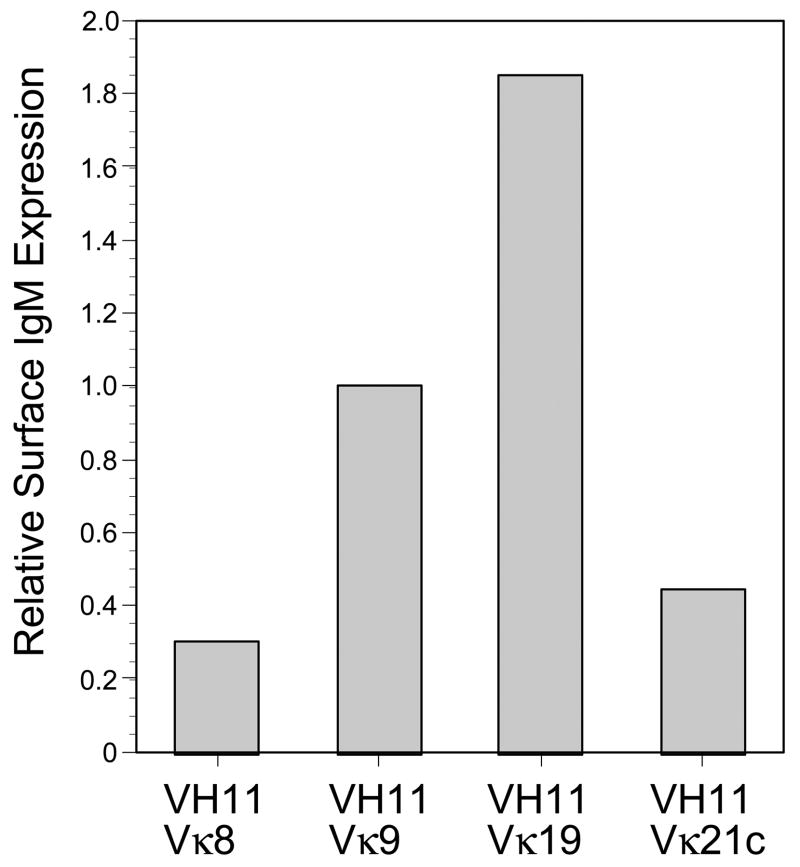
Finally, we sought to compare the effectiveness of VH11 pairing with four different light chains by measuring the expression of assembled Ig on the surface of pro-B cells simultaneously transduced with VH11 and each light chain. The N38 Abelson pro-B cell line (verified to be μ–by cytoplasmic staining, data not shown) was transduced with VH11μ-IRES-CFP and LC-IRES-GFP, cells were cultured overnight, and then surface IgM expression was determined by flow cytometry. Surface expression was assessed as a ratio of surface IgM staining to GFP expression in order to correct for variation in levels of retroviral transduction from one experiment to the next. Vκ9 light chain was used as a control, set to 1.0, as we assume that it can pair effectively with VH11. Subsequent experiments paired VH11μ with Vκ8, Vκ19, and Vκ21c light chains. Vκ8 was selected as a B2-subset typical light chain. Vκ19 and Vκ21c light chains were chosen due to their presence being detected on B1-subset cells from previous experiments. As shown in Figure 4, the pairing of VH11 heavy chain with Vκ8 light chain was not efficient, with surface expression approximately 75% less than the control VH11Vκ9 pairing (Figure 5). Interestingly, however, the VH11Vκ19 pairing exhibited a higher than expected surface expression, almost twice as high as the VH11Vκ9 pairing. This may indicate that this light chain is more efficient at pairing with the VH11 heavy chain than Vκ9. Finally, the Vκ21c light chain exhibited a surface expression pattern similar to that seen with the VH11Vκ8 pairing. Thus we found two light chains that paired less well than Vκ9 with VH11-μ, but, unexpectedly, one that was more effective.
Figure 4.
Cell surface IgM expression on pre-B cells transduced with VH11 and several different light chains. Transduced cells were stained with APC-anti-IgM(331.12) and then gated for VH11 (CFP+) and kappa light chain (GFP+) expression. Fluorescence intensity determined by flow cytometry, normalized for transduction level variation as described in the text. Level on Vκ9 cells arbitrarily set to 1.0.
Figure 5.
