Abstract
For the past decades, numerous works have been dedicated to the development of signal processing methods aimed at measuring the degree of association between EEG signals. This interdependency parameter, which may be defined in various ways, is often used to characterize a functional coupling between different brain structures or regions during either normal or pathological processes. In this paper we focus on the time-frequency characterization of the interdependency between signals. Particularly, we propose a novel estimator of the linear relationship between nonstationary signals based on the cross correlation of narrow band filtered signals. This estimator is compared to a more classical estimator based on the coherence function. In a simulation framework, results show that it may exhibit better statistical performances (bias and variance or mean square error) when a priori knowledge about time delay between signals is available. On real data (intracerebral EEG signals), results show that this estimator may also enhance the readability of the time-frequency representation of relationship and thus can improve the interpretation of nonstationary interdependencies in EEG signals. Finally, we illustrate the importance of characterizing the relationship in both time and frequency domains by comparing with frequency-independent methods (linear and nonlinear).
Keywords: Algorithms; Brain; physiopathology; Computer Simulation; Diagnosis, Computer-Assisted; methods; Electroencephalography; methods; Epilepsy; diagnosis; physiopathology; Humans; Models, Neurological; Models, Statistical; Reproducibility of Results; Sensitivity and Specificity; Signal Processing, Computer-Assisted; Stochastic Processes
Keywords: Coherence, Time-Frequency, Functional Coupling, Statistical Coupling, Neuroscience, Nonlinear System
I. Introduction
Electroencephalographic (EEG) signals provide information about cerebral activity with an excellent time resolution (in the order of 1 ms). Quantitative analysis of EEG signals is generally performed using signal processing (SP) methods which may substantially complement the visual inspection of time series.
For the past decades, numerous works have been dedicated to the development of SP approaches aimed at evaluating the degree of association (in a general sense, i.e. interdependency). Indeed, the statistical relationship between signals acquired from different brain structures or regions may be used to characterize the functional coupling between recorded sites during normal (cognitive, for instance) or pathological (epileptic, for instance) processes.
These methods may be divided into two categories depending on whether or not the nonlinear nature of the relationship is taken into account.
Linear methods were developed first. Many estimators based on linear cross-correlation or coherence function were proposed and used to study functional couplings between brain regions during cognitive tasks (Chapman et al. [1]) or during epileptic processes like seizures. In this latter field, works based on the coherence function were initiated by Brazier [2] who studied the propagation of epileptic activities from intracerebral recordings. They were followed by Gotman [3] who studied interhemispheric relations in partial seizures and by Duckrow et al. [4] and Franaszczuk et al. [5, 6] who analyzed possible synchronization mechanisms occurring at the seizure onset.
The development of nonlinear methods is more recent (Pikovsky et al. [7]). A first family of methods based on mutual information (Mars et al. [8]) or on nonlinear regression (Pijn et al. [9], Wendling et al. [10]) was first introduced in the EEG field. A second family is currently developing, based on works related to the study of nonlinear dynamical systems and chaos (Iasemidis [11], Lehnertz [12]).
Recently, a comparative study of different synchronization measures in real EEG data was performed by Quian Quiroga et al. [13]. Authors first confirmed that linear methods (coherence and cross-correlation) as well as nonlinear methods (mutual information, phase synchrony) are useful in EEG analysis and provide information that is not accessible by the visual inspection. They also showed that all these measures (except entropy) may give similar results, qualitatively.
If nonlinear methods have the capability to account for the nonlinearity of relationship, they are generally independent from frequency, a key parameter in the EEG analysis that can be related to the oscillatory behavior of recorded neural populations. On the opposite, linear methods can not be used to analyze the nonlinear property of relationship but they can characterize its dependence on frequency. However, as underlined by Zaveri et al. [14] who give a review on the use of coherence in the EEG field, proposed estimators generally have strong bias and variance which make the interpretation of real data intricate. To alleviate these difficulties, frequency bands may be defined. For instance, classical delta, theta, alpha, beta and gamma EEG bands can be used to average the coherence function (Razoumnikova [15]) or to compute the cross-correlation of filtered signals (Nikolaev et al. [16], Wendling et al. [17]). Again, this is not entirely satisfactory since the choice of frequency bands becomes critical in this case (relevant phenomena may overlap two bands).
