SUMMARY
We have analyzed the molecular basis of sugar reception in Drosophila. We define the response spectrum, concentration dependence, and temporal dynamics of sugar-sensing neurons. Using in situ hybridization and reporter gene expression we identify members of the Gr5a-related taste receptor subfamily that are co-expressed in sugar neurons. Neurons expressing different Gr5a-related genes send overlapping but distinct projections to the brain and thoracic ganglia. Genetic analysis of receptor genes shows that Gr5a is required for response to one subset of sugars and Gr64a for response to a complementary subset. A Gr5a;Gr64a double mutant shows no physiological or behavioral responses to any tested sugar. The simplest interpretation of our results is that Gr5a and Gr64a are each capable of functioning independently of each other within individual sugar neurons and that they are the primary receptors used in the labellum to detect sugars.
INTRODUCTION
A major problem in neurobiology is how an animal decides what to eat. The fruit fly Drosophila evaluates gustatory input to assess the nutritive value of a potential food source. In particular, the detection of sugars is a crucial factor in determining whether a food source is accepted. Despite its critical importance to the survival of the species, little is known about the molecular basis of sugar perception in the fly. A central goal in the field has been to define the receptors that mediate sugar detection.
Sugars, salts, bitter compounds, and certain other molecules are detected by gustatory neurons, which are widely distributed in the body of the fly (Stocker, 1994; Vosshall and Stocker, 2007). Neurons that influence feeding behavior are present in the labellum as well as the tarsal segments of each of the legs. Activation of either labellar or tarsal gustatory neurons with a sugar solution results in proboscis extension, which is a component of feeding behavior (Dethier, 1976; Rodrigues and Siddiqi, 1978).
Gustatory neurons are housed in sensory hairs called sensilla (Falk et al., 1976; Nayak and Singh, 1983; Stocker, 1994). Each half of the labellum is covered with ~31 prominent taste hairs, arranged in a stereotypical pattern, and a number of smaller structures called taste pegs (Falk et al., 1976; Ray et al., 1993; Shanbhag et al., 2001). Each of the 31 sensilla is typically innervated by four gustatory neurons and a single mechanosensory neuron. Physiological analysis has shown that one of the chemosensory neurons is activated by sucrose and other sugars, and has been referred to as the “sugar” neuron (Rodrigues and Siddiqi, 1978; Fujishiro et al., 1984; Wieczorek and Wolff, 1989). Another neuron is activated by salts and has been named the “salt” neuron (Rodrigues and Siddiqi, 1978; Fujishiro et al., 1984). A third neuron is activated by pure water but not by solutions of high osmolarity; it has been named the “water” neuron (Rodrigues and Siddiqi, 1978; Fujishiro et al., 1984). The fourth chemosensory neuron responds to aversive compounds such as caffeine, and has been named the “bitter” neuron (Meunier et al., 2003).
In Drosophila, a large, highly diverse family of gustatory receptor (Gr) genes was identified by genomic analysis (Clyne et al., 2000). The family consists of 60 genes encoding 68 predicted seven-transmembrane-domain receptors (Robertson et al., 2003). In previous studies we and others identified one of these receptors, Gr5a, as a receptor for trehalose, a disaccharide sugar (Dahanukar et al., 2001; Ueno et al., 2001; Chyb et al., 2003). Gr5a is expressed in a large number of gustatory neurons in the labellum (Chyb et al., 2003), and recent studies have shown that Gr5a serves as a marker for the sugar neuron in each sensillum (Marella et al., 2006). Bitter neurons express Gr66a (Thorne et al., 2004; Wang et al., 2004), also a member of the Gr gene family, which is required for physiological and behavioral responses to caffeine (Moon et al., 2006). Promoter expression analysis of several other gustatory receptor genes in the labellum suggested that all of those tested were co-expressed with Gr66a in subsets of bitter neurons (Thorne et al., 2004; Wang et al., 2004).
Axonal projections of Gr5a-positive and Gr66a-positive neurons have been mapped to the sub-esophageal ganglion (SOG) of the brain (Thorne et al., 2004; Wang et al., 2004). The two classes of neurons project to non-overlapping regions in the SOG, suggesting that at the first level of processing, attractive and aversive inputs may be segregated. Evidence that Gr5a neurons mediate attractive signals and Gr66a neurons mediate aversive signals was provided by expression of a capsaicin receptor in each of these classes of neurons (Marella et al., 2006). In the first instance, flies showed behavioral attraction to capsaicin and in the second instance they were repelled by it.
Gr5a-labeled neurons are responsive not only to trehalose, but to sucrose and other sugars (Wang et al., 2004; Marella et al., 2006). Physiological and behavioral analysis showed that sucrose response is not affected in flies lacking Gr5a (Dahanukar et al., 2001), suggesting that these neurons express at least one other receptor; however, other receptors in sugar neurons were not identified.
Here we examine the responses of sugar neurons in the largest sensilla of the labellum, the “L” sensilla. Of 50 compounds tested, including 34 diverse sugars, we identify a small number, primarily disaccharides and oligosaccharides, which elicit robust electrophysiological responses in sugar neurons. We determine by in situ hybridization and reporter gene expression that two other Gr genes, both phylogenetically related to Gr5a, are co-expressed with Gr5a in sugar neurons. Projection patterns of the neurons expressing each reporter gene are distinct, providing a mechanism by which information from different sub-populations of sugar cells in the periphery could be spatially represented in the brain.
Having found co-expression of Gr5a-related genes in sugar neurons, we examine mutants of Gr5a and two related genes by electrophysiology and behavioral analysis. We find that Gr5a is required for detection of a small subset of sugars including trehalose. We generate deletion mutants lacking Gr64a and find that it is required for response to a complementary subset of sugars. Strikingly, flies lacking both Gr5a and Gr64a do not show electrophysiological or behavioral responses to any tested sugar. These results demonstrate that the sugars divide into two classes that are dependent either on Gr5a or on Gr64a for their responses. The simplest interpretation of our results is that these two receptors are capable of operating independently of each other in an individual sugar neuron, and that they constitute the primary basis of sugar reception in the fly.
RESULTS
Responses of sugar-sensitive neurons depend on sugar identity and intensity
To define the response spectrum of labellar sugar neurons, we examined the electrophysiological responses of individual labellar taste sensilla to a large panel of compounds. We chose a set of 50 tastants that include most naturally occurring monosaccharides, as well as a number of disaccharides, oligosaccharides, sugar alcohols, sugar acids, and nucleotides, as well as two proteins shown to evoke feeding behavior in vertebrates (Kim and Kinghorn, 2002). The compounds were chosen from chemical classes that are known to elicit physiological responses in the sugar-sensitive neuron (Wieczorek and Wolff, 1989), or that evoke an acceptance behavior response in Drosophila (Rodrigues and Siddiqi, 1978). We also included two nucleotides, because studies have shown that the sugar-sensitive neuron in larger flies responds to certain nucleotides (Furuyama et al., 1999). All compounds were tested at a concentration of 100 mM, with the exception of ethanol (25%), monellin (0.1%) and thaumatin (0.1%).
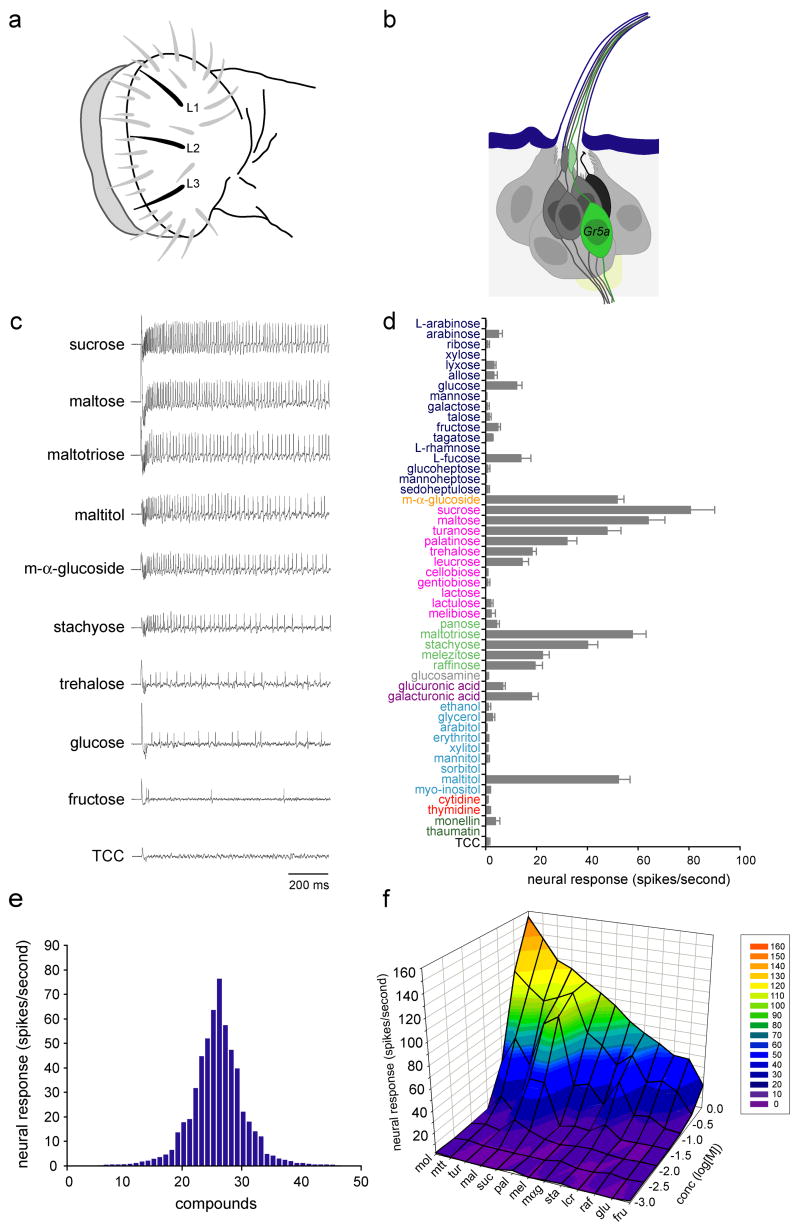
We tested L-type sensilla, of which there are three on each half of the labellum (Ray et al., 1993) (Figures 1a and 1b). These sensilla are highly stereotyped in their positions and can be recorded from conveniently. A diverse subset of sugars elicits responses from these sensilla, in each case from a single neuron (Figures 1c and S1). In order to confirm that the same neuron is activated by each sugar, we tested binary mixtures of sugars and found that in every case the activity of only a single neuron could be detected: all action potentials were of uniform amplitude, and no overlapping spikes, distinguishable by altered amplitude or irregular shape, were observed (Figure S1). As a control we tested a mixture of sucrose and sodium chloride, two stimuli that have been shown to activate different neurons in each sensillum (Fujishiro et al., 1984). This mixture elicited a response from two neurons as determined by the presence of actions potentials of two different amplitudes in the spike train (Figure S1). Thus all sugars appear to activate the same neuron.