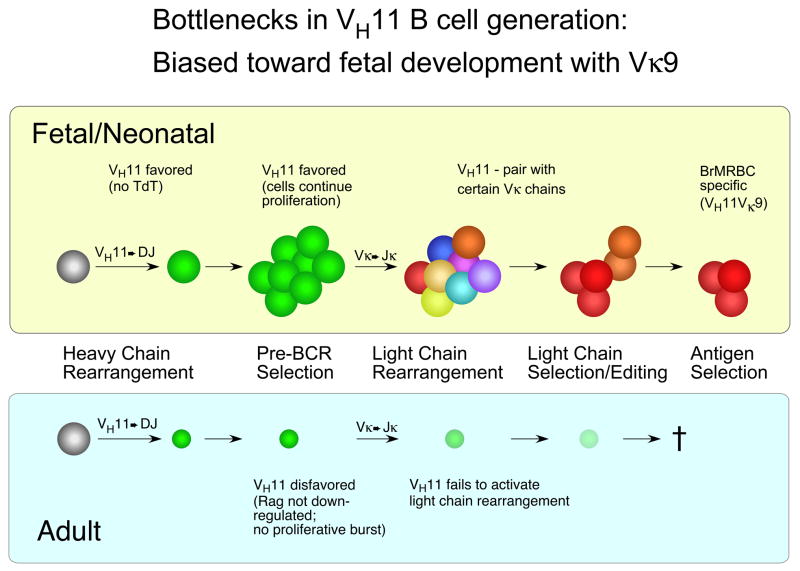
Model for VH11Vκ9 natural autoantibody B cell generation, showing the two bottlenecks discussed in the text and illustrating a fetal bias in production.
Discussion
B-1 B cells do not conform to the conventional model of B cell development. Rather than a diverse repertoire of BCRs, where selection of B cells with somatically hypermutated combining site BCRs in the germinal center reaction can generate antibody and memory B cells of high affinity to a vast diversity of potential antigenic determinants, B-1 cells instead express a restricted and predominantly germline-encoded repertoire. This divergence appears partly due to the distinctive restricted timing of much of B-1 B cell development to the fetal/neonatal period, when pre-B selection, mediated through the pre-BCR, exhibits a different response to strong signaling, favoring different heavy chains compared to conventional development ongoing in bone marrow of adult mice. The production of B cells with VH11 BCRs, as a significant component of the natural autoantibody B cell pool, typifies this B-1 development.
First, the progression of B cells with VH11 rearrangements is clearly restricted to entry into the B-1 B cell pool, as shown by anti-idiotype staining (Figure 1). It is striking that most VH11+ B cells also co-express the Vκ9 light chain and so exhibit specificity for the senescent red blood cell determinant revealed by proteolytic treatment with bromelain (“anti-BrMRBC”). This is not dependent on the distinctive peritoneal microenvironment, as VH11+Vκ9+ B cells are found in both the spleen and peritoneal cavity. Significantly, examination of the newly formed B cells in bone marrow show an under-representation of B cells with surface expression of VH11, likely of about 10-fold. We do observe a greater absolute number of VH11+Vκ9+ B cells in the peritoneal cavity compared to the spleen (Figure 2), so it is possible that the B-1 pool in the spleen actually enters the spleen from an initial population generated in the peritoneal cavity.
Examination of VH11 expression in a VH11-μ heavy chain transgenic mouse, the BR5 line, provides a clue as to the distinctive origin of most VH11Vκ9 B cells. As shown in Figure 3, most (>90%) of the B cells in spleen of adult animals do not express the transgene on the cell surface, but instead bear a heavy chain produced by rearrangement of the endogenous IgH locus. However, the B cell pool in newborn BR5 mice shows a rather different distribution, with over 70% of the B cells expressing VH11. We hypothesize that this may be due to ineffective pairing of the transgene heavy chain with surrogate light chain (SLC), so that such cells are handicapped in bone marrow development, responsible for most B cells in the spleen of adult mice. The failure to mediate conventional pre-BCR signaling effectively would allow recombinase activating (RAG1/2) gene expression and rearrangement of the endogenous IgH loci during the stages in B cell development where chromatin is in an open configuration. Such rearrangement, if productive, can generate a heavy chain with the capability to pair effectively with SLC, thereby signaling further development and eventually contributing to the mature B cell pool in spleen.