In this paper, we present a novel estimator for characterizing the evolution of linear relationship between signals both in the time and frequency domains. This estimator is based on the computation of the Pearson Product-Moment correlation between EEG signals filtered, in narrow and overlapping frequency bands, using a continuous filter bank. Asymptotically, it gives the same value as that obtained with the classical coherence estimator (squared modulus of coherence function, periodogram method). However, application to simulated nonstationary signals shows that its performances (in terms of bias and variance) can be better. In addition, contrary to aforementioned methods, no assumption on frequency bands is required. The method has also been applied to real EEG signal intracerebrally recorded in epileptic patients candidate to surgery (Stereoelectroencephalography, SEEG). Results show that the method is able to track the evolution of relationship between signals in the time-frequency plane with a good resolution. They also show that the method may help to better analyze paroxysmal phenomena that occur at the onset of epileptic seizures.
II. Problem Statement and Methods
We are interested in measuring the degree of statistical time-frequency relationship between real signals. Our reference method is the coherence function computed in a sliding window. We compare this method with the linear correlation coefficient computed between corresponding outputs of a narrow-band filter bank applied to the two signals of interest.
We show, in the case of stochastic wide sense stationary signals, that these two kinds of measure correspond asymptotically to the same theoretical quantity.
We compare then these two estimators (in term of bias and variance) in different simulated situations for which the target is either derived by mathematical calculation or estimated by Monte-Carlo simulation. To make the two estimators comparable, the lengths of observation window and the frequency resolution (the shape and length of weighting functions) have to be the same.
Two kinds of simulation models are chosen to encompass different physiological situations that may be encountered in real data. In the first simulation (model M1), shared information of two analyzed signals has stochastic nature; whilst in the second simulation (model M2), it has deterministic nature.
The delay parameter between channels (generally optimized in correlation method) can be imposed in the case of having a priori information about its value (possibly zero); we investigate here the effect of this information on the estimator performances.
Finally, the application of these measures to real signals recorded during an epileptic seizure with typical signatures are presented and commented.
Problem statement
We assume an observation vector X(t) = {x1(t), …, xM(t)}; t ∈ [0, T] where xi(t) is the signal recorded from the ith sensor. The problem is to characterize the statistical relationship, both in the time and frequency domains, between nonstationary signals xi(t) and xj(t), t ∈ [0, T] for a given pair (i, j) ∈ {1 … M}2; i ≠ j. Indeed, this relationship may be interpreted as a functional coupling between two brain sites recorded by sensors i and j. The coupling evolution provides essential information about the organization of epileptic processes during seizures.
Numerous functionals Ψ(Pt, δ), where Pt, δ is the joint probability of (xi, xj) over an interval of [t−δ/2, t + δ/2[, can be introduced for characterizing the statistical relationship between xi and xj in the time-frequency plane. In this paper we focus on the comparison of two of them: i) the squared modulus of a local coherence function |ρij(t, f)|2 and ii) a local linear correlation coefficient , computed at the outputs of narrow band-pass filter, as the function of frequency and time, which is maximized for time delay. To our knowledge, the application of this latter method in the EEG field has never been reported.
Coherence function and frequency-dependent correlation coefficient
The squared modulus of the local coherence function is defined as below (Haykin et al. [18]):
| (1) |
where f is the frequency, γxixj, γxixi, and γxjxj designate the local power cross-spectral density or power auto-spectral densities during an interval [t−δ/2, t + δ/2] defined as:
where FT(x̃i) is the Fourier transform computed with respect to θ of locally weighted signal x̃i(θ, t) = xi(θ)h0(θ−t), θ ∈ ℝ with h0(θ), θ ∈ [−δ/2, δ/2] a weighting function (hamming window for example), and * stands for complex conjugate.
