Abstract
Congestive heart failure remains the leading cause of morbidity and mortality in the developed world. Current therapies do not address the underlying pathophysiology of this disease, namely the progressive loss of functional cardiomyocytes. The notion of repairing or regenerating lost myocardium via cell based therapies remains highly appealing. The recent identification of adult stem cells, including both cardiac stem/progenitor cells and bone marrow stem cells, has triggered an explosive interest in utilizing these cells for physiologically relevant cardiomyogenesis. Enthusiasm for cardiac regeneration via cell therapy has further been fueled by the many encouraging reports in both animals and human studies. Further intensive research in basic science and clinical arenas are needed in order to make this next great frontier in cardiovascular regenerative medicine a reality. In this review, we focus on the role of bone marrow derived stem cells and cardiac stem/progenitor cells in cardiomyocyte homeostasis and myocardial repair and regeneration, as well as provide a brief overview of current clinical trials utilizing cell-based therapeutic approaches in patients with heart disease.
Despite advances in the treatment of congestive heart failure (CHF), morbidity and mortality remain inappropriately high 1 This medical epidemic has only continued to escalate, given an overall aging population and the greater number of patients surviving an initial myocardial infarction (MI). The pathophysiology of post-MI heart failure is driven by the loss of cardiomyocytes, either due to acute ischemic necrosis or chronic apoptosis, and the inability of the remaining cardiomyocytes to adequately compensate. As such, the concept of repairing or regenerating lost myocardium via cell based therapies (so termed “cardiomyoplasty”) remains highly appealing. Over the past decade, much research has focused upon identifying the ideal cell type with which to promote myocardial regeneration. Thus far, several cells types have been investigated in animal models including, but not limit to, fetal cardiomyocytes 2,3, fibroblasts, skeletal myoblasts 4-7 and endothelial progenitor cells 8,9. Results with all these cell types have generally been encouraging with regards to beneficial post-MI remodeling; albeit none have resulted in definitive differentiation into physiologically-significant, force-generating cardiomyocytes. Five years ago, striking reports suggested, for the first time, that bone marrow derived stem cells (hematopoietic stem cells) may have the potential to regenerate significant amounts of lost myocardium in mice following MI 10,11, creating overwhelmingly enthusiasm and subsequent skepticism in the field of cardiac repair and regeneration. More recently, the identification of resident cardiac stem/progenitor cells by several groups, including ours 12-15, has brought about a second wave of the scientific interest. These findings have advanced our understanding of myocardial biology and physiology and have introduced the new paradigm of the heart as a non-terminally differentiated organ. Despite the inability of myocardial tissue to adequately ‘self-heal’ following acute injury, as well as controversy regarding the ideal cell type for therapy, and a lack of understanding regarding the underlying cellular mechanisms mediating cardiac regeneration, we remain cautiously optimistic that cell based therapies may be sufficiently developed to effectively regenerate myocardium following cardiac injury.
In this review, we focus on the role of bone marrow derived stem cells and cardiac stem/progenitor cells in cardiomyocyte homeostasis and myocardial repair and regeneration, as well as provide a brief overview of current clinical trials utilizing cell-based therapeutic approaches in patients with heart disease.
Cardiac Stem/Progenitor Cells
For decades, the adult heart has been thought to exist as a terminally differentiated organ with limited proliferative capacity. Cardiomyocytes undergo hypertrophy, rather then hyperplasia, in response to hemodynamic stress, in contrast to other tissues, such as liver, intestine, and skeletal muscle. These long-held tenets of myocardial biology have recently been challenged and a new paradigm of the heart as a partially self-renewing organ has been proposed. While evidence challenging the old belief has fermented for some time 16,17, the identification of resident cardiac stem/progenitor cells (summarized in Table 1) has brought this new concept to the scientific forefront 12,13,15,18-20 and suggests the capacity of adult myocardium to maintain physiological homeostasis, at least partially, through resident cardiac stem cells.
Table 1.
Summery of reported cardiac stem/progenitor cells
| Surface Markers | Gene Expression | Self renewal | In-vitro differentiation | In-vivo differentiation | Species | Reference | |
|---|---|---|---|---|---|---|---|
| SP | Sca-1+, CD31+, CD34 -, c-kit-*, CD45-, Isl1- | Nkx2.5, GATA4, MEF2C, Tie2 | Yes | CM, CE, SMC | CM, EC | Mice, pig, human | Hierlihy et al 2002 18 Pfister et al 2005 15 Mouquet et al 2005 49 Martin et al 2004 13 Messina et al 2003 14 |
| c-kit | Sca-1+, SP+** Lin-, CD45-, CD31-, CD34- | Nkx2.5, GATA4, MEF2C | Yes | CM, EC, SMC | CM, EC, SMC | Mice, rat, dog, pig, human, | Urbanek et al 2003 107 Beltrami et al 2003 12 Linke et al 2005 108 |
| Sca-1 | CD31+, CD34- Lin-, CD45-, c-kit-*** | Nkx2.5***, GATA4, MEF2C, Tie2 | ND | CM | CM | Mice, dog | Oh et al 2003 19 Matsuura et al 2004 109 |
| Isl-1 | Lin-, CD45-, c-kit-, CD34-, CD31-, SP- | Nkx2.5, GATA4 | Yes | CM | ND | Mice, rat, human | Laugwitz et al 2005 20 |
CM: cardiomyocyte. EC: endothelial cell. SMC: smooth muscle cell. ND: not determined
C-kit might be cleaved by cardiac tissue enzymatic digestion. Martin et al found no expression of CD31, while Pfister et al reported two sub populations of CD31+ and CD31- among CSP. These differences may due to slight variation in isolation procedures used between groups.