The effectiveness of VH11-μ pre-BCR signaling can be assessed using a model system that we described previously [37]. In the experiment presented here, we introduced heavy chains into a pro-B cell line that lacks heavy or light chain expression by retroviral transduction. By using a retrovirus where the heavy chain gene is linked to a fluorescent reporter (eGFP) by an IRES, it is possible to purify heavy chain expressing cells rapidly after transduction using electronic cell sorting. This experiment showed a very significant (about 20-fold) decrease in pre-existing TdT levels as a consequence of introducing a heavy chain capable of effective pairing with SLC (Figure 4). Importantly, the comparable experiment using a VH11-μ heavy chain shows a less than 2-fold decrease, consistent with its poor capacity to assemble with SLC and thereby only weakly mediate one of the normal features of pre-B cell progression, down-regulation of TdT. Thus, it is not unexpected that most of the B cells in a VH11 transgenic mouse will express endogenous heavy chain.
However, what is surprising is the relative paucity of B cells expressing both VH11 and endogenous heavy chain (Figure 3). We speculated that this might indicate a preference of VH11 heavy chain for specific light chains that are only infrequently found in bone marrow selected B cells. One way to test this idea was to co-transduce light chain together with VH11 heavy chain into the pro-B cell line and then measure the decrease in TdT, mediated by BCR, rather than pre-BCR signaling. Indeed, co-transduction of a Vκ9 light chain, cloned from a B-1 derived hybridoma secreting natural autoantibody binding to senescent red blood cells, resulted in a very strong down-regulation of TdT level, about 7-fold compared to VH11 alone. Interestingly, co-transduction of a different kappa light chain, Vκ8, cloned from an anti-influenza hybridoma (38) and likely derived from a B-2 type B cell, did not result in as significant a decrease in TdT levels, only about 2-fold compared to VH11 alone. This poor down-regulation implies formation of a less than ideal BCR, providing some evidence supporting the hypothesis that VH11 does not pair equally well with all light chains.
Thus, in addition to the pre-BCR selection bottleneck in bone marrow, that restricts much of VH11 B cell generation to fetal B-1 type development, it seems likely that a second bottleneck, requirement for distinctive light chains, further biases development of VH11 B cells. We tested this by directly assessing the efficiency of VH11-μ to assemble with different light chains, co-transducing VH11-μ together with several different light chains, then staining the resulting cells for expression of IgM on the cell surface. We reasoned that poor assembly would result in low levels of surface IgM, and more efficient assembly would result in progressively higher levels. We included Vκ9 as a positive control of a light chain capable of mediating effective VH11 B cell development. As predicted by our model, we found two light chains, Vκ8 and Vκ21c, that resulted in lower level IgM expression, compared with Vκ9. However, unexpectedly, we also identified a light chain, Vκ19, that mediated significantly higher expression compared with Vκ9. One possible explanation for the relatively rare occurrence of VH11Vk19 B cells normally is that this level of BCR expression may not be tolerated by fetal B-1 development, leading to their deletion. This possibility could be tested by retroviral transduction of normal pro-B cells, using cells from fetal liver and bone marrow of RAG-deficient mice, then monitoring the fate of such cells in vitro and in vivo.
The combination of these two bottlenecks, and their overall effect on the development of VH11Vκ9+ B-1 B cells, is depicted in Figure 6. Notably, the first bottleneck of inefficient SLC pairing with VH11 heavy chain would only occur later, within adult bone marrow development. Here, the failure to form an effective pre-BCR due to weak assembly of VH11-μ with SLC would halt development prior to the proliferative burst, with RAG expression continuing. The result could be recombination on the other IgH allele which, if productive and encoding a heavy chain capable of assembling with SLC, might rescue the VH11-containing cell. Subsequent light chain rearrangement and expression would likely result in a B cell with only the non-VH11 μ chain assembled with light chain on the cell surface, similar to the endogenous-only B cells dominating the B cell pool in the spleen of adult BR5 transgenic mice.
The second proposed bottleneck, restricted pairing of VH11 heavy chain with highly specific light chains, would take place earlier, during fetal liver B1 ontogeny. Here, VH11 rearrangements would be favored and such cells would continue their proliferation as a result of not forming a strongly-signaling pre-BCR. Cells would progress to rearranging light chain genes, and then pairing with VH11 heavy chain would be tested. Here, the VH11 heavy chain would screen the light chains expressed for the few that meet its stringent requirements, signaling with sufficient, but not excessive, strength. We suggest that Vk9 is one of a relatively small number of light chains with this property. This would result in the eventual formation of the B1 repertoire, where the VH11Vκ9 natural autoantibody is well represented and subject to positive selection by self-antigen. This final requirement, selection by antigen, is important to note, as experiments with another B-1 derived BCR, anti-thymocyte autoantibody, has demonstrated the key role of antigen-dependent selection in B-1 B cell development [39].