Figure 1. Responses of sugar neurons.
(a) Labellar sensilla. The three L-type sensilla are in black. (b) An L-type sensillum, showing the sugar neuron (green), other gustatory neurons (dark gray), the mechanosensory neuron (black) and supporting cells (light gray). (c) Sample traces of recordings from L-type sensilla. A control trace is shown using the diluent, tri-choline citrate (TCC), alone. (d) Responses to a panel of 50 compounds. Sugars were D-isomers except as indicated. Chemicals are color coded: monosaccharides (dark blue), a glucoside (orange), disaccharides (pink), oligosaccharides (light green), glucosamine (gray), sugar acids (violet), alcohols (light blue), nucleotides (red), proteins (dark green), diluent control (black). All compounds were tested at a concentration of 100 mM except for ethanol (25% v/v), monellin (0.1% w/v), thaumatin (0.1% w/v) and TCC (30 mM). For all stimuli, 10≤n≤15. Error bars indicate SEM. (e) Tuning curve for L-type sensilla. The 50 stimuli are arranged along the X-axis according to the strengths of the responses that they elicit. Those that elicit the strongest responses are placed near the center, and those that elicit the weakest responses are placed near the edges. (f) Concentration-dependent responses to a panel of 13 sugars. n=6. Maltitol (mol), maltotriose (mtt), turanose (tur), maltose (mal), sucrose (suc), palatinose (pal), melizitose (mel), m-α-glucoside (mαg), stachyose (sta), leucrose (lcr), raffinose (raf), glucose (glu) fructose (fru).
The response spectrum of the sugar neuron in L-type sensilla is shown in Figure 1d, and a tuning curve is shown in Figure 1e. Of 50 compounds tested, 2 generated responses of ≥60 spikes per second, 4 generated responses of 40–60 spikes per second, 3 generated responses of 20–40 spikes per second, and an additional 6 generated responses of ~10–20 spikes per second. The distribution of responses varied among classes of sugars. Among the disaccharides (pink), sucrose and maltose elicited the strongest responses at this concentration: ≥60 spikes per second. Other disaccharides, turanose, palatinose, trehalose and leucrose, elicited moderate responses of ~15–50 spikes per second. Among oligosaccharides (light green), maltotriose and stachyose elicited responses of ≥40 spikes per second. All the disaccharides and oligosaccharides that elicited responses comprise units of glucose and/or fructose. Monosaccharides (dark blue), however, did not evoke very high responses. A glucoside, m-α-glucoside, elicited a response >50 spikes per second. Among alcohols (light blue), only maltitol, a sugar alcohol, elicited a strong response (52.2 ± 4.6 spikes per second; n=12). The nucleotides that we tested did not generate responses. We also tested 18 amino acids, none of which elicited responses above background levels (not shown). Each of the three L-type sensilla gave similar responses.
Of the 15 compounds that elicited responses of ≥10 spikes per second at 100 mM, we selected 12 to examine across a wide range of concentrations (Figure 1f). The structures of these sugars are shown in Figure S2. We also included an additional sugar, fructose, which did not elicit high responses at this concentration but is behaviorally attractive to flies (Rodrigues and Siddiqi, 1978). At lower concentrations only a few of the sugars elicited responses. Sucrose (suc), maltose (mal) and maltotriose (mtt) had the lowest response thresholds, eliciting responses at concentrations of 10 mM or less. At a concentration of 1 M, the highest concentration tested, the responses varied widely: maltitol (mol), maltotriose and turanose (tur) yielded the highest responses, whereas glucose and fructose yielded the lowest responses at this concentration. Moreover, the sugars that elicited the highest responses at a concentration of 1M, maltitol and maltotriose, were distinct from those that elicited the highest responses at a concentration of 100 mM, sucrose and maltose. Turanose showed a higher threshold than maltotriose or maltose, but also a steep concentration-dependence, as evident from the deep fold in the surface of the dose-response graph (Figure 1f).
In the case of sugar stimuli that elicited strong responses, such as sucrose (Figure 1c), neurons showed a high rate of initial firing, followed by a quick decay in firing rate over the course of 100 ms; the rate then showed a sustained period of firing that decreased in rate only gradually. The dynamics of the neural responses to sugars over the course of a 1 sec stimulation period are quantitated for 12 sugars across a range of concentrations in Figure S3.
Co-expression of Gr5a-related receptors in sugar neurons
One receptor, Gr5a, has been shown to be required in sugar neurons for response to trehalose (Dahanukar et al., 2001; Ueno et al., 2001). To identify the receptors that underlie the entire response spectrum of sugar neurons, we first sought to identify additional Gr genes that they express. The co-expression of a number of Gr genes has previously been analyzed using Gr promoter-GAL4 lines, but of 8 genes tested in this manner for co-expression with either Gr5a or Gr66a in the labellum, a receptor that is expressed in bitter neurons, all appeared to be co-expressed in subsets of bitter neurons, rather than in sugar neurons (Thorne et al., 2004; Wang et al., 2004).
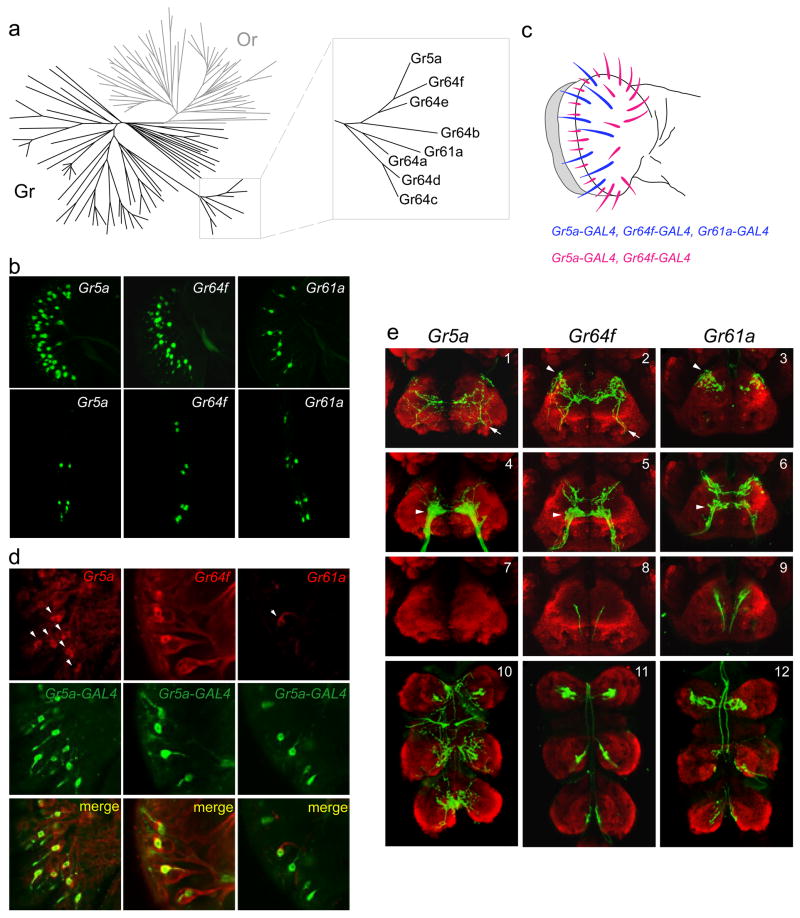
Phylogenetic analysis of the 68 gustatory receptors revealed that seven are most closely related to Gr5a in sequence, sharing 25% to 40% amino acid identity with Gr5a (Robertson et al., 2003) (Figure 2a). We successfully engineered Gr promoter-GAL4 lines for two of these Gr5a-related receptor genes and asked whether they were expressed in sugar neurons by comparing their expression patterns to that of Gr5a.
Figure 2. Expression of Gr5a-related receptors.
(a) Phylogenetic tree depicting the chemoreceptor superfamily in Drosophila, indicating odor receptors (Or) in gray and gustatory receptors (Gr) in black. Inset shows the Gr5a sub-family. Adapted from van der Goes van Naters and Carlson (2006). (b) GFP reporter expression in the labellum (top) or the three distal-most segments of forelegs (bottom), driven by Gr5a-GAL4 (left), Gr64f-GAL4 (center) or Gr61a-GAL4 (right). (c) A receptor-to-sensillum map of Gr5a, Gr64f and Gr61a based on the data represented in (b). (d) In situ hybridizations with probes against the indicated mRNAs (red) to labella of Gr5a-GAL4;UAS-GFP flies that were simultaneously stained for GFP (green). (e) Axonal projections of neurons labeled by the indicated drivers (green). The neuropil is stained with nc82 (red). Shown here are optical sections of anterior views of the SOG (1–9, dorsal is up) and the thoracic ganglion (10–12, anterior is up).
In the labellum, the Gr5a promoter drives expression in most if not all sensilla (Figure 2b, top left). We found that Gr64f-GAL4 is also expressed in most if not all labellar sensilla (Figure 2b, top center), in a pattern similar to that of Gr5a-GAL4. In contrast, Gr61a-GAL4 drives expression in only ~8–10 labellar sensilla, including the three L-type sensilla (Figure 2b, top right). In the legs, Gr5a-GAL4 is expressed in ~10 neurons in the foreleg (Figure 2b, bottom left), ~4 neurons in the midleg and ~4 neurons in the hindleg. Gr64f-GAL4 and Gr61a-GAL4 drivers labeled ~14 neurons in the foreleg and ~6 neurons each in the midleg and the hindleg, as if some leg neurons express Gr64f and Gr61a, but not Gr5a (Figure 2b, bottom panels, and data not shown).