SP phenotype was not addressed through Hoechst exclusion but through MDR-1 expression.
Matsuura et al reported 40% and 10% of Sca-1 cells expressing CD45 and CD34, respectively.
The report by Hierlihy and colleagues 18 in 2002 was the first identifying the presence of a stem cell-like population in adult hearts based on their specific ability to efflux Hoechst dye. Such Hoechst-effluxing capacity was first introduced to identify highly enriched hematopoetic stem cell populations, termed side population (SP) stem cells, from bone marrow 21. Recently, this methodology has been utilized to identify tissue specific stem/progenitor cells in various adult organs including pancreas, pituitary, testis, mammary gland, lung, liver, skeletal muscle, liver, lung as well as heart (for review see 22,23). Using immunohistochemistry analysis, Hierlihy et al found that adult myocardium retains a specific SP cells population, capable of tissue specific differentiate into cardiomyocytes, in-vitro 18. In 2003, Beltrami et al thoroughly described a population of cardiac stem cells (c-kit+ cells) found in clusters and residing among cardiomyocytes in adult hearts 12. In-vitro, cardiac c-kit+ cells appear to be clonogenic and were able to undergo self renewal and differentiation into cardiac cell lineages (cardiomyocytes, endothelial, smooth muscle cell). More importantly, these c-kit+ cell, when implanted in mouse hearts following MI, retained the capacity for differentiation into cardiomyocytes, in-vivo. These in-vivo data are of both scientific and clinical significance, as they strongly implicate the regeneration potential of cardiac stem cells in injured hearts. In the same year of Beltrami’s report, Oh et al employed a different stem cell marker, Sca-1, to identify yet another population of resident cardiac progenitor cells in adult hearts 19. Similarly, these Sca1+ cells were found to be capable of differentiation into cardiomyocytes, in-vitro and in-vivo, in response to 5-azacytidine and myocardial ischemia, respectively 19. In addition to the initial observation identifying SP cells in adult myocardium, several groups, including ours, have confirmed the presence of such progenitor cells population in adult hearts 13,15. Martin et al reported expression of α-sarcomeric actinin in cardiac SP cells co-cultured with other cardiac cells as well as demonstrated the presence of SP cells in human myocardium 13,18. Work performed by Tomita and colleagues documented the generation of neurosphere like clusters, referred to as “cardiospheres”, from neonatal cardiac SP cells 24. Similar to the cardiospheres described by Messina et al 14, cardiospheres derived from cardiac SP cells have been shown to harbor clonogenic cells with remarkable multi-lineage differentiation potential 24. These cardiospheres expressed cardiac, smooth muscle and interestingly, neuronal genes and proteins. Data from our group demonstrated not only the capacity for biochemical, but also functional, cardiomyogenic differentiation in cardiac SP cells 15. More importantly, our study demonstrated that among cardiac SP cells, cardiomyogeneic differentiation is restricted to cells negative for CD31 expression and positive for Sca-1 expression (CD31-/Sca-1+ SP cells) 15 While the in-vitro cardiomyogenic differentiation potential of cardiac SP cells has been consistently demonstrated, less is known about the ability of these cells to undergo cardiomyogeneic differentiation, in-vivo. Recently, Komuro and colleagues studied the homing and differentiation efficiency of intravenously injected cardiac SP cells in a myocardial cryoinjury rat model 25. Neonatal rat cardiac SP cells were found to be able to home to areas of injured myocardium and undergo differentiation into cardiomyocytes, endothelial cells, smooth muscle cells and fibroblasts. Another potential marker for cardiac stem/progenitor cells, Isl-1 (LIM homeodomain transcription factor), was more recently reported by Laugwitz et al 20. These cells were found to harbor similar cardiomyogenic potential in-vitro, though were phenotypically distinct from SP cells. As more information becomes available regarding cardiac stem/progenitor cells, a key question remains whether these seemingly unique progenitor populations described above are truly distinct from each other, or represent the same population of progenitor cells at different stages in the differentiation process.