While this model of selection by light chain pairing is intriguing, more work needs to be done with additional light chains to determine how widespread this inability is. Double transgenic animals, expressing VH11 and different light chains, could also be constructed. This would allow an in vivo examination of the concept, both in the fetal liver and bone marrow environments. It would also be interesting to attempt to alter the amino acid sequence of key sites in the prototypic Vκ9 light chain. By pairing these altered light chains with the VH11 heavy chain and observing TdT expression and/or relative surface IgM levels, one could ascertain how critical light chain structure is for assembly with VH11-μ heavy chain. This information could be used to further pinpoint and delineate the specifics of the interactions between the two polypeptide chains in this prototypic configuration.
These findings illustrate an interesting dichotomy of B-cell receptor repertoire selection that is dependent upon developmental stage of and/or cellular microenvironment. If the first round of B-cell development results in a very limited range of BCR molecules, it is logical to ask what purpose these receptors might serve, and why a shift towards a more diverse population is observed as a mouse matures. Possibly, the purpose of these receptors may be gleaned from their specificity. This predisposition towards specific rearrangements may be a relic of a time prior to “modern” Ig gene rearrangement and BCR diversity. This has been described previously as a “layered” immune system, where more complex system are “layered” on more primitive ones [40].
Another point to consider it that the maternal immune system is providing passive protection and antibodies for the fetus. The fetus is essentially in an immune-privileged site, largely sequestered from environmental antigenic exposure. The shift to a diverse repertoire, therefore, could be explained by the necessity for a more capable and comprehensive immune response to an antigen-rich environment after birth than was possible with an initial ‘hard-wired’ and limited receptor system. This system, intermediate between innate and adaptive immunity – hard-wired by rearrangement-dependent “memory” selection into the germline over evolutionary time, more “primitive” than the adaptive bone marrow B cell development system, entails constraints on the peripheral population as well. These B cells cannot be afforded the same flexibility of affinity maturation afforded to bone marrow generated (B-2) B cells, due to possible selection for self-reactivity (which would pose an inherent danger of autoimmunity). Nevertheless, there is an inherent potential in any autoreactive cell populations for development of expanded clones that could become targets for transformation events. If this is the case, then B-1 cells could be the founding cell population of leukemic malignancies (i.e, B-CLLs). Remarkably, despite such a potential negative consequence, this system has been maintained as a component of the immune system over the evolutionary period spanning mice and humans. This testifies to its inherent utility over the millennia, outweighing any potential risks, maintaining a niche in the immune system for fetal B-1 B cells.
The data presented herein should be compared and contrasted with other and related issues in this special volume which deals with natural autoantibodies, loss of tolerance and the production of autoantibodies [41–54].
Table 2.
Primers used for Q-PCR.
| forward | reverse | probe | |
|---|---|---|---|
| β-actin | CACCGAGGCCCCCCT | CAGCCTGGATGGCTACGTACA | AACCCTAAGGCCAACCGTGAAAAGATGA |
| TdT | CTATGAGTGCGCCTGTGCC | GCCGAAGGTCTCTCTCAAACTG | TGCTTGGATGGACCGGCTCCA |
Primer pair/specific oligonucleotide probes used in quantitative PCR. Probes labeled with 6FAM and BHQ1.