To ask whether these Gr5a-related receptors are co-expressed in the same neuron within taste sensilla, we first carried out a double-label analysis with Gr66a, a receptor that is expressed in bitter taste neurons and that is required for response to caffeine (Moon et al., 2006). We generated a construct in which upstream regulatory sequences of Gr66a were fused to multiple copies of a RFP reporter gene (Wang et al., 2004). The expression pattern of RFP in transgenic flies bearing this construct is consistent with the pattern described for Gr66a-GAL4 (Thorne et al., 2004; Wang et al., 2004). We crossed the RFP transgene into each of the three Gr promoter-GAL4;UAS-GFP lines described above and examined RFP and GFP expression. Consistent with earlier studies (Wang et al., 2004), Gr5a-GAL4 drives expression in neurons that are distinct from those labeled by the Gr66a construct in the labellum (Figure S4a, left), and in the legs (not shown). Gr61a-GAL4- and Gr64f-GAL4-positive neurons also do not overlap with the Gr66a-positive neurons (Figure S4a), suggesting that Gr61a and Gr64f are not expressed in bitter neurons, but in sugar neurons along with Gr5a. In order to test more directly whether Gr61a and Gr64f are indeed co-expressed with Gr5a in sugar neurons, we wished to use in situ hybridization to label Gr61a or Gr64f mRNA using flies in which the Gr5a neurons were labeled with GFP.
Although there has been very little prior success in detecting Gr gene expression by in situ hybridization in the labellum (Clyne et al., 1999; Dunipace et al., 2001; Scott et al., 2001; Thorne et al., 2004; Wang et al., 2004), we were able to detect Gr5a mRNA in a single neuron of each labeled sensillum (Figure 2d). We first asked whether the Gr5a in situ hybridization probe labeled neurons that were positive for GFP driven by Gr5a-GAL4. We found co-labeling of Gr5a mRNA and GFP, supporting the fidelity of our Gr5a driver (Figure 2d). We then found that in situ hybridization probes for Gr64f and Gr61a also labeled populations of Gr5a-GAL4 neurons, indicating that Gr64f and Gr61a are co-expressed with Gr5a in many cells (Figure 2d). As a final test of coexpression, we tested all pairwise combinations of the drivers in the legs and found evidence confirming that the Gr61a-GAL4-labeled neurons coincided with the Gr64f-GAL4-labeled neurons, and that Gr5a-GAL4 drives expression in a subset of these neurons (Figure S4b).
This evidence for co-expression of Gr5a, Gr64f and Gr61a is consistent with our sensillar mapping analysis based on the expression of individual Gr-GAL4 drivers (Figures 2b and 2c). The mapping data further suggests that in the labellum there may be two distinct classes of sugar neurons: one class that is positive for Gr5a, Gr64f and Gr61a, and a larger class that is positive only for Gr5a and Gr64f (Figure 2c).
Different classes of sugar neurons show overlapping but distinct axonal projection patterns
The peripheral expression patterns of the Gr5a-GAL4, Gr61a-GAL4 and Gr64f-GAL4 drivers raised the possibility that there may be distinct classes of sugar neurons. To investigate this possibility further, we asked whether there were any differences in axonal projections of neurons labeled with each driver. Projections in the CNS derive not only from labellar sensilla but also from other taste sensilla, including those on legs and in internal mouthparts (Stocker and Schorderet, 1981; Shanbhag and Singh, 1992; Stocker, 1994).
Previous studies have shown that axons of Gr5a-labeled labellar neurons project to a discrete region in the sub-esophageal ganglion (SOG) of the brain (Thorne et al., 2004; Wang et al., 2004) (Figure 2e4, arrowhead). In addition, Gr5a-GAL4 is expressed in neurons in taste pegs, which project to a region antero-lateral to that of the labellar projections (Figure 2e1, arrow).
We found that both Gr64f-GAL4 and Gr61a-GAL4 neurons project to regions that overlap with the projections of Gr5a-GAL4 labellar neurons (Figures 2e4, 2e5 and 2e6, arrowheads). However, projection patterns of both Gr64f-GAL4 and Gr61a-GAL4 neurons also have features that are not shared with Gr5a-GAL4 neurons. Both drivers labeled classes of gustatory neurons in the internal mouthparts, which project to the tritocerebrum (Stocker and Schorderet, 1981), antero-dorsal to the SOG (Figures 2e2 and 2e3, arrowheads). Both drivers also labeled leg neurons that show V-shaped projections in a more posterior region of the SOG (Figures 2e8 and 2e9). While most leg neurons in flies project to thoracic ganglia, these V-shaped projections in the SOG resemble projections from the forelegs of the blowfly, visualized by retrograde dye-filling methods (Edgecomb and Murdock, 1992). The presence of these projections in the Gr64f-GAL4 and Gr61a-GAL4 lines but not in the Gr5a-GAL4 line is consistent with our identification of leg neurons that label with Gr64f-GAL4 and Gr61a-GAL4 but not Gr5a-GAL4 (Figures 2b, bottom panels and S4b). Like Gr5a-GAL4, Gr64f-GAL4 also drives expression in a subset of neurons that innervate peg sensilla in the labellum (Figures 2e1 and 2e2 arrows); these projections are not labeled by Gr61a-GAL4. These results, taken together, are consistent with the existence of multiple classes of taste neurons, distinguishable by their expression of different Gr5a-related-GAL4 drivers.
We have also examined axonal projections of neurons in the thoracic ganglion; no previous descriptions of thoracic projections with Gr-GAL4 drivers are available, to our knowledge. In Drosophila, the thoracic ganglion consists of three bilaterally symmetrical leg neuromeres that are fused together (Stocker, 1994). We found that drivers for all three receptors labeled axon terminals in the ventral region of each of the neuromeres (Figures 2e10, 2e11 and 2e12). The projections of Gr5a-neurons were elaborate, and there appeared to be numerous termini distributed over a portion of each neuromere (Figure 2e10). In contrast, the axon terminals of Gr64f-GAL4 and Gr61a-GAL4 neurons were concentrated in a dense pattern in each neuromere (Figures 2e11 and 2e12). The projection patterns of Gr64f-GAL4 and Gr61a-GAL4 were very similar, consistent with the observation that Gr64f-GAL4 and Gr61a-GAL4 are co-expressed in leg neurons.
We were unable to obtain functional promoter lines for most other genes in the Gr64 cluster, which left open the possibility that other Gr5a-related receptors are also expressed in sugar neurons. However, analysis of promoter lines for nearly all of the other Gr genes suggested that few if any of the other 60 Gr receptors are expressed in sugar neurons (AD, L. Weiss, JYK & JRC, unpublished data).
The trehalose receptor is required for responses to several sugars
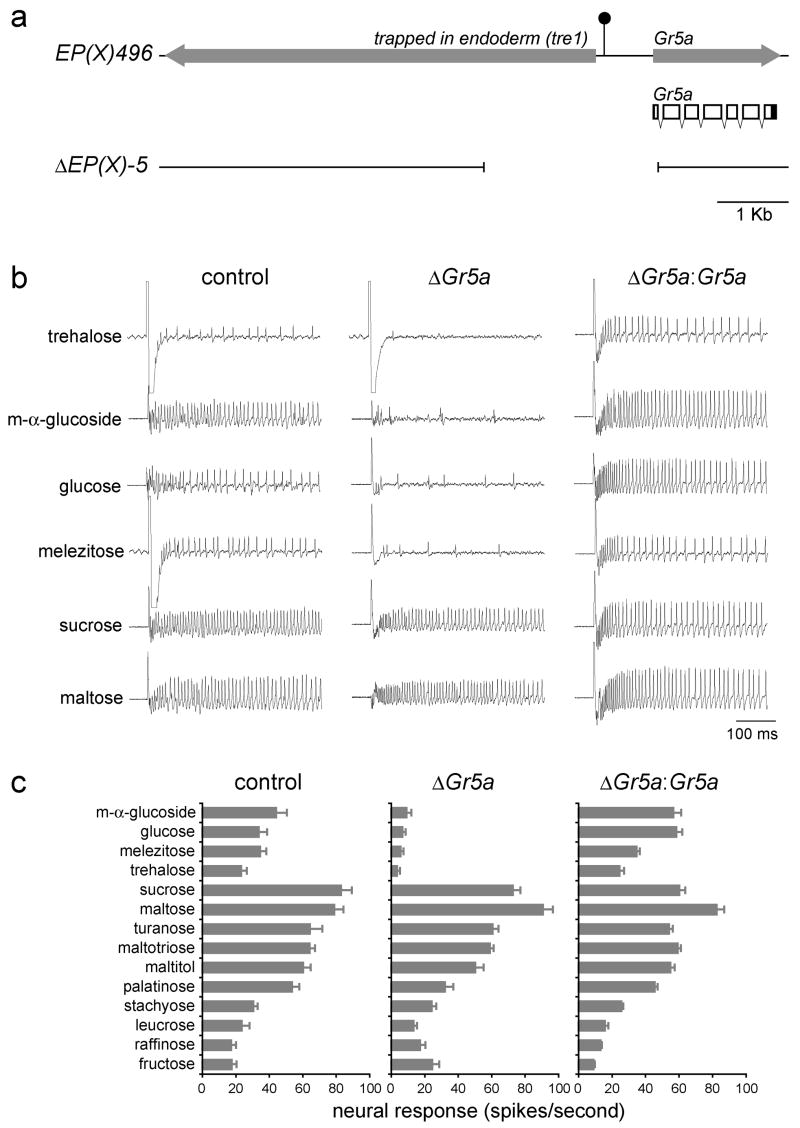
If only a small number of Gr genes are expressed in sugar neurons, but these neurons respond to a large number of sugars with diverse structures (Figures 1, S1 and S2), it seems likely that individual receptors mediate responses to multiple sugars. In a previous study, we showed that Gr5a is necessary for response to trehalose (Dahanukar et al., 2001). However, response to only one other sugar, sucrose, was measured in Gr5a mutant flies. We therefore extended our genetic analysis of the role of Gr5a in mediating sugar responses by testing Gr5a mutant flies with a diagnostic panel of 14 sugars. We used a deletion line, ΔEP(X)-5 (ΔGr5a), which was generated by imprecise excision of a P element that lies in the region upstream of Gr5a (Dahanukar et al., 2001; Ueno et al., 2001) (Figure 3a). We tested the panel of sugars on individual L-type sensilla in each of the two strains. We confirmed that mean responses to trehalose are reduced in Gr5a mutant flies from 23.2 ± 3.4 (n=10) to 3.4 ± 1.7 (n=10) spikes per second (Figures 3b and 3c). We found that mean responses to three other sugars, m-α-glucoside, glucose, and melizitose, were dramatically reduced (Figures 3b and 3c).