The local linear correlation coefficient is defined as follows:
| (2) |
where
| (3) |
is the linear correlation coefficient between signals xi,f and xj,f outputs of a band-pass filter whose impulse response is h(t, f) = h1(t)cos(2πft), t ∈ ℝ (characterized by a central frequency f). τm and τM are respectively minimum and maximum a priori bounds for the time delay between signals xi,f and xj,f. Since τm and τM are very small relatively to δ, we can neglect their effects and choose h1 identical to h0.
In theory, both quantities |ρij(t, f)|2 and are equal to 0 for xi independent from xj and increase, up to a maximum value of +1, for increasing linear dependence between xi and xj.
In the stationary case, and if δ → ∞ we have:
where ρij(f) is the classical coherence function associated to a pair of stationary random signals (Bendat and Piersol [19]). Furthermore, when relaxing the constraint τm≤τ≤τM to τ ∈ ℝ and for h1(t)cos(2πft), t ∈ ℝ, being the impulse response of a very-narrow band filter, (1) and (2) become asymptotically equivalent as demonstrated in appendix A. So, in the following, |ρij(t, f)|2 will be taken as a reference and we introduce estimators for (1) and (2) to measure it.
In practice, to be computed on digital signals sampled at frequency Fs, an estimation Ψ̂t,δ(xi, xj) of Ψ(Pt,δ) must be introduced in each case. For discrete time signals |ρij(t, f)|2 in (1) becomes |ρij[t, f]|2, t ∈ ℤ and |f|<FS/2. Estimators use N0 = [δFS] (where [.] designates the integer part of its argument) samples of xi[n] ≜ xi(n/FS), n/FS ∈ [t−δ/2, t + δ/2] for i =1, 2, (N0 is thus the length of a discrete sliding observation window)
Typically, the squared modulus of the local coherence function is estimated using the periodogram method over a sliding window (divided into Nb overlapped blocks) with the central point at time t:
| (4) |
where is the Discrete Fourier transform (DFT) of xi[n] weighted by Hamming window, with length L, in kth block of the sliding observation window.
The estimation of the local linear correlation coefficient is:
| (5) |
where y.,f[t] = x.,f[t−k] − x̄.,f and x̄.,f is the mean of x.,f over a sliding window of duration H. Here H = N0 − L to ensure using the same information from observed signals (it is worth to mention that here also we have neglected the effects of τm and τM because they are very small compared to L).
For 0 < f < Fs/2, an appropriate filter bank can be derived using the Short-Time Fourier Transform (STFT), as described in appendix B. To ensure to have the same frequency resolution for two estimators, the discrete impulse response of the band-pass filter is set to h[n]cos(2πfn/FS) where h is the Hamming window used in (4).
Comparison of the two estimators
First, we compare the performance of two estimators in terms of bias and MSE (mean square error) through simulations in which the linear relationship between simulated signals xi and xj may vary as a function of time. In each simulation, target values for |ρ[t, f]|2 are derived mathematically or computed by Monte-Carlo simulation using a large number of realizations. Then, both estimators are evaluated on real SEEG signals recorded from internal brain structures during seizure in a case of temporal lobe epilepsy (TLE).
1) Simulated signals: Comparative analyses of the two estimators are based upon two simulation models M1 and M2. These models are simple but rely on some physiological considerations.
Let Si and Sj be two sensors whose outputs are xi[t] and xj[t]. Furthermore, let Pi and Pj be two neural populations recorded respectively by sensor Si and Sj (Pi and Pj belong to two distinct brain structures). Our physiological hypothesis is that we have two partitions Pi = Pi1 ∪ Pi2 and Pj = Pj1 ∪ Pj2 where the two subpopulations Pi1 and Pj1 are interconnected by long neuronal fibers (the axons of pyramidal cells, see Martin [20]) and may synchronize with each other during ictal activities probably with a short mean time delay τ between activities of Pi1 and Pj1 (Nunez and Cutillo [21]). Pi2 and Pj2 are the neuronal subpopulations corresponding to the background activity without statistical relation with activities of Pi1 and Pj1. In our simulations, and without loss of generality, we imposed τm = τM = 0. The ictal activity of Pi1 and Pj1 may have, in many instances, less complexity (as defined in nonlinear dynamic systems theory; see Kantz and Schreiber [22]) than the background activities of Pi2 and Pj2.