Bone Marrow Derived Adult Stem Cells
The bone marrow is known to be excellent reservoir for many adult stem cells, and bone marrow derived stem cells have been employed to treat hematologic disorders for decades. Recent reports have demonstrated that bone marrow derived stem cells are able to traverse cell lineage boundaries and transdifferentiate into hepatocytes, endothelial cells, skeletal muscle, and neurons upon proper stimulation 26-28. While the ability of bone marrow derived stem cells to transdifferentiate into cardiomyocytes remains highly controversial, much of the recent progress in regenerative cardiovascular research, both in animal and humans, has been achieved using bone marrow derived stem cell populations, including hematopoietic stem cells (HSC), mesenchymal stem cells (MSC), and endothelial progenitor cells (EPC).
Hematopoietic stem cells (HSC)
HSC can be isolated from bone marrow cells through selective sorting for a particular set of surface receptors (Lineage-, c-kit+, Sca-1+, CD34lo, CD38hi) 29,30 and represent the prototypic adult stem cell population. The ability of HSC to reconstitute the hematopoietic system of a myeloablated host led to the first clinical application of adult stem cells more than three decades ago 31. Despite the failure of studies to definitely prove differentiation of HSC into cardiomyocytes, in-vitro, several studies in mice have demonstrated the potential of HSC to differentiate into cardiomyocytes or vascular cells following cardiac injury, in-vivo 32-34.
Mesenchymal stem cells (MSC)
Within the bone marrow stroma resides a subset of non-hematopoietic cells that have the potential to differentiate into cells of mesenchymal origin 35,36. These mesenchymal stem cells (MSC) represent approximately 0.001 to 0.01% of the total nucleated marrow cell population, a concentration 10-fold lower than their hematopoietic counterparts. MSC are self-renewing and expandable in-vitro using standard cell culture techniques. Immunophenotypically, MSC lack the typical hematopoietic antigens (CD45, CD34, CD14) but express specific adhesion molecules (ALCAM/CD44) and antigens (SH2/SH3/SH4/STRO-1) 37,38. At first, MSC were thought to contribute solely to the formation of the stromal microenvironment in the bone marrow and maintain HSC survival and function. However, subsequent studies have suggested that MSC are themselves capable of multipotency, with differentiation into chondrocytes, osteoblasts, astrocytes, neurons, skeletal muscle and, notably, cardiomyocytes 26,39-41.
Endothelial progenitor cells (EPC)
Endothelial progenitor cells (EPC) represent a subset of hematopoietic stem cells that are able to acquire an endothelial phenotype, in-vitro 42-45. EPC express the hematopoietic stem cell markers CD133, CD34 and the endothelial marker Flk-1 (VEGFR-2) 44. EPC can be isolated directly from the bone marrow or from the peripheral circulation and expanded, in-vitro.
Cardiac Cellular Homeostasis: Physiological and Pathological States
The identification of resident cardiac stem/progenitor cells evokes a new understanding of the mechanisms by which the adult heart may maintain cellular homeostasis. It is still a matter of debate whether cardiac cellular homeostasis is maintained solely by endogenous stem/progenitor cells or via extra-cardiac sources, notably bone marrow derived stem cells. In particular, the observation of male (host) cells in male patients transplanted with female hearts (mix-gender donor hearts) 46,47 suggests the potential role of extra-cardiac stem cells in the turnover of the cardiac cells. Interestingly, it has been proposed that the chimerism observed in humans may possibly result from cardiac progenitor cells residing in host atria which were kept intact during cardiac transplantation, and not from circulating bone marrow stem cells 48. More recent data in animal models, however, have suggested that bone marrow derived stem cells contribute little in maintaining the homeostasis of cardiac cells during normal post natal growth as well as normal adulthood 34,49,50
In contrast, bone marrow derived stem cells likely play a significant role in maintaining cardiac cells homeostasis, including the turnover of cardiac stem/progenitor cells, cardiomyogenesis, and angiogenesis, following myocardial injury 49-51. Jackson et al 34 demonstrated the ability of bone marrow SP cells to undergo cardiomyogenic differentiation, albeit at a very low frequency, and angiogenesis, following MI. Utilizing a murine model of GFP-labeled bone marrow, we also have found that bone marrow derived stem cells (SP cells) homed to areas of injured heart as early as three days following MI 49. These bone marrow derived cells may not only contribute to active myocardial repair, as have been suggested by several groups 8,9,32,33,52-56, but also participate in the reconstitution of the cardiac progenitor cell pool 49. This is further supported by additional recent work 50, which has utilized genetic mouse models to demonstrate an increase in cardiac c-kit+ cells, recruited from bone marrow, following MI 50 Moreover, using a rat model of heterotropic gender-mismatched cardiac transplantation, Wang et al also demonstrated that bone marrow derived stem cells are attracted to areas of myocardial ischemic injury and participate in cardiac repair 51 These experimental data were further supported by observations in human mix-gender cardiac transplants, which suggest greater cardiac chimerism may occur in patients with MI 57. In summary, the current literature suggests that cardiac injury may serve as a necessary and potent stimulant for the recruitment and potential cardiomyogeneic differentiation of endogenous bone marrow derived stem cells.