Figure 3. The trehalose receptor, Gr5a, mediates responses to several sugars.
(a) The Gr5a genomic region. The filled arrows indicate the trapped in endoderm (Tre1) and Gr5a transcription units. The filled circle depicts the P element insertion in EP(X)496. Shown below is the transcript structure for Gr5a, indicating both protein-coding (hollow) and untranslated (filled) regions. Sequences deleted in ΔEP(X)-5 are indicated at the bottom. (b) Sample traces from the indicated genotypes. (c) Sugar response profiles of L-type sensilla from EP(X)496 (control), ΔEP(X)-5 (ΔGr5a) or from ΔEP(X)-5;Gr5a-GAL4;UAS-Gr5a (ΔGr5a:Gr5a) flies. Sugars were tested at 100 mM, except for glucose and fructose, which were tested at 300 mM. For all graphs, n=10–11. Error bars indicate SEM.
To determine whether the loss of Gr5a is responsible for the reduction in sugar responses in Gr5a mutant flies, we engineered a UAS-Gr5a construct using Gr5a cDNA sequences. We employed the Gr5a-GAL4 transgene described above to drive expression of the Gr5a cDNA. As shown in Figure 3c, responses to trehalose were restored to wild-type levels (24.4 ± 2.8; n=11).
Furthermore, rescue occurred for all the other sugar responses that were reduced in Gr5a mutants, demonstrating that responses to trehalose, as well as to m-α-glucoside, glucose, and melizitose, are mediated primarily by Gr5a.
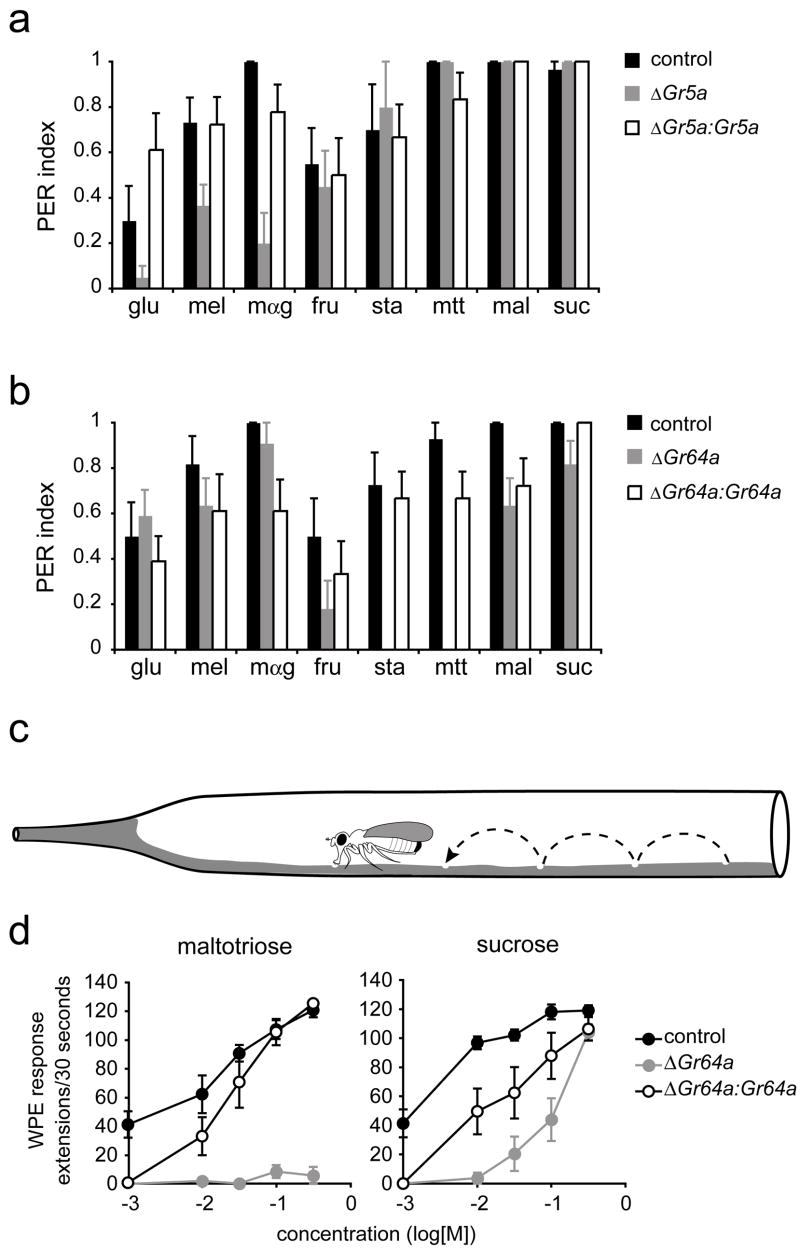
Gr64a is a broadly tuned receptor required for responses to sucrose and other sugars
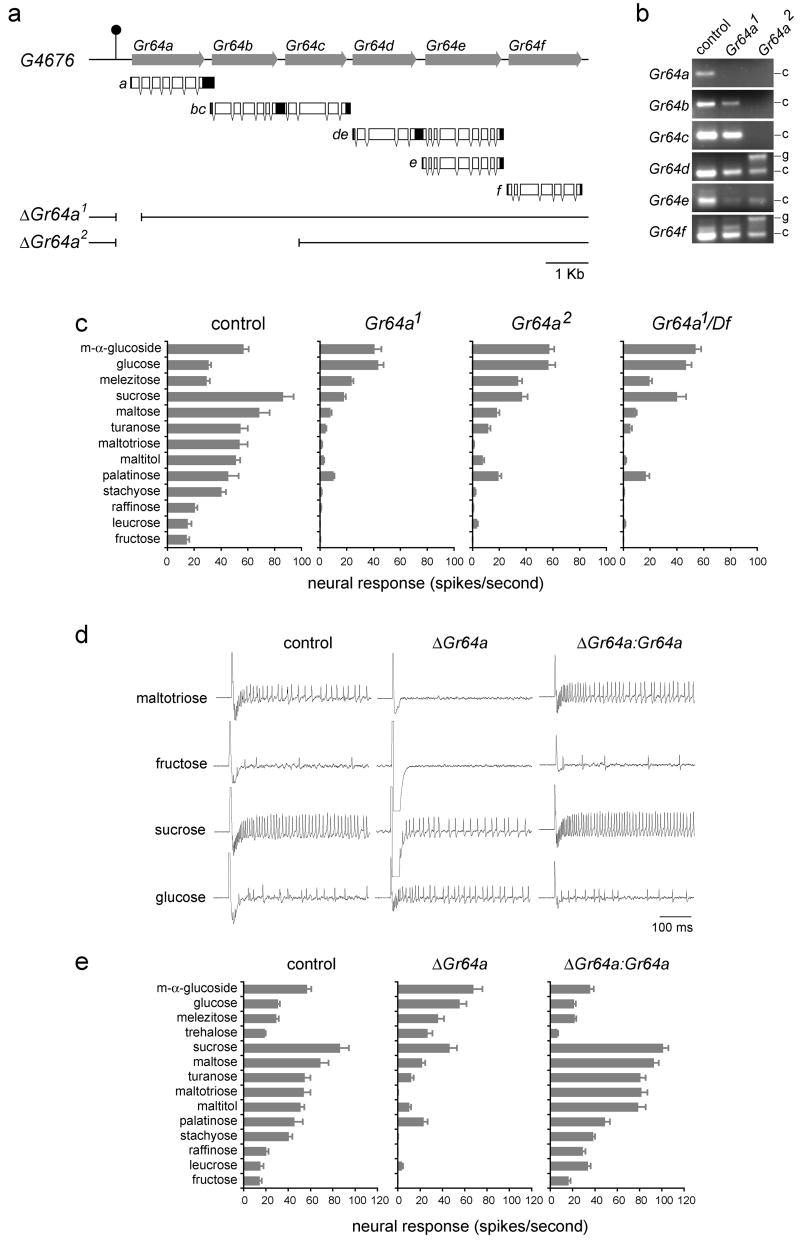
Responses to many sugars are unaffected in Gr5a mutant flies (Figure 3c). We have found that at least two other Gr5a-related receptors are expressed in sugar neurons (Figure 2). We reasoned that one or more Gr5a-related receptors might be responsible for the sugar responses that remain in flies lacking Gr5a. All of the Gr5a-related receptors are encoded by genes that lie on the third chromosome. These include Gr61a and a tightly linked cluster of six genes, Gr64a-Gr64f (Figure 4a), which is the largest cluster of genes in the entire chemoreceptor family of this species (Robertson et al., 2003). Gr64a-Gr64f are all transcribed in the same orientation, and the protein-coding sequences of each gene lie within ~200 bp of each other.
Figure 4. Gr64a mediates responses to several sugars.
(a) The Gr64a-Gr64f genomic region. The filled arrows indicate the Gr64a-Gr64f genes; transcript structures are shown below indicating protein-coding (hollow) and untranslated (filled) regions. The filled circle depicts the P element insertion site in G4676. Sequences deleted in Gr64a1 and Gr64a2 are indicated below. (b) RT-PCR expression analysis of Gr64a-Gr64f in precise excision (control), Gr64a1/Gr64a1, and Gr64a2/Gr64a2 flies as indicated. The positions of the cDNA (c) and genomic (g) products are indicated on the right. All products agree with the predicted sizes. (c) Sugar response profiles of L-type sensilla from precise excision (control), Gr64a1/Gr64a1, Gr64a2/Gr64a2, and Gr64a1/Df(3L)GN34 male flies. (d) Sample traces from the indicated genotypes. (e) Sugar response profiles of L-type sensilla from precise excision (control), Gr64a1/Gr64a2 (ΔGr64a), and Gr5a-GAL4,UAS-Gr64a/UAS-Gr64a;Gr64a1/Gr64a2 (ΔGr64a:Gr64a). Sugars were tested at 100 mM, except for glucose and fructose, which were tested at 300 mM. n=9–12. Error bars indicate SEM.
Due to the uncommon nature of this cluster, we examined the structure of the transcripts encoded by these genes. Interestingly, 5′- and 3′-RACE experiments revealed an unusual feature of this gene cluster: some of the receptors are encoded in bi-cistronic messages (Figure 4a). We recovered two species of such mRNAs, one that included the coding regions of both Gr64b and Gr64c, and a second that included Gr64d and Gr64e. Bi-cistronic messages are uncommon in Drosophila; of ~14,000 annotated genes, only ~50 have been predicted to be transcribed in this manner (Misra et al., 2002). Encoding two receptors on a single transcript may be an efficient mechanism of ensuring their co-expression.