Acknowledgments
We thank J. Oesterling for technical assistance with flow cytometry and A. Cywinski for help with sequence analysis. This work was supported by grants from the National Institutes of Health (AI 26782, AI 40946) to R. Hardy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci. 1984;81:2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayakawa K, Carmack CE, Hyman R, Hardy RR. Natural autoantibodies to thymocytes: origin, VH genes, fine specificities, and the role of Thy-1 glycoprotein. J Exp Med. 1990;172:869–878. doi: 10.1084/jem.172.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Advances in Immunology. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 4.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 6.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline. Relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J Exp Med. 1988;168:687–698. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmack CE, Shinton SA, Hayakawa K, Hardy RR. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reininger L, Kaushik A, Izui S, Jaton JC. A member of a new VH gene family encodes antibromelinized mouse red blood cell autoantibodies. Eur J Immunol. 1988;18:1521–1526. doi: 10.1002/eji.1830181008. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Carmack CE, Shinton SA, Riblet RJ, Hayakawa K. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J Immunol. 1989;142:3643–3651. [PubMed] [Google Scholar]
- 10.Pennell CA, Arnold LW, Haughton G, Clarke SH. Restricted Ig variable region gene expression among Ly-1+ B cell lymphomas. J Immunol. 1988;141:2788–2796. [PubMed] [Google Scholar]
- 11.Pennell CA, Mercolino TJ, Grdina TA, Arnold LW, Haughton G, Clarke SH. Biased immunoglobulin variable region gene expression by Ly-1 B cells due to clonal selection. Eur J Immunol. 1989;19:1289–1295. doi: 10.1002/eji.1830190721. [DOI] [PubMed] [Google Scholar]
- 12.Mercolino TJ, Locke AL, Afshari A, Sasser D, Travis WW, Arnold LW, Haughton G. Restricted immunoglobulin variable region gene usage by normal Ly-1 (CD5+) B cells that recognize phosphatidyl choline. J Exp Med. 1989;169:1869–1877. doi: 10.1084/jem.169.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986;16:450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa K, Hardy RR, Stall AM, Herzenberg LA, Herzenberg LA. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B Cells are Distinct from Progenitors for Other B Cells. The Journal of Experimental Medicine. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991;88:11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantor AB, Merrill CE, Herzenberg LA, Hillson JL. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol. 1997;158:1175–1186. [PubMed] [Google Scholar]
- 18.Landau NR, Schatz DG, Rosa M, Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987;7:3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y-S, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster I, Gu H, Rajewsky K. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. Embo J. 1988;7:3693–3703. doi: 10.1002/j.1460-2075.1988.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haughton G, Arnold LW, Whitmore AC, Clarke SH. B-1 cells are made, not born. Immunology Today. 1993;14:84–87. doi: 10.1016/0167-5699(93)90064-R. discussion 87–91. [DOI] [PubMed] [Google Scholar]
- 22.Wasserman R, Li YS, Shinton SA, Carmack CE, Manser T, Wiest DL, Hayakawa K, Hardy RR. A novel mechanism for B cell repertoire maturation based on response by B cell precursors to pre-B receptor assembly. J Exp Med. 1998;187:259–264. doi: 10.1084/jem.187.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melchers F, ten Boekel E, Seidl T, Kong XC, Yamagami T, Onishi K, Shimizu T, Rolink AG, Andersson J. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- 24.Melchers F. Fit for life in the immune system? Surrogate L chain tests H chains that test L chains. Proc Natl Acad Sci U S A. 1999;96:2571–2573. doi: 10.1073/pnas.96.6.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blank M, Shoenfeld Y. B cell targeted therapy in autoimmunity. J Autoimmun. 2007;28:62–68. doi: 10.1016/j.jaut.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Broker BM, Klajman A, Youinou P, Jouquan J, Worman CP, Murphy J, Mackenzie L, Quartey-Papafio R, Blaschek M, Collins P, et al. Chronic lymphocytic leukemic (CLL) cells secrete multispecific autoantibodies. J Autoimmun. 1988;1:469–481. doi: 10.1016/0896-8411(88)90068-6. [DOI] [PubMed] [Google Scholar]
- 27.Cabiedes J, Cabral AR, Lopez-Mendoza AT, Cordero-Esperon HA, Huerta MT, Alarcon-Segovia D. Characterization of anti-phosphatidylcholine polyreactive natural autoantibodies from normal human subjects. J Autoimmun. 2002;18:181–190. doi: 10.1006/jaut.2001.0575. [DOI] [PubMed] [Google Scholar]
- 28.Cabral AR, Cabiedes J, Alarcon-Segovia D. Hemolytic anemia related to an IgM autoantibody to phosphatidylcholine that binds in vitro to stored and to bromelain-treated human erythrocytes. J Autoimmun. 1990;3:773–787. doi: 10.1016/s0896-8411(05)80043-5. [DOI] [PubMed] [Google Scholar]