Mobilization and Homing of Marrow Derived Stem Cell
It is well recognized that, despite the existence of cardiac stem/progenitor cells, this endogenous capacity for regeneration is insufficient to mediate repair following severe cardiac injury. Thus, the ability of injured myocardium to recruit extra-cardiac stem cells following injury is critical to aid in myocardial repair and regeneration. At least three major compartments can be thought of to regulate this complicated orchestra, the injured myocardium, the bone marrow, and the peripheral circulation. The injured myocardium is responsible for releasing the signals via peripheral blood to signal the mobilization of the extra-cardiac stem cells from the major reservoir, bone marrow, into peripheral circulation. Following mobilization, these circulating bone marrow-derived stem cells are then able to follow a trail marked by specific signals, subsequently exit the circulation, and home to injured sites to initiate the cardiac repair process (Figure 1). These three players involved in mobilization and homing process must work together to achieve functional significant stem cell-mediated repair and regeneration.
Figure 1.
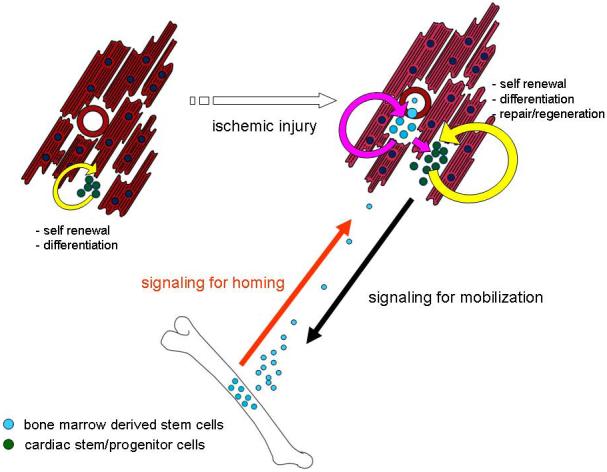
A schematic representation of cell-based myocardial repair. Signals for mobilization and homing must work in an integrated fashion among the myocardium, peripheral blood, and bone marrow to achieve functionally significant stem cell-mediated repair and regeneration.
The precise time course, kinetics and factors stimulating bone marrow mobilization remain the subject of intense investigation; nonetheless, several crucial factors have been shown to promote the mobilization of bone marrow-derived stem cells into peripheral circulation, including granulocyte colony-stimulating factor (G-CSF), granulocyte / macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF) and erythropoietin (EPO) (for review, 58). Myocardial ischemia is known to induce several classically ‘mobilizing cytokines’, including, but not limit to, G-CSF 59,60,61, SCF 59-61, VEGF 61-65, SDF-1 59,61,65,66, and EPO 67,68 and these cytokines may be responsible for the observed homing of bone marrow-derived stem cells following MI. Mobilization of EPC through cytokine stimulants increases EPC concentration in the peripheral circulation substantially 68. In addition to well-recognized HSC mobilizing agents such as G-CSF and SCF, VEGF, and EPO, statins have been shown to promote EPC recruitment 68-71. Moreover, given the capacity of bone marrow-derived stem cells to home to sites of injury, it has been suggested that mobilization of bone marrow-derived stem cells through systemically delivered cytokine stimulants may represent a less invasive strategy to activate and deliver stem cells following MI. Therefore, these cytokines/factors and their respectively receptors can be targeted to promote stem cell mobilization and homing for therapeutics applications. Herein, we highlight several key signalling factors to demonstrate the potential of manipulating these signalling axises to achieve functionally significant cell-based cardiac repair.
G-CSF and SCF/c-kit
SCF, also known as steel factor, is a ligand for c-kit, a receptor expressed in stem cell and tissue progenitor cells, including resident cardiac stem cells. Similar to G-CSF and GM-CSF, SCF is a hematopoietic factor that is well known to regulate proliferation, differentiation and survival of bone marrow derived stem cells 72,73 Orlic et al was the first to use a combined therapy of G-CSF and SCF in a murine model of MI and demonstrated a significant improvement in LV remodelling, cardiac function, and animal survival with five days of treatment 33. Improved outcome was associated with significant bone marrow derived cardiomyogenesis 33 These results, however, were not reproduced when G-CSF and SCF were given as a single dose at 4 hours following MI to non-human primates 74 While G-CSF and SCF/c-kit represent important factors for the recruitment of bone marrow derived stem cells following MI, actual results from various groups have been controversial at best; owing to the timing of cytokine administration and the dose utilized, as well as the actual model system. Nonetheless, the best “proof of concept” approach demonstrating the importance and involvement of the SCF/c-kit axis in bone marrow mobilization and cardiac repair has taken advantage of a transgenic mouse model overexpressing mutant c-kit (kitw/kitw-v) 50. In kitw/kitw-v mice, the mobilization and homing of bone marrow-derived stem cells to the heart is markedly impaired following MI, despite elevated circulating levels of SCF. This deficiency further results in early cardiac failure and death. Intriguingly, this dysfunction can be rescued by bone marrow transplantation with wild type cells, thus restoring the capacity for homing by bone marrow derived stem cells. While certainly essential, proper manipulation of the SCF/c-kit axis for clinical benefit remains a goal of the future.