In order to generate flies lacking one or more of the receptor genes in the Gr64 cluster, we took advantage of a P element insertion located 375 bp upstream of the translational initiation codon of Gr64a. We mobilized the P element and recovered 2 lines that have deletions within the Gr64 cluster (Figure 4a). One line, Gr64a1, has a small deletion that removes part of the protein-coding region of Gr64a, including the translational initiation codon. A second line, Gr64a2, bears a larger deletion that removes the entire protein-coding regions of Gr64a and Gr64b, as well as part of the protein-coding region of Gr64c. To determine whether the expression of any other genes in the cluster was affected by either deletion, we performed an RT-PCR analysis. In Gr64a1 we could not detect the Gr64a transcript but were able to detect expression of all other genes in the cluster (Figure 4b). In Gr64a2, expression of Gr64a, Gr64b and Gr64c was abolished, but we were able to amplify products from all other genes.
To investigate the function of Gr64a in sugar reception, we began by characterizing the phenotype of Gr64a1 mutant flies. We tested the electrophysiological responses of L-type labellar sensilla in these flies against the diagnostic panel of sugars (Figure 4c). Virtually no responses remained for maltotriose, stachyose, raffinose, leucrose and fructose, suggesting that responses to these sugars are mediated primarily by Gr64a. Responses were lowered, but not completely abolished, for sucrose, maltose, turanose, maltitol and palatinose, suggesting that multiple receptors may contribute to these responses. Strikingly, mean responses to m-α-glucoside, glucose and melezitose, which are mediated via Gr5a, were not reduced in Gr64a1 mutant flies; response to glucose was not reduced and may even be increased to some extent.
When we examined the sugar response profile in Gr64a2 flies with the larger deletion that also removes Gr64b and Gr64c, we found that the electrophysiological phenotype was no more severe than that in Gr64a1 mutant flies (Figure 4c). The simplest interpretation is that Gr64a, but not Gr64b or Gr64c, is required for these responses. To test whether Gr64a1 might cause a partial loss of function of other genes in the cluster required for these responses, we tested it in a heterozygous combination with a large deletion that removes the entire cluster and found no further reduction in sugar responses (Figure 4c).
To confirm the role of Gr64a in mediating sugar responses, we carried out a transgenic rescue experiment. We found that expression of Gr64a restored the sugar response profile in Gr64a mutant flies (Figures 4d and 4e), as determined by driving expression of a Gr64a cDNA in sugar neurons with Gr5a-GAL4. Responses to all the affected sugars were restored. Responses to m-α-glucoside, glucose, melizitose and trehalose, which are dependent on Gr5a and not Gr64a, were not significantly different from the levels observed in control flies. As an additional control, we tested the effect of the Gr5a-GAL4 driver alone and found that it did not alter the response profile of the sugar neurons in Gr64a mutants (not shown). Finally, we note that in all genetic analysis of Gr64a deletion mutants we used a precise excision as a control strain to reduce the possibility of genetic background effects.
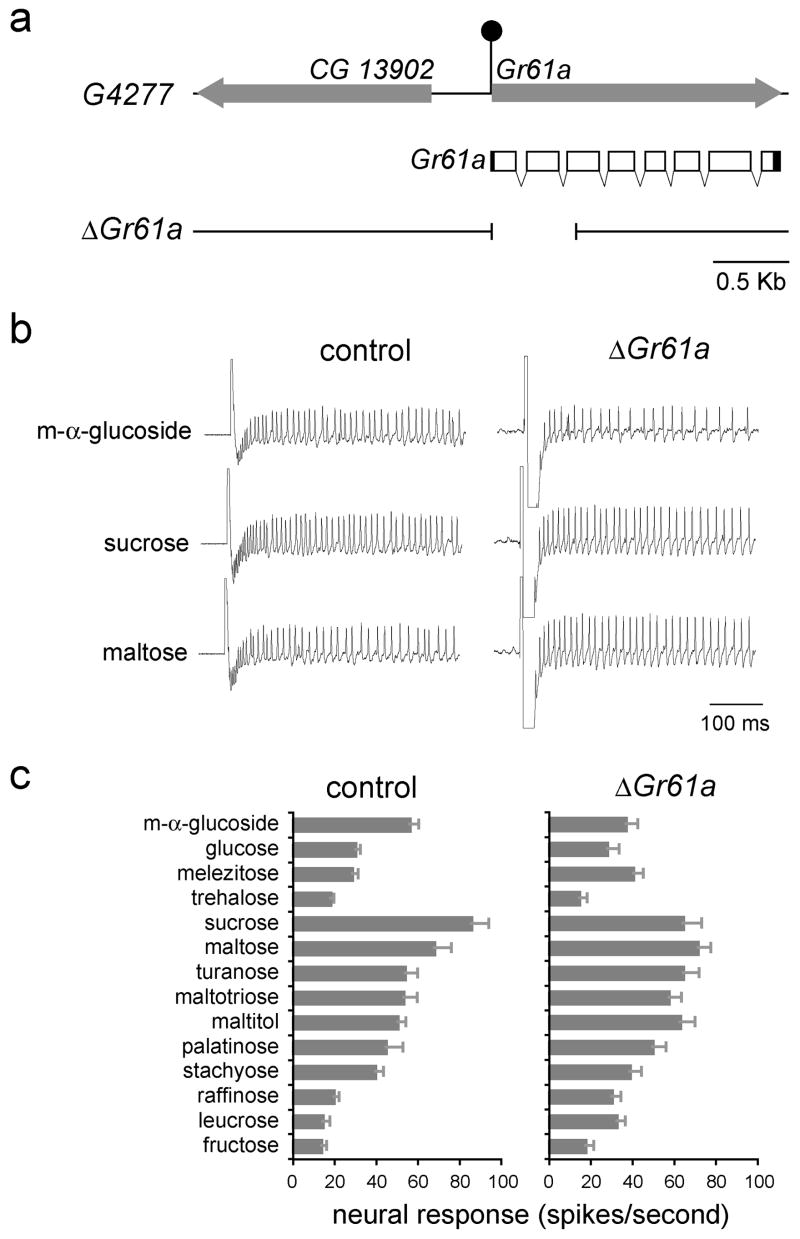
Normal responses of labellar neurons to most sugars in the absence of Gr61a
Since Gr61a is co-expressed with Gr5a in at least some taste neurons in the labellum (Figures 2c and 2d), we wished to investigate the role of Gr61a in sugar responses. We isolated a deletion mutant lacking the translational initiation codon of Gr61a by performing a P element excision screen similar to that described above (Figure 5a).
Figure 5. Most sugar responses are not reduced by loss of Gr61a.
(a) The Gr61a genomic region. The filled arrows indicate transcription units. Shown below is the transcript structure for Gr61a, indicating both protein-coding (hollow) and untranslated (filled) regions. The filled circle depicts the P element insertion in G4277. Sequences deleted in ΔGr61a are indicated below. (b) Representative traces from control flies and Gr61a1/Gr61a1 (ΔGr61a) flies. (c) Sugar response profiles of L-type sensilla from control and Gr61a1/Gr61a1 (ΔGr61a) flies. Sugars were tested at 100 mM, except for glucose and fructose, which were tested at 300 mM. n=9–10. Error bars indicate SEM.
We examined the response profile of L-type sensilla in Gr61a mutant flies. Electrophysiological recordings revealed that the responses in the mutant flies were very similar to those in control flies, suggesting that Gr61a is not essential in labellar neurons for responses to any of the sugars tested (Figures 5b and 5c). Mean responses to m-α-glucoside (37±5.8 spikes per second, n=10) and sucrose (64.4±8.9 spikes per second, n=10) were somewhat reduced (p<0.05, ANOVA, post-hoc Tukey test) as compared to the levels in control flies (56.2±4.4 and 85.8±8.5 spikes per second, respectively, n=9). However, expression of Gr61a cDNA in sugar neurons did not restore responses to sucrose and m-α-glucoside (not shown). Our results thus suggest that Gr61a is not required for response to any of the tested sugars.
Gr5a and Gr64a mediate acceptance behavior to distinct subsets of sugars
How are the activities of taste receptors translated into behavioral responses? To investigate this question, we employed two different behavioral paradigms. First, we used an established proboscis response assay (Rodrigues and Siddiqi, 1978). Sugar stimuli were applied to the labellum, and responses were measured by observing extension of the proboscis, which is interpreted as a sign of acceptance (Dethier, 1976). We tested a panel of eight sugars, including glucose, melizitose and m-α-glucoside, whose electrophysiological responses are dependent on Gr5a, as well as fructose, stachyose, maltotriose, maltose and sucrose, whose electrophysiological responses are dependent on Gr64a. Consistent with the electrophysiological results, the mean proboscis extension responses (PERs) of Gr5a mutants to glucose, melizitose and m-α-glucoside were reduced, but responses to other sugars were not affected (Figure 6a). Conversely, responses of Gr64a mutants to glucose, melezitose and m-α-glucoside were normal. In these mutants the mean responses to fructose, stachyose, maltotriose, maltose and sucrose were lower (Figure 6b), although the reduction to sucrose was limited in this assay at the tested concentrations. In rescue experiments, expression of either Gr5a or Gr64a under the control of the Gr5a promoter increased the mean behavioral response to all sugars whose responses had been reduced (Figures 6a and 6b).
Figure 6. Gr5a and Gr64a mediate behavioral responses to sugars.
(a,b) Proboscis extension responses. (a) EP(X)496 (control), ΔEP(X)-5 (ΔGr5a) and ΔEP(X)- 5;Gr5a-GAL4;UAS-Gr5a (ΔGr5a:Gr5a); n=8–15 for all sugars except for stachyose, n=5–9. (b) Precise excision (control), Gr64a1/Gr64a1 (ΔGr64a) and Gr5a-GAL4/UAS-Gr64a;Gr64a1/Gr64a1 (ΔGr64a:Gr64a). n=9–14. Error bars indicate SEM. All sugars were tested at 100 mM. glucose (glu), melezitose (mel), m-α-glucoside (mαg), fructose (fru), stachyose (sta), maltotriose (mtt), maltose (mal), sucrose (suc). (c) The walking proboscis extension (WPE) assay. Not drawn to scale. (d) Proboscis extensions in the walking assay. Genotypes are: precise excision (control), Gr64a1/Gr64a2 (ΔGr64a) and Gr5a-GAL4/UAS-Gr64a;Gr64a1/Gr64a2 (ΔGr64a:Gr64a). n=9–10. Error bars indicate SEM.