These facts motivated the choice of model M1 and M2. In model M1 activities of Pi1 and Pj1 are random (non-deterministic), while in model M2 they are quasi-deterministic and may frequently exhibit dynamics similar to nonlinear oscillator’s outputs.
Model M1 is described by the following equations:
| (6) |
where B1, B2 and B3 are three independent wide sense stationary (IWSS) random signals having power spectral density (PSD) γi(f), i=1,2,3 and 0≤α[t]≤1 represents the time-varying degree of relationship between generated signals. B3 is interpreted as the shared physiological activity whereas B1 and B2 represent independent background activities.
The second model M2 is proposed to encompass the case in which a deterministic signal influences both x1 and x2 signals. In M2, simulated signals x1 and x2 are given by:
| (7) |
where B1 and B2 are as before two background activities; C[t] and α[t] are respectively the deterministic signal and the degree of relationship.
2) Estimation of the bias, variance and MSE: Theoretical values of |ρ[t, f]|2 are mathematically derived when α[t] is a constant in the model M1. For the cases of non-constant α[t] in M1 or M2 model, |ρ[t, f]|2 is computed by Monte-Carlo simulation. They are used as target to estimate the bias and mean square error (MSE) of both estimators on signals generated with model M1 and M2. Bias, variance and MSE are respectively defined by E(θ̂−θ), E{(θ̂−E(θ̂))2} and E{(θ̂−θ)2} where θ = |ρ[t, f]|2and θ̂ = |ρ̂[t, f]|2or θ̂ = R̂2[t, f]. These expectations are estimated over a sufficiently large number of realizations (also by Monte-Carlo simulation).
The effects of the various parameters used in the estimator |ρ̂ij[t, f]|2 and R̂ij2[t, f] were studied: the width of the sliding window, number of blocks for calculation of DFT, percentage of overlapping, and the time resolution of the STFT. For comparison purpose, the width of the sliding windows as well as the length and shape of the weighting functions were chosen the same for the two estimators.
3) Real SEEG signals: An illustrative example of nonstationary real EEG signals was chosen for direct comparison of both methods on real data. In this example, signals are recorded from two internal limbic structures (anterior hippocampus and amygdala) in a patient suffering from mesial TLE during a seizure. These two structures are anatomically interconnected. Several studies already reported that they may play an active role in the triggering of seizures in the temporal lobe.
In the presented case, they were found to be involved at the onset of seizures and to give rise to a characteristic electrophysiological pattern (spiking background activity followed by a rapid tonic discharge that gradually slows down, see Maldonado et al. [23]). Recordings were performed using multiple lead electrodes (10 to 15 leads, length: 2 mm, diameter: 0.8 mm, 1.5 mm apart) placed intracranially according to Talairach’s stereotactic method [24]. The positioning of electrodes was determined from available non-invasive information and hypothesis about the localization of his epileptogenic zone (defined as the cerebral regions simultaneously discharging at seizure onset). Signals are sampled at 256 Hz, digitized with precision of 16 bits/sample and stored on hard disk for further processing.
III. Results
Model M1, stationary situation
In the case where α is not dependent on time, signals x1 and x2 are stationary and the theoretical value of |ρ[t, f]|2 can be derived:
where γ̃Bi(f) is the PSD of product of discrete time signal Bi and weighting window h0 in section 2.2. Here, we choose γB1(f) = γB2(f) = γB3(f) and hence:
Bias and MSE values (variance is equal to MSE minus squared bias) were computed for different lengths of the sliding window duration N0 and different coupling parameter α (N0 = 256 × k1, k1= 2,…,5; α = 0.1 × k2, k2 = 0, …,10). The length L, of blocks used in (4), was set to 256 points. Consequently, the frequency resolution of both estimators |ρ̂[t, f]|2 and R̂2[t, f] is the same.