SDF-1/CXCR4
SDF-1, and its receptor CXCR4, have recently been suggested to also be important in regulating the mobilization of stem cells. Using gain and loss of function approaches, Moore et al has demonstrated that inhibition of SDF-1/CXCR4 by neutralizing antibodies retards the mobilization of stem cells 75; while overexpression of CXCR4 augments the migration of progenitor cells 76 More recently, several groups have suggested that the SDF-1/CXCR4 axis may be involved in the mobilization of bone marrow-derived stem cells and homing to injured myocardium following MI 77,78, whereas the mechanisms regulating this mobilization remain less clear. SDF-1 may upregulate secondary agents, including metalloproteinase-9 (MMP-9), causing the release of SCF and subsequently mobilization of c-kit+ cells into the circulation 79 Alternatively, Misao and colleagues have suggested that the beneficial effects of G-CSF mediated cardiac repair following MI is through the upregulation of SDF-1 and subsequently recruitment of CXCR4+ cells 80 Altogether, these reports demonstrate the complex interplay that exists between these mobilizing / homing signals. To take advantage of these secreted factors/cytokines to improve post-MI cardiac repair and regeneration, thorough investigation of the timing of the release and interactions among signalling factors is required.
Potential Mechanisms of Stem Cell-mediated Myocardial Repair/Regeneration
Over the past decade, many groups have employed a spectrum of bone marrow-derived stem cell populations, including total bone marrow, HSC, MSC, and EPC for the treatment of post-MI heart failure in both animal models as well as in human clinical trials. Interestingly, while only few groups have observed differentiation of bone marrow derived stem cell into cardiomyocytes, most groups have reported a beneficial effect on post-MI remodelling. As such, these data are certainly both encouraging, given the improvement in objective measures such as cardiac structure and function, yet disappointing, as they fail to demonstrate physiologically relevant cardiac differentiation. This has brought into question the ultimate goal of cell-based therapies. Certainly our central objective is to improve cardiac function, and by doing so, patient outcomes. To that end, cell based therapies may be beneficial not only in the regeneration of lost myocardium, via direct trans-differentiation, but also in protecting existing viable myocardium or repairing damaged myocardium, via paracrine influences.
Stem Cells (Trans)differentiation
The foremost purpose of cell-based therapies remains the regeneration of lost cardiac cells via differentiation. Using genetic markers and/or labelled fluorescent dyes, several groups have reported the transdifferentiation of bone marrow derived HSC into cardiomycoytes 32-34 However, these results, subsequently, have been called into question by others, who have failed to identify HSC-derived cardiomyocytes 81-83. In addition to HSC, MSC have also been suggested to retain the capacity for cardiomyogenic differentiation both in-vitro with proper stimulation and in-vivo 40,55,56,84,85. Similar to criticisms voiced regarding HSC, the ability of MSC to trans-differentiate into cardiomyocytes have been challenged 86. Furthermore, many studies have suggested that cell fusion, rather than trans-differentiation, of bone marrow derived cells may explain observed phenotypic changes 87-90 Regardless of the mechanism responsible, fusion vs. trans-differentiation, it is generally agreed that the number of reported cardiomyocytes derived from exogenously delivered bone marrow stem cells remains relatively low and cannot physically account for observed functional improvements. As such, one alternative proposed mechanism is stem cell-mediated paracrine effects (discussed in the following section). In contrast to cardiomyogenesis, by in large, most groups have observed bone marrow derived stem cells to contribute to angiogenesis, an observation made over 10 years by Asahara and colleagues 42,91 and more recently, by many other laboratories 8,92-95. Finally, an alternative mechanism by which bone marrow derived stem cell populations may contribute to myocardial repair is via maintenance of cardiac-specific stem cells pool following injury. Indeed, our group has recently found that bone marrow derived SP cells home to injured myocardium following MI and under go phenotypic changes to adapt a cardiac SP cell phenotype. Such cardiac SP cells, may in-turn, contribute to the capacity of the heart for long-term endogenous cardiac repair.
Paracrine Influence
As suggested above, the beneficial effects of stem cell therapy on post-MI cardiac function remain disproportionate to the degree of cardiomyogeneic differentiation. Such observations have lead to the hypothesis that potential paracrine effects may hold a prominent role in stem cell therapy. Such paracrine influences may include secretion of factors that either attenuate apoptosis of endogenous cardiomyocytes 96,97 and EC 8, promote angiogenesis 52,98, and/or activate resident cardiac stem / progenitor cells 80. Uemura and colleagues suggested that hypoxia-induced apoptosis may be attenuated in cardiomyocytes co-cultured with total bone marrow cells, in-vitro 97. Moreover, mesenchymal stem cells also may produce angiogenic factors such as VEGF and bFGF, as well as chemotactic factors, including as MCP-1 and PGF, that serve to recruit monocytes and promote angiogenesis 99. While the “paracrine hypothesis” of stem cell therapy seems rational given prior observations, to date, it remains unexplored with largely indirect supportive evidence.