We developed a second behavioral paradigm and investigated further the role of Gr64a in the behavioral response to sucrose. In the proboscis extension assay, the fly is immobilized and the stimulus is applied to the labellum by the experimenter. We desired a paradigm in which behavior would more closely mimic that of the fly in its natural environment. For this purpose, we established an assay of acceptance behavior (Figure 6c) in which the fly is allowed to walk freely on an agarose-coated surface. The test sugar is included in the agarose. We counted the number of proboscis extensions in the 30-second period after the fly was introduced onto the medium. As a test of the paradigm, we measured response to maltotriose (mtt), which showed dramatically reduced responses in Gr64a mutants in both physiological and PER assays when tested at 100 mM (Figures 4c and 6b). Using the new assay, we found that behavioral responses to maltotriose were severely affected in Gr64a mutants across a wide range of concentrations (Figure 6d). Response to sucrose was also strongly reduced, but not at the highest concentration tested. For both sugars, rescue of the behavioral phenotypes occurred with expression of a UAS-Gr64a transgene, but rescue was partial, perhaps because of insufficient expression levels of Gr64a, or because the Gr5a-GAL4 transgene did not drive expression in all the neurons that require Gr64a for this behavior, particularly in the legs.
The labellar sugar response spectrum is dependent on two receptors
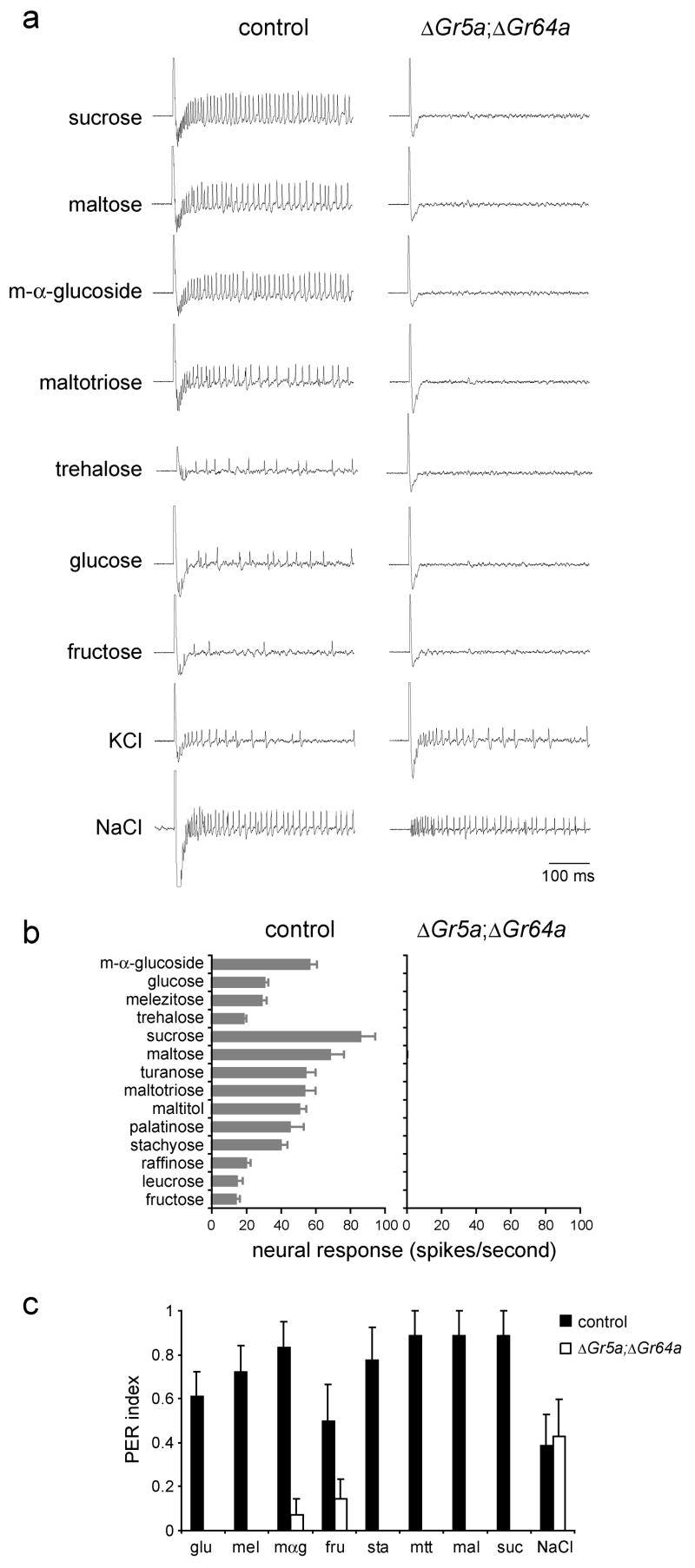
Our analysis of physiological and behavioral responses of mutant flies showed that Gr5a and Gr64a mutations produced defects in responses to complementary subsets of sugar stimuli. The results raised the possibility that essentially all sugars depend on either Gr5a or Gr64a. To test this possibility we created a Gr5a;Gr64a double mutant.
When we examined flies that were mutant for both Gr5a and Gr64a, we found that neurons in L-type sensilla had lost completely the ability to respond to all of the sugars in our panel (Figures 7a and 7b). Furthermore, we tested eight of the sugars in the panel—fructose, glucose, m-α-glucoside, maltose, maltotriose, stachyose, sucrose, and trehalose—at a concentration of 1 M and found that they did not evoke any responses even at this higher concentration (not shown). We confirmed the integrity of the sensilla by testing with 1 mM KCl, which activates the water neuron (Hiroi et al., 2002), and with 400 mM NaCl, which activates both the salt and the bitter neurons (Hiroi et al., 2002; Meunier et al., 2003) (Figure 7a). We then extended our analysis of the Gr5a;Gr64a mutant to include all morphological types of sensillum (L,M,S,I,P) (Ray et al., 1993), including nearly all of the individual L,M,I, and P sensilla, and found no activity in response to sucrose in any of them (not shown).
Figure 7. Labellar sugar responses depend on either Gr5a or Gr64a.
(a)Sample traces of physiological recordings from precise excision (control), or ΔEP(X)-5;Gr64a2/Gr64a2 (ΔGr5a; ΔGr64a) flies as indicated. (b) Response profiles. All sugars were tested at 100 mM, except for glucose and fructose, which were tested at 300 mM. KCl was tested at 1 mM, and NaCl at 400 mM. n=9–12 for control, n=6 for mutants. Error bars indicate SEM. (c) Proboscis extension responses of control flies (precise excision), or ΔGr5a; ΔGr64a (ΔEP(X)-5;Gr64a2/Gr64a2) flies as indicated. None of the responses of the mutant exceeded a control response to pure water (p<0.05). All sugars were tested at 100 mM. glucose (glu), melezitose (mel), m-α-glucoside (mαg), fructose (fru), stachyose (sta), maltotriose (mtt), maltose (mal), sucrose (suc). NaCl was tested at 5 mM. n=7–9. Error bars indicate SEM.
We then used the proboscis extension response assay to ask whether flies mutant for both Gr5a and Gr64a were capable of behavioral responses to sugars. We found that Gr5a;Gr64a flies did not respond to any of the eight sugars in our panel, although their behavioral response to 5 mM sodium chloride was not affected (Figure 7c). Higher concentrations of sucrose, glucose or fructose also did not evoke proboscis extensions in the mutant flies (mean responses to 1 M solutions were 0.0, 0.0, and 0.14 ± 0.14 respectively, n=7). Taken together, these results demonstrate that all of the tested sugars are dependent on Gr5a or Gr64a for neuronal and behavioral responses.
DISCUSSION
Sugar neurons respond selectively to a diverse subset of sugars
We have systematically analyzed the physiological responses of L-type sugar neurons to a large panel of compounds. We found that the strongest responses are elicited by a small subset of sugars, including certain disaccharides and oligosaccharides.
Sucrose generated the strongest responses among a panel of 50 compounds tested at 100 mM. Sucrose is present at comparable concentrations in many fruits, including citrus, peaches and pineapples (U.S. Department of Agriculture, 2006). Turanose, palatinose and leucrose are all isomers of sucrose and also elicit responses of various strengths. Many of the sugars that evoke responses, including glucose and trehalose, are found in fruits and vegetables or in yeasts and may thus be encountered by the fly in its natural environment.
The responses depend on sugar concentration as well as identity. The neurons are sensitive to a number of sugars over concentrations that span three orders of magnitude. The dose-response curves of different sugars, however, are distinct: they differ in threshold, slope, and maximal firing rate observed. Many of these sugars are present in fruits at concentrations of 100–300mM (U.S. Department of Agriculture, 2006), and at these concentrations the responses lie well within the dynamic range of the neurons. Surprisingly, responses to fructose and glucose, which are particularly abundant in fruits, are much weaker than those of sucrose, even when compared at concentrations that have equal caloric values. However, the concentrations of both fructose and glucose are typically higher than that of sucrose in fruits such as apples, bananas and grapes, suggesting that sugar neurons may be most sensitive to changes in sugar concentrations over a range that is ecologically relevant.
Expression of Gr5a-related receptors
Molecular analysis has revealed co-expression of Gr61a and Gr64f with Gr5a (Figures 2b, 2c and 2d), and genetic analysis of a double mutant has provided evidence for co-expression of Gr64a with Gr5a in sugar neurons. These results suggest that at least some labellar sugar neurons, including those of L-type sensilla, co-express four receptors of the Gr5a subfamily.
Molecular and genetic evidence indicates that Gr5a is expressed in essentially all labellar sensilla. Molecular analysis has provided evidence that Gr64f is also broadly expressed, and functional evidence suggests that Gr64a is as well. Specifically, an electrophysiological survey showed that all labellar sensilla in wild type respond to sucrose (data not shown and J. Perry & J.C., unpublished data), a sugar that acts via Gr64a. We have found that in a Gr5a;Gr64a mutant all morphological types of sensillum (L,M,S,I,P) showed no activity in response to sucrose; moreover, we tested nearly all of the L, M, I, and P sensilla, suggesting that Gr64a acts in all, or almost all, of the 31 sensilla on the labellum (not shown). Furthermore, the double mutants are also behaviorally unresponsive to sugars. Thus Gr5a and Gr64a seem likely to be expressed in all or almost all sugar neurons in the labellum, and perhaps Gr64f is as well.