The overlapping percentage of two successive blocks in the computation of |ρ̂[t, f]|2 was chosen by minimizing the mean square error (MSE) between theoretical and estimated values. The optimal overlapping rate was found to be equal to 0.8 although the MSE does not decrease considerably for percentages beyond 50% (this result corroborates those published in Carter [25]).
Bias and MSE values were first computed by Monte-Carlo simulation over the time-frequency plane (1 frequency bin corresponds to 1 Hz) and were found to be approximately constant. This result shows that in this case both estimators are independent from frequency and time. Thus, bias and MSE values can be averaged over time-frequency. Fig. 1 represents the bias and the MSE of both methods for 4 different values of N0 and 11 values of α uniformly distributed between 0 and 1.
Fig. 1.
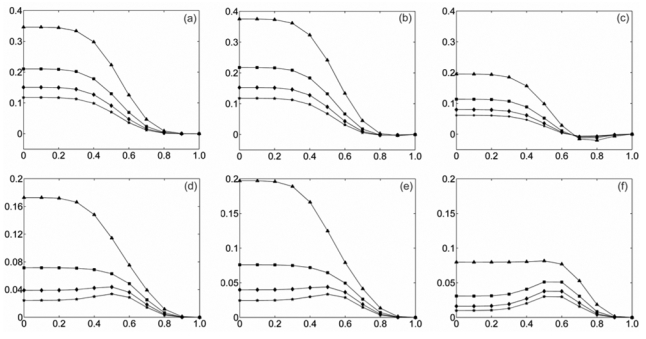
Bias and MSE of both estimators for model M1 (stationary situation, constant α[t]) for different duration N0 of the sliding window a) Bias of |ρ̂[t, f]|2. b) Bias of R̂2[t, f] with τM = −τm = 5. c) Bias of R̂2[t, f] with τm = τM = 0. d) MSE of |ρ̂[t, f]|2. e) MSE of R̂2[t, f] with τM = −τm = 5. f) MSE of R̂2[t, f] with τm = τM = 0. These curves are obtained by Monte Carlo simulation for long duration signals and by averaging over time-frequency plane.
As depicted on this figure, bias and MSE are approximately equal when we use the constraint τM = −τm = 5 and for τm = τM =τ0 (corresponding to the case where the time delay is known and equal to τ0; here τ0 = 0 = 0) the R̂2[t, f] has clearly smaller bias and MSE than |ρ̂[t, f]|2.
This result was obtained for two different kinds of PSD: similar bias and MSE curves were obtained when B1, B2 and B3 are white Gaussian noises or colored Gaussian noises with a 1/f power spectrum distribution similar to that of normal background EEG activity.
Model M1, nonstationary situation
Arbitrary profiles of evolutionary relationship were chosen for generating nonstationary signals x1 and x2. A scenario is provided in Fig. 2-a (dashed line) in which the evolution of α[t] was defined on four successive periods: linear increases from 0 to 1, constant period where α[t] = 1, constant period where α[t] = 0, linear decreases from 1 to 0. The target curve (Fig. 2-a, solid line) was computed from (Haykin et al. [18]):
Fig. 2.