Current Translational Approaches for Bone Marrow Based Therapies
While the ideal cell type for stem cell-based therapies remains to be determined, to date, bone marrow derived stem cells, isolated from whole bone marrow aspirate, remains the most commonly used cell type for human studies. This is largely due to its easy accessibility and well characterized properties by haematologists for over thirty years. Among bone marrow populations, total mononuclear bone marrow cells and circulating endothelial progenitor cells have recently or are currently being employed in many Phase I and/or II trials (Table 2). Current methods of delivery include direct intramyocardial injection, via both endocardial catheter-based and epicardial surgical-based approaches, and more recently, percutaneous (catheter-based) intracoronary injection. Alternatively, indirect mobilization has also been attempted with peripheral delivery of cytokines, notably G-CSF.
Table 2.
Summary of Major Cell-Based / Cytokine Clinical Trials
| Study | Method of delivery | Patients treated/ control | Placebo/ control | Cell type Cell number or Dose | Time of cell delivery (days post-MI) | Results | Reference |
|---|---|---|---|---|---|---|---|
| Strauer | Intracoronary | 10/10 30 (CPC) | control | BM-MNC 9 × 106 to 2.8 × 107 CPC |
7 | Improved contractility and reduced infarct size | Strauer et al 2002 110 |
| TOPCARE-AMI | Intracoronary | 29 (BM-MNC) | N/A | 1.3 × 107 BM-MNC 2.4 × 108 |
3 to 7 | Improved EF and reduced infarct size | Assmus et al 2002 111 Britten et al 2003 112 Schachinger et al 2004 113 |
| BOOST | Intracoronary | 30/30 | control | BM-MNC 24 × 109 |
6 | Improved EF at 6 months No difference at 18 months |
Wollert et al 2004 100 Meyer et al 2006 101 |
| Janssens | Intracoronary | 33/34 | placebo | BM-MNC 3.0 × 108 cells |
1 | No effect | Janssens et al 2006 114 |
| Chen | Intracoronary | 34/35 | placebo | MSC 48 × 1010 to 60 × 1010 |
18 | Improved and perfusion at 3 months | Chen et al 2004 115 |
| REPAIR-AMI | Intracoronary | 102/102 | placebo | BM-MNC 2.4 × 108 |
4 | Improved EF and reduced infarct size at 4 months | Schachinger et al 2006 103 |
| ASTAMI | Intracoronary | 100 | control | BM-MNC 8.7 × 107 |
5 to 8 | No difference at 6 months | Lunde et al 2006 102 |
| FIRSTLINE-AMI | Mobilization | 25/25 | control | G-CSF 10μg/Kg BW |
0 to 6 1QD |
Improved EF and remodeling at 4 months | Ince et al. 2005 104 |
| STEMMI | Mobilization | 39/39 | placebo | G-CSF 10μg/Kg BW |
0 to 6 1QD |
No difference at 6 months | Ripa et al 2006 106 |
| REVIVAL II | Mobilization | 56/58 | placebo | G-CSF 10μg/Kg BW |
0 to 5 1QD |
No difference at 6 months | Zohlnhoefer et al 2006 105 |
BM-MNC: unfractionated bone marrow mononuclear cells; CPC: Circulating progenitor cells; MSC: mesenchymal stem cells; 1QD: one subcutaneous G-CSF injection per day; EF: left ventricular ejection fraction.
In just a few years, cell based therapy has evolved at an explosive pace, from early in-vitro cell studies to animal models of myocardial infarction, and now to several early phase clinical trials. Overall, most initial non-randomized clinical trials, while designed for safety rather than efficacy, have encouragingly suggested a moderate improvement in heart function following stem cell therapy. The first randomized trial of intracoronary bone marrow derived stem cells, the BOOST I trial 100, demonstrated an early benefit in left-ventricular ejection fraction at 6 months post-cell therapy as assessed by cardiac MRI. However, due to continued improvement in the control group, the benefit in treated patients relatively to the control group was lost at 18 months follow up 101. Two larger clinical trials investigating intracoronary delivery of bone marrow cells have also been initiated, with early results presented at the Scientific Sections of American Heart Association in late 2005. In the ASTAMI trial, a randomized trial of one hundred patients 102 with acute MI, bone marrow mononuclear cells was delivered 6 days post-PTCA. At 6 months follow-up, no improvement in left ventricular ejection fraction or infarct size was observed. In somewhat contrast, the REPAIR-AMI 103, a randomized, placebo control trial with over two hundred patients following acute-MI, suggested a small, albeit significantly important, improvement in left ventricular ejection fraction by ventriculography. While both trials still are ongoing, many potential explanations for observed differences, including severity ventricular dysfunction, the timing of cell delivery, and the method cells isolation (quality of the cells), have been proposed and are currently undergoing investigation in animal models. Mobilization of stem cells from the bone marrow represents an alternative cell-based therapy that has also recently been investigated in clinical trials. The FIRSTINE-AMI 104 has demonstrated not only the safety and feasibility of bone marrow mobilization using G-CSF in MI patients after reperfusion, but also suggested a potential improvement in left ventricular ejection fraction and an attenuation of left ventricular dilation. Importantly, this trial showed that treatment with G-CSF did not augment post-percutaneous coronary intervention restenosis rate. However, subsequent randomized placebo controlled clinical trials, REVIVAL II 105 and STEMMI 106, have failed to reproduce the benefits previously seen in early human studies. Although these trials failed to demonstrate positive outcomes, no adverse events, including vessel restenosis, were observed. Reasons for these negative results remain to be determined, though, inappropriate cytokine dosing and inadequate timing of the cytokine administration have been proposed as potential explanations.