Gr61a, however, appeared to be restricted in its expression among labellar sensilla, both by in situ hybridization and by analysis of a Gr61a-GAL4 driver. These results suggest a subdivision of labellar sugar neurons into two classes based on the presence or absence of Gr61a. We have not been able to define a function for Gr61a; however, mutational analysis suggests that it does not play a role in responses to any of the sugars in our panel. It is possible that Gr61a is required for response to other sugars or sugar-derivatives that we have not yet tested, or for responses to another class of behaviorally attractive compounds. Further electrophysiological analysis with an expanded panel of tastants may provide insight into whether there are functional differences among sugar-sensing neurons and whether these differences correlate with the expression of Gr61a.
Sugar responses are mediated by either of two receptors, Gr5a or Gr64a
Gr5a and Gr64a are both required for normal responses of sugar neurons, but for different subsets of sugars. Flies lacking Gr5a are severely defective in physiological and behavioral responses to one subset of sugars, including trehalose; flies lacking Gr64a are severely defective in responses to a complementary subset of sugars, including sucrose. All tested sugars fall into one of these two subsets. These results suggest that Gr5a and Gr64a function as distinct receptors in the same neurons, rather than as obligate heterodimeric co-receptors, as in the mammalian sugar receptor T1R2+T1R3 (Nelson et al., 2001).
It is possible that Gr5a and Gr64a function as heterodimeric receptors with other members of the Gr family, such as Gr64f. Two recent studies report deletions of part or all of the Gr64 cluster that result in reduced behavioral responses to trehalose; the phenotype is rescued by supplying a transgene containing five of the six receptors encoded by this cluster (Slone et al., 2007), but not by Gr64a alone (Jiao et al., 2007). These data support the idea that one of the receptors in this cluster other than Gr64a may function in concert with Gr5a to mediate trehalose response. There is precedent for such interactions from Or proteins, which dimerize with the non-canonical receptor Or83b (Neuhaus et al., 2005; Benton et al., 2006).
We were surprised by the neat sub-division of sugars into those dependent on Gr5a and those dependent on Gr64a. A simple structural criterion to distinguish the two classes of sugars is not immediately evident upon inspection. The Gr64a-dependent sugars are remarkably diverse in structure, with some containing glucose units and some containing fructose subunits; they ranged in size from one to four subunits. Gr5a-dependent sugars also vary in size, subunit composition, and linkage types.
In Gr5a mutants there are some weak residual responses to the affected subset of sugars; likewise, in Gr64a mutants some of the affected sugars continue to elicit some response. Since there is no residual response in the Gr5a; Gr64a double mutant, the simplest interpretation of our results is that each receptor provides the residual function observed when the other is eliminated, i.e., the two receptors exhibit some limited redundancy.
Gr5a and Gr64a share 28% amino acid identity and 47% amino acid similarity. Both receptors are evolutionary conserved and are found in all of the 12 Drosophila species for which genome sequences are available, with the exception that D. pseudoobscura appears to have lost Gr5a. The receptor most closely related to Gr5a is Gr64f (40% amino acid identity), and the receptor most closely related to Gr64a is Gr61a (36% amino acid identity). Although we have found evidence that Gr64f and Gr61a are both expressed in sugar neurons (Figure 2), we have not identified functions for them. We cannot exclude the possibility of a role for Gr61a or Gr64f in response to compounds we have not tested, such as glycoproteins or glycolipids, or in neurons whose responses we have not measured, such as those of internal chemosensory cells. We note that in mammals, an amino acid receptor (T1R1+T1R3) comprises a subunit, T1R3, of the heterodimeric sugar receptor (T1R2+T1R3) (Nelson et al., 2001; Nelson et al., 2002). However, L-type sensilla did not respond to any of 18 amino acids tested, making it unlikely that either Gr61a or Gr64f mediates responses to this class of compounds.
Classic physiological and biochemical studies led to the proposal of a “fructose” site in sugar-sensing neurons (Tanimura and Shimada, 1981). Our studies provide a molecular and genetic identity to this site: fructose response is completely abolished by loss of Gr64a and is completely restored by the addition of a Gr64a transgene. Our results also provide a molecular explanation for our earlier finding that sucrose responses were not affected in a Gr5a mutant. These results suggested the presence of another receptor within the sugar neuron, a receptor that has now been identified as Gr64a.
We note that two recent studies have identified a role for members of the Gr64 cluster in mediating sugar responses (Jiao et al., 2007; Slone et al., 2007), particularly that of Gr64a in response to sugars including sucrose, maltose and glucose (Jiao et al., 2007). Consistent with our observations, physiological and behavioral responses to sucrose were restored to wild type levels in transgenic rescue experiments; we did not observe a role for Gr64a in glucose response. One of these studies also provided biochemical evidence that Gr5a-related receptors are expressed in sugar-sensitive neurons (Jiao et al., 2007).
In summary, the simplest interpretation of our results is that Gr5a and Gr64a are the primary sugar receptors in the labellum of the adult fly. Each is capable of mediating response to a subset of sugars independently of the other, and together they are able to identify the food sources that are sufficiently rich in caloric value as to sustain the life of the fly.
EXPERIMENTAL PROCEDURES
Stocks
Flies were raised at room temperature (23±2°C) for electrophysiological recordings and behavioral experiments, and at 25°C for GFP visualization. w;UAS-mCD8-GFP, used as a source of GFP, and the deficiency that uncovers the Gr64 cluster, Df(3L)GN34, were obtained from the Bloomington Stock Center.
Isolation of mutants
Flies containing a transposable P element, P{EP}, 375 bp upstream of Gr64a (G4676) or 8 bp upstream of Gr61a (G4277) were obtained from Genexel, Korea. Both lines were used for imprecise excision screens in which the P elements were mobilized using Δ2–3. For G4676, ~2200 w− progeny were screened by PCR to identify deletions. PCR products were sequenced to identify deletion breakpoints. A line in which this P element was excised precisely was used as a control in the electrophysiology and behavior experiments, and is designated as “control” in the text. For G4277, ~450 w− progeny were screened. For RT-PCR analysis, total RNA was isolated from ~120 proboscises using QIAshredder and RNeasy (Qiagen). Samples were treated with DNaseI before performing RT-PCR using standard techniques.
Constructs and Transgenic Flies
Total RNA was isolated from ~200 proboscises of Canton-S flies as described previously (Clyne et al., 1999). 5′- and 3′-RACE experiments were performed using the SMART 5′RACE kit (Clontech) or standard oligo-dT primers. PCR products obtained after 35 thermocycles were AT-cloned and sequenced to determine intron-exon junctions and predicted protein sequences. The bi-cistronic mRNAs were identified in multiple, independent PCR reactions. Results of 3′-RACE for Gr64b and Gr64d were confirmed with independent 5′-RACE reactions for Gr64c and Gr64e. Full-length cDNAs were then generated by standard molecular techniques and cloned into pUAST (Brand and Perrimon, 1993).
For Gr promoter-GAL4 constructs, upstream DNA sequences were amplified from Canton-S genomic DNA and cloned into pG4 (Brand and Perrimon, 1993). Primer sequences used for Gr61a were 5′-GGTACCCAGCAGATCATCCATGTC and 5′-GCGGCCGCGCTCCTCAGCTCTGACCG (~5 Kb), and for Gr64f were 5′-GGTACCCAGCGATTGTCTCTTAGCTG and 5′-GCGGCCGCCCTAGGACCTGCTGGG (~10 Kb). For Gr66a, primers were as described (~2 Kb) (Dunipace et al., 2001). The mRFP clone was generously provided by Roger Tsien. IRES sequences were amplified from Ubx using the primers 5′-GGAAGCTTAATTAACAGCAAAGTGCAAT and 5′-GGAAGATCTCTGGCGGTAAGAATCTTGGC. Three copies of the RFP coding region interrupted by IRES sequences were cloned downstream of Gr66a promoter sequences. The construct also included sequences of the SV40 3′ UTR.
All DNA constructs were injected into w1118 flies, unless otherwise indicated. At least two independent lines for each transgene were tested in electrophysiology experiments, with similar results.
In situ hybridization and immunohistochemistry
In situ hybridization and immunohistochemical localization on the labellum were performed as described previously for maxillary palps (Goldman et al., 2005) with the following modifications: whole heads were first fixed in 4% paraformaldehyde in 1XPBS+0.2% Triton X-100 on ice for 3 hours, after which the labella were dissected and post-fixed at room temperature for 15 minutes. Adult brains were dissected and prepared as described (Python and Stocker, 2002). Samples were immunostained with nc82 monoclonal antibody (a gift from Alois Hofbauer) and a polyclonal antibody against GFP (Invitrogen). All tissues were visualized using a Bio-Rad 1024 laser-scanning confocal microscope.
Tastants
Chemicals were of the highest purity available, typically of 98–99% purity, and were obtained from: Sigma/Aldrich (D-allose, L-arabinose, D-arabinose, D-arabitol, D-(+)-cellobiose, cytidine, D(−)-fructose, L-(−)-fucose, D-(+)-galactose, D-(+)-galacturonic acid, β-gentiobiose, D-(+)-glucose monohydrate, D-glucuronic acid, D-glucoheptose, D-(−)-glucosamine hydrochloride, α-lactose, lactulose, D-leucrose, D-lyxose, maltitol, D-(+)-maltose monohydrate, maltotriose hydrate, D-mannoheptose, D-(+)-mannose, D-(+)-melezitose hydrate, melibiose, methyl-α-D-glucopyranoside, monellin, myo-inositol, palatinose, D-panose, sedoheptulose anhydride monohydrate, potassium chloride, sodium chloride, D-sorbitol, stachyose hydrate, sucrose, D-(−)-tagatose, D-talose, D-(+)-trehalose, D-turanose, thaumatin, thymidine, D-(+)-raffinose pentahydrate, L-rhamnose monohydrate, D-ribose, D-xylitol, D-xylose, L-amino acid kit); Riedel (D-mannitol); American Bioanalytical (glycerol); Pharmco-Aaper (ethyl alcohol).