- 29.Hentati B, Payelle-Brogard B, Jouanne C, Avrameas S, Ternynck T. Natural autoantibodies are involved in the haemolytic anaemia of NZB mice. J Autoimmun. 1994;7:425–439. doi: 10.1006/jaut.1994.1031. [DOI] [PubMed] [Google Scholar]
- 30.Kaushik A, Mayer R, Fidanza V, Zaghouani H, Lim A, Bona C, Dighiero G. Ly1 and V-gene expression among hybridomas secreting natural autoantibody. J Autoimmun. 1990;3:687–700. doi: 10.1016/s0896-8411(05)80036-8. [DOI] [PubMed] [Google Scholar]
- 31.Levine JS, Subang R, Koh JS, Rauch J. Induction of anti-phospholipid autoantibodies by beta2-glycoprotein I bound to apoptotic thymocytes. J Autoimmun. 1998;11:413–424. doi: 10.1006/jaut.1998.0235. [DOI] [PubMed] [Google Scholar]
- 32.Plater-Zyberk C, Brennan FM, Feldmann M, Maini RN. ‘Fetal-type’ B and T lymphocytes in rheumatoid arthritis and primary Sjogren’s syndrome. J Autoimmun. 1989;2(Suppl):233–241. doi: 10.1016/0896-8411(89)90135-2. [DOI] [PubMed] [Google Scholar]
- 33.Youinou P. B cell conducts the lymphocyte orchestra. J Autoimmun. 2007;28:143–151. doi: 10.1016/j.jaut.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Youinou P, Mackenzie L, le Masson G, Papadopoulos NM, Jouquan J, Pennec YL, Angelidis P, Katsikis P, Moutsopoulos HM, Lydyard PM. CD5-expressing B lymphocytes in the blood and salivary glands of patients with primary Sjogren’s syndrome. J Autoimmun. 1988;1:185–194. doi: 10.1016/0896-8411(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa K, Asano M, Shinton SA, Gui M, Wen LJ, Dashoff J, Hardy RR. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J Exp Med. 2003;197:87–99. doi: 10.1084/jem.20021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527–535. doi: 10.1016/s1074-7613(00)80268-x. [DOI] [PubMed] [Google Scholar]
- 37.Wasserman R, Li YS, Hardy RR. Down-regulation of terminal deoxynucleotidyl transferase by Ig heavy chain in B lineage cells. J Immunol. 1997;158:1133–1138. [PubMed] [Google Scholar]
- 38.Carmack CE, Camper SA, Mackle JJ, Gerhard WU, Weigert MG. Influence of a V kappa 8 L chain transgene on endogenous rearrangements and the immune response to the HA(Sb) determinant on influenza virus. J Immunol. 1991;147:2024–2033. [PubMed] [Google Scholar]
- 39.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 40.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 41.Avrameas S, Ternynck T, Tsonis I, Lymperi P. The immune system as emerges from studies on natural autoantibodies. J Autoimmun. 2007 in press. [Google Scholar]
- 42.Cohen I. Biomarkers, self-antigens and the immunological homunculus. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.016. in press. [DOI] [PubMed] [Google Scholar]
- 43.Lan R, Mackay I, Gershwin M. Regulatory T cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang K, Burow A, Kurrer M, Lang P, Recher M. Balance of the innate immune response in autoimmune disease. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.018. in press. [DOI] [PubMed] [Google Scholar]
- 45.Lutz H. Homeostatic roles of naturally occurring antibodies. An overview. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.007. in press. [DOI] [PubMed] [Google Scholar]
- 46.Milner J, Ward J, Keane-Myers A, Min B, Paul W. Repertoire-dependent immunopathology. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papadimitraki E, Bertsias G, Boumpas D. Toll like receptors and autoimmunity: A critical appraisal. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.09.001. in press. [DOI] [PubMed] [Google Scholar]
- 48.Pasquali J-L, Soulas-Sprauel P, Korganow A-S, Martin T. Auto-reactive B cells in transgenic mice. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.006. in press. [DOI] [PubMed] [Google Scholar]
- 49.Peng Y, Martin D, Kenkel J, Zhang K, Ogden C, Elkon K. Innate and adaptive immune response to apoptotic cells. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz-Argüelles A, Brito G, Reyes-Izquierdo P, Pérez-Romano B, Sánchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.012. in press. [DOI] [PubMed] [Google Scholar]
- 51.Ryan K, Patel S, Stephens L, Anderton S. Death, adaptation and regulation: the three pillars of immune tolerance restrict the risk of autoimmune disease caused by molecular mimicry. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.014. in press. [DOI] [PubMed] [Google Scholar]
- 52.Vollmers H, Brändlein S. Natural antibodies and cancer. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.013. in press. [DOI] [PubMed] [Google Scholar]
- 53.Zelenay S, Fontes M, Fesel C, Demengeot J, Coutinho A. Physiopathology of natural auto-antibodies: The case for regulation. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.011. in press. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Z-H, Tzioufas A, Notkins A. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun. 2007 doi: 10.1016/j.jaut.2007.07.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]