Conclusions
Myocardial infarction results in cell death and it replacement of cardiomyocytes with non-contractile scar tissue. The optimal goal for cell-based cardiac repair is to restore cardiac structure and function through regeneration of functionally-competent cardiomyocytes. To rebuild a normal and functional cardiac tissue requires not only highly integrated cardiomyogenesis and angiogenesis, but also a proper matrix network system, to ensure synchronized contraction and relaxation with native myocardium. While the task of achieving such goal is daunting, the therapeutic potential of myocardial regeneration remains enormous. Further intensive research, in basic science and clinical arenas, as well as carefully constructed clinical trials, are needed in order to make this next great frontier in cardiovascular medicine a reality.
Acknowledgements
This work was supported by research grants funding from the National Institutes of Health (R.L.). O.P. is a recipient of a Swiss National Science Foundation Grant (PBBSB-101349), and F.M. is a recipient of a Federation Francaise de Cardiologie grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 2.Scorsin M, et al. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg. 2000;119:1169–75. doi: 10.1067/mtc.2000.104865. [DOI] [PubMed] [Google Scholar]
- 3.Koh GY, et al. Stable fetal cardiomyocyte grafts in the hearts of dystrophic mice and dogs. J Clin Invest. 1995;96:2034–42. doi: 10.1172/JCI118251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain M, et al. Cell therapy attenuates deleterious ventricular remodeling and improves cardiac performance after myocardial infarction. Circulation. 2001;103:1920–7. doi: 10.1161/01.cir.103.14.1920. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DA, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 6.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–23. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12–8. [PubMed] [Google Scholar]
- 8.Kocher AA, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto A, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 10.Orlic D. Adult BM stem cells regenerate mouse myocardium. Cytotherapy. 2002;4:521–5. doi: 10.1080/146532402761624674. [DOI] [PubMed] [Google Scholar]
- 11.Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann N Y Acad Sci. 2003;996:152–7. doi: 10.1111/j.1749-6632.2003.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami AP, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Martin CM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–75. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Messina E, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 15.Pfister O, et al. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 16.McDonnell TJ, Oberpriller JO. The response of the atrium to direct mechanical wounding in the adult heart of the newt, Notophthalmus viridescens. An electron-microscopic and autoradiographic study. Cell Tissue Res. 1984;235:583–92. doi: 10.1007/BF00226956. [DOI] [PubMed] [Google Scholar]
- 17.Rumyantsev PP, Borisov A. DNA synthesis in myocytes from different myocardial compartments of young rats in norm, after experimental infarction and in vitro. Biomed Biochim Acta. 1987;46:S610–5. [PubMed] [Google Scholar]
- 18.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–43. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 19.Oh H, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laugwitz KL, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–45. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 23.Challen GA, Little MH. A Side Order of Stem Cells: The SP Phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 24.Tomita Y, et al. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170:1135–46. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada H, Matsuura K, Oyama T, Iwanaga K, Takahashi T, Goto T, Nagai T, Komuro I. Homing and differentiation of intravenously transplanted cardiac side population cells in injured myocardium. Circulation. 2005;111:801. [Google Scholar]
- 26.Ferrari G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 27.Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 28.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 29.Osawa M, et al. In vivo self-renewal of c-Kit+ Sca-1+ Lin(low/-) hemopoietic stem cells. J Immunol. 1996;156:3207–14. [PubMed] [Google Scholar]
- 30.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 31.Goldman JM, Horowitz MM. The international bone marrow transplant registry. Int J Hematol. 2002;76(Suppl 1):393–7. doi: 10.1007/BF03165291. [DOI] [PubMed] [Google Scholar]
- 32.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 33.Orlic D, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–9. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson KA, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow.Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 36.Tavassoli M, Crosby WH. Transplantation of marrow to extramedullary sites. Science. 1968;161:54–6. doi: 10.1126/science.161.3836.54. [DOI] [PubMed] [Google Scholar]
- 37.Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 38.Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 39.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–13. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makino S, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira RF, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–61. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asahara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 43.Gehling UM, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- 44.Peichev M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 45.Shi Q, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–7. [PubMed] [Google Scholar]
- 46.Quaini F, et al. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- 47.Murry CE, Whitney ML, Laflamme MA, Reinecke H, Field LJ. Cellular therapies for myocardial infarct repair. Cold Spring Harb Symp Quant Biol. 2002;67:519–26. doi: 10.1101/sqb.2002.67.519. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz RS, Curfman GD. Can the heart repair itself? N Engl J Med. 2002;346:2–4. doi: 10.1056/NEJM200201033460102. [DOI] [PubMed] [Google Scholar]