Electrophysiology
Extracellular single-unit recordings were performed as described previously (Dahanukar et al., 2001). Newly eclosed flies were transferred to fresh vials with standard cornmeal agar medium, and flies were aged for 5–10 days at room temperature. Action potentials were recorded from L-type sensilla of male flies using TasteProbe (Syntech, Hilversum, Netherlands). Neural response was quantified by counting the number of impulses generated in the 500-ms period beginning 200 ms after onset of stimulation. KCl and NaCl were dissolved in water. All other tastants were dissolved in an aqueous solution containing 0.03 M tricholine citrate (Sigma) as electrolyte. Stock solutions were stored in glass vials at −20°C. For recordings, aliquots of 500 μl were stored at 4°C and used for no longer than one week. For all experiments, no more than three sensilla were tested on a single fly, and a maximum of 15 stimuli were tested on a single sensillum. Amino acids were tested at 25 mM.
Behavioral assays
For the PER, newly eclosed flies were transferred to fresh vials with standard cornmeal agar medium and maintained at room temperature for 5–6 days. Flies were then starved for 20–24 hours in vials with water-saturated Kimwipes. An airstream was used to lodge single males into yellow pipette tips (1–200μl) and a notch was made in the tip to mark the position of the center of the eye. The fly was then removed, the pipette tip was cut at the notch, and the fly was introduced back into the tip such that its head protruded through the opening. A wick made from a Kimwipe was saturated with sugar solution and used to contact the labellum (Shiraiwa and Carlson, 2007). Flies were initially tested with a negative control (water) and a positive control (100 mM sucrose or 100 mM m-α-glucoside). Only those that responded appropriately, i.e. did not extend their proboscis in response to water, but did so upon contact with the positive control, were tested further. Except for the positive control, sugars were tested blind. Flies were periodically checked with the negative and positive control stimuli to ensure that the responses were consistent through the duration of the experiment. Responses were scored as follows: proboscis extension (1), no extension (0), weak and/or inconsistent extension (0.5). At least 10 flies were tested for each genotype.
For the walking proboscis extension assay, flies were maintained and starved as above. Sugar solutions were added to aliquots of 1% agarose solution kept at 55°C such that the final concentration of agarose was 0.8%. The solution was aspirated into 5 3/4” Pasteur pipettes and immediately expelled such that the inner surface of the pipette was coated with a thin layer of agarose. The Pasteur pipettes were prepared ~1–3 hours prior to the experiment. Individual flies were introduced into the pipettes, and proboscis extensions were examined under a stereo-zoom microscope and counted for a 30-second period (Cheng et al., 1992). Data points greater than two standard deviations from the mean were discarded.
Supplementary Material
Supplementary Figure 1. Recordings from sensilla stimulated with binary mixtures.
Sample physiological recordings from sensilla stimulated with binary mixtures, showing neuronal activity in the 200–500 ms period following contact with the stimulus. The amplitudes and shapes of action potentials are uniform in the case of binary mixtures of sugars, but two distinct sizes of action potentials are detected in response to a mixture of sucrose and sodium chloride: dots indicate positions of action potentials of one of the neurons. sucrose (suc), m-α-glucoside (mαg), glucose (glu), melezitose (mel), trehalose (tre), maltose (mal), turanose (tur), maltotriose (mtt), maltitol (mol), palatinose (pal), stachyose (sta), raffinose (raf), leucrose (lcr), fructose (fru).
Supplementary Figure 2. Sugar structures.
Structures of sugars included in the diagnostic panel.
Supplementary Figure 3. Temporal dynamics of sugar responses in L sensilla.
Surface plots of neural responses to indicated sugars and concentrations over a 1-second period. Impulses were counted in 50-ms bins, starting 15 ms after contact of the recording electrode with the taste sensillum.
Supplementary Figure 4. Expression of Gr5a-related receptors.
(a) Double-labeling with the indicated Gr-GAL4 transgenes (green) and Gr66a-RFP (red). In all cases, genotypes were Gr-GAL4/Gr66a-RFP, Gr66a-RFP; UAS-GFP/Gr66a-RFP, Gr66a-RFP.
(b) GFP reporter expression in the three distal-most segments of forelegs, driven by combinations of Gr-GAL4 drivers as indicated. The mean numbers of cells detected in each case were: Gr5a (5.6), Gr61a (12), Gr64f (9.7), Gr5a+Gr61a (9.8), Gr5a+Gr64f (10) and Gr61a+Gr64f (9.6). Genotypes were Gr-GAL4/UAS-GFP; Gr-GAL4/UAS-GFP.
Acknowledgments
We thank Wynand van der Goes van Naters for building a preamplifier, and for help with electrophysiology; A. Ray and J. Perry for discussions and helpful suggestions and members of the Carlson lab for comments on the manuscript. This research was supported by NIH grant GM63364 to J.R.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cheng E, Chang C-M, Sun HY. An improved proboscis extension assay. Drosophila. Information Newsletter. 1992;6 [Google Scholar]
- Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci U S A. 2003;100:14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- Dethier VG. The Hungry Fly. Cambridge: Harvard University Press; 1976. [Google Scholar]
- Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- Edgecomb RS, Murdock LL. Central projections of axons from taste hairs on the labellum and tarsi of the blowfly, Phormia regina Meigen. J Comp Neurol. 1992;315:431–444. doi: 10.1002/cne.903150406. [DOI] [PubMed] [Google Scholar]
- Falk R, Bleiser-Avivi N, Atidia J. Labellar taste organs of Drosophila melanogaster. J Morphol. 1976;150:327–342. doi: 10.1002/jmor.1051500206. [DOI] [PubMed] [Google Scholar]
- Fujishiro N, Kijima H, Morita H. Impulse frequency and action potential amplitude in labellar chemosensory neurons of Drosophila melanogaster. J Insect Physiol. 1984;30:317–325. [Google Scholar]
- Furuyama A, Koganezawa M, Shimada I. Multiple receptor sites for nucleotide reception in the labellar taste receptor cells of the fleshfly Boettcherisca peregrina. J Insect Physiol. 1999;45:249–255. doi: 10.1016/s0022-1910(98)00120-6. [DOI] [PubMed] [Google Scholar]
- Goldman AL, van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose, identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, Kinghorn AD. Highly sweet compounds of plant origin. Arch Pharm Res. 2002;25:725–746. doi: 10.1007/BF02976987. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- Misra S, Crosby MA, Mungall CJ, Matthews BB, Campbell KS, Hradecky P, Huang Y, Kaminker JS, Millburn GH, Prochnik SE, et al. Annotation of the Drosophila melanogaster euchromatic genome: a systematic review. Genome Biol. 2002;3:research0083.0081–0083.0022. doi: 10.1186/gb-2002-3-12-research0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Nayak SV, Singh RN. Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int J Insect Morphology & Embryology. 1983;12:273–291. [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Störtkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Python F, Stocker RF. Adult-like complexity of the larval antennal lobe of D. melanogaster despite markedly low numbers of odorant receptor neurons. J Comp Neurol. 2002;445:374–387. doi: 10.1002/cne.10188. [DOI] [PubMed] [Google Scholar]
- Ray K, Hartenstein V, Rodrigues V. Development of the taste bristles on the labellum of Drosophila melanogaster. Dev Biol. 1993;155:26–37. doi: 10.1006/dbio.1993.1003. [DOI] [PubMed] [Google Scholar]
- Robertson HM, Warr CG, Carlson JR. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100:14537–14542. doi: 10.1073/pnas.2335847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues V, Siddiqi O. Genetic-Analysis of Chemosensory Pathway. Proc Indian Acad Sci [B] 1978;87:147–160. [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shanbhag S, Singh RN. Functional implications of the projections of neurons from individual labellar sensillum of Drosophila melanogaster as revealed by neuronal marker horseradish peroxidase. Cell Tissue Res. 1992;267:273–282. [Google Scholar]
- Shanbhag SR, Park SK, Pikielny CW, Steinbrecht RA. Gustatory organs of Drosophila melanogaster: fine structure and expression of the putative odorant-binding protein PBPRP2. Cell Tissue Res. 2001;304:423–437. doi: 10.1007/s004410100388. [DOI] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. Journal of Visualized Experiments. 2007;3 doi: 10.3791/193. http://www.jove.com/Details.htm?ID=193&VID=155. [DOI] [PMC free article] [PubMed]
- Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1–8. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–523. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Tanimura T, Shimada I. Multiple receptor proteins for sweet taste in Drosophila discriminated by papain treatment. J Comp Physiol. 1981;141:265–269. [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Agriculture, A. R. S. USDA National Nutrient Database for Standard Reference. 2006 Release 19. http://www.ars.usda.gov/ba/bhnrc/ndl.
- Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- Van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Wieczorek H, Wolff G. The labellar sugar receptor of Drosophila. J Comp Physiol [A] 1989;164:825–834. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Recordings from sensilla stimulated with binary mixtures.
Sample physiological recordings from sensilla stimulated with binary mixtures, showing neuronal activity in the 200–500 ms period following contact with the stimulus. The amplitudes and shapes of action potentials are uniform in the case of binary mixtures of sugars, but two distinct sizes of action potentials are detected in response to a mixture of sucrose and sodium chloride: dots indicate positions of action potentials of one of the neurons. sucrose (suc), m-α-glucoside (mαg), glucose (glu), melezitose (mel), trehalose (tre), maltose (mal), turanose (tur), maltotriose (mtt), maltitol (mol), palatinose (pal), stachyose (sta), raffinose (raf), leucrose (lcr), fructose (fru).
Supplementary Figure 2. Sugar structures.
Structures of sugars included in the diagnostic panel.
Supplementary Figure 3. Temporal dynamics of sugar responses in L sensilla.
Surface plots of neural responses to indicated sugars and concentrations over a 1-second period. Impulses were counted in 50-ms bins, starting 15 ms after contact of the recording electrode with the taste sensillum.
Supplementary Figure 4. Expression of Gr5a-related receptors.
(a) Double-labeling with the indicated Gr-GAL4 transgenes (green) and Gr66a-RFP (red). In all cases, genotypes were Gr-GAL4/Gr66a-RFP, Gr66a-RFP; UAS-GFP/Gr66a-RFP, Gr66a-RFP.
(b) GFP reporter expression in the three distal-most segments of forelegs, driven by combinations of Gr-GAL4 drivers as indicated. The mean numbers of cells detected in each case were: Gr5a (5.6), Gr61a (12), Gr64f (9.7), Gr5a+Gr61a (9.8), Gr5a+Gr64f (10) and Gr61a+Gr64f (9.6). Genotypes were Gr-GAL4/UAS-GFP; Gr-GAL4/UAS-GFP.