- 49.Mouquet F, et al. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–2. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 50.Fazel S, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–77. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, et al. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol. 2006;40:736–45. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Kamihata H, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 53.Kawamoto A, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–7. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 54.Mangi AA, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 55.Shake JG, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73:1919–25. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 56.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 57.Hocht-Zeisberg E, et al. Cellular repopulation of myocardial infarction in patients with sex-mismatched heart transplantation. Eur Heart J. 2004;25:749–58. doi: 10.1016/j.ehj.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 59.Leone AM, et al. Endogenous G-CSF and CD34(+) cell mobilization after acute myocardial infarction. Int J Cardiol. 2006;111:202–8. doi: 10.1016/j.ijcard.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 60.Dewald O, et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–77. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wojakowski W, et al. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–20. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 62.Namiki A, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–95. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 63.Couffinhal T, et al. Mouse model of angiogenesis. Am J Pathol. 1998;152:1667–79. [PMC free article] [PubMed] [Google Scholar]
- 64.Banai S, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–9. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, et al. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768–74. doi: 10.1136/hrt.2005.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Askari AT, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 67.Vogt C, Noe G, Rich IN. The role of the blood island during normal and 5-fluorouracil-perturbed hemopoiesis. Blood Cells. 1991;17:105–21. discussion 121-5. [PubMed] [Google Scholar]
- 68.Heeschen C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 69.Kalka C, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86:1198–202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 70.Llevadot J, et al. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walter DH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–24. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 72.Ulich TR, et al. Hematologic effects of stem cell factor in vivo and in vitro in rodents. Blood. 1991;78:645–50. [PubMed] [Google Scholar]
- 73.Anderson DM, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–43. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 74.Norol F, et al. Influence of mobilized stem cells on myocardial infarct repair in a nonhuman primate model. Blood. 2003;102:4361–8. doi: 10.1182/blood-2003-03-0685. [DOI] [PubMed] [Google Scholar]
- 75.Petit I, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 76.Kahn J, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 77.Kucia M, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbott JD, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 79.Heissig B, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Misao Y, et al. Importance of recruitment of bone marrow-derived CXCR4(+) cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–65. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Murry CE, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 82.Balsam LB, et al. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 83.Limbourg FP, et al. Haematopoietic stem cells improve cardiac function after infarction without permanent cardiac engraftment. Eur J Heart Fail. 2005;7:722–9. doi: 10.1016/j.ejheart.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 84.Tomita S, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 85.Hakuno D, et al. Bone marrow-derived regenerated cardiomyocytes (CMG Cells) express functional adrenergic and muscarinic receptors. Circulation. 2002;105:380–6. doi: 10.1161/hc0302.102593. [DOI] [PubMed] [Google Scholar]
- 86.Davani S, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108(Suppl 1):II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 87.Terada N, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–5. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 88.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 89.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 90.Vassilopoulos G, Russell DW. Cell fusion: an alternative to stem cell plasticity and its therapeutic implications. Curr Opin Genet Dev. 2003;13:480–5. doi: 10.1016/s0959-437x(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 91.Asahara T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 92.Urbich C, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–6. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 93.Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–7. doi: 10.1038/nm963. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003;100:2426–31. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang JS, Shum-Tim D, Chedrawy E, Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration: pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi T, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 97.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 98.Fuchs S, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–32. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 99.Kinnaird T, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 100.Wollert KC, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 101.Meyer GP, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 102.Lunde K, et al. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J. 2005;39:150–8. doi: 10.1080/14017430510009131. [DOI] [PubMed] [Google Scholar]
- 103.Schachinger V, Tonn T, Dimmeler S, Zeiher AM. Bone-marrow-derived progenitor cell therapy in need of proof of concept: design of the REPAIR-AMI trial. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S23–8. doi: 10.1038/ncpcardio0441. [DOI] [PubMed] [Google Scholar]
- 104.Ince H, et al. Prevention of left ventricular remodeling with granulocyte colony-stimulating factor after acute myocardial infarction: final 1-year results of the Front-Integrated Revascularization and Stem Cell Liberation in Evolving Acute Myocardial Infarction by Granulocyte Colony-Stimulating Factor (FIRSTLINE-AMI) Trial. Circulation. 2005;112:I73–80. doi: 10.1161/CIRCULATIONAHA.104.524827. [DOI] [PubMed] [Google Scholar]
- 105.Zohlnhofer D, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. Jama. 2006;295:1003–10. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- 106.Ripa RS, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–92. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- 107.Urbanek K, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–5. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Linke A, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–71. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsuura K, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 110.Strauer BE, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–8. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 111.Assmus B, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 112.Britten MB, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–8. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 113.Schachinger V, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 114.Janssens S, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 115.Chen SL, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–8. [PubMed] [Google Scholar]