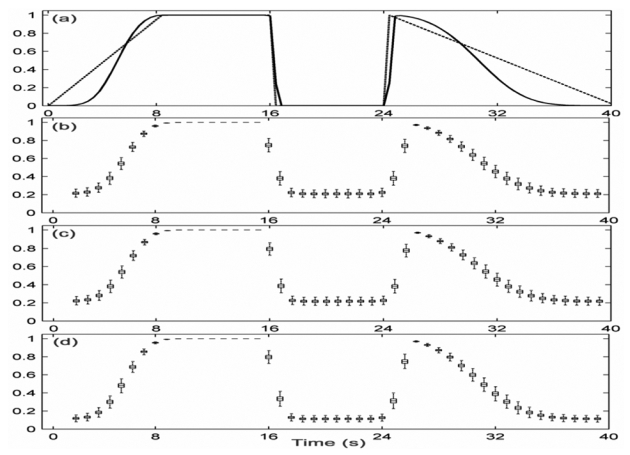
Performances of both estimators for model M1 (nonstationary situation, time-varying α[t] for N0 =768. a) Time course of parameter α[t] (dashed line) and target relationship curve (solid line) averaged over frequency axis. Standard boxplots obtained by Monte-Carlo simulation for different estimators: b) |ρ̂[t, f]|2, c) R̂2[t, f] with τM = −τm = 5, d) R̂2[t, f] with τm = τM = 0.
where denotes the STFT’s of the signal xi, and the superscript (n) denotes the nth of N=10000 realizations of two signals. In the computation of STFT we have used Hamming temporal window of length of L=256 to ensure having the same frequency resolution for target and for both estimators.
As in the stationary case, both estimators were found to be frequency independent and thus estimated values of bias and variance were obtained by averaging along the frequency axis in order to facilitate their comparison to the target curve. Quantitative results displayed in Fig. 2, using boxplots, show that R̂2[t, f] has smaller bias and variance values compared to those of |ρ̂[t, f]|2 when the time delay is known and equal to τ0 (τm = τM = τ0; here τ0 = 0) and that the two estimators have similar performances, when the constraint τM = −τm = 5 is used, with however a better behavior of R̂2[t, f] around the rupture occurring at 16s and after the time instant 24 s.
Model M2, multi-component nonstationary situation
The common part C of x1 and x2 in (7) was extracted from a SEEG recording during an ictal period. It corresponds to a reproducible quasi-deterministic pattern observed during different ictal episodes of a patient.
The independent background activities B1 and B2of M2 where simulated by surrogating (randomization of phase in Fourier transform of same signal, see Schreiber and Schmitz [26]) a segment of real background SEEG of patient.
A large number of realizations (x1[k], x2[k]), 0≤k≤K, K/FS = 40s,FS =200Hz were simulated with , where denotes the temporal mean square value of C (estimated over its time support) and is the variance of the surrogated data.
The target |ρ[t, f]|2 was computed by Monte-Carlo procedure as in M1 for the case of time-varying α[t]. The visual inspection of the results, depicted on Fig. 3, reveals that the two estimators have similar behaviors (Fig. 3-b vs. 3-d) for τM = −τm= 5 and for known time delay τm = τM = τ0 (here τ0 = 0) the R̂2[t, f] seems to exhibit less bias than |ρ̂[t, f]|2. In an attempt at quantifying the differences between the two estimators, mean of absolute values of bias, mean of variances and mean of MSE values over the time-frequency plane were calculated. The obtained values, reported in Table I, indicate that the R̂2[t, f] estimator has better performances than |ρ̂[t, f]|2.
Fig. 3.
Model M2, nonstationary multi-component situation, a) Target time-frequency relationship obtained by Monte-Carlo simulation. Different estimations of target: b) |ρ̂[t, f]|2, c) R̂2[t, f] with τm = τM = 0, d) R̂2[t, f] with τM = −τm = 5.
Real EEG signals
Results obtained on simulated signals using relatively short duration analysis windows show that statistical performances of R2 estimator i) are equivalent to those of the coherence estimator when R2 is maximized with respect to time delay τ and ii) are better (in terms of bias and mean square error) than those obtained with the coherence estimator when time delay τ is known.
This ability of both estimators to characterize a relationship between signals in the time-frequency plane is of great interest in the analysis of intracerebral SEEG signals recorded in epileptic patients candidate to surgery. Indeed, interdependencies between signals can be interpreted in terms of functional couplings between brain structures (possibly well localized in frequency) and thus provide essential information about the organization of the epileptogenic zone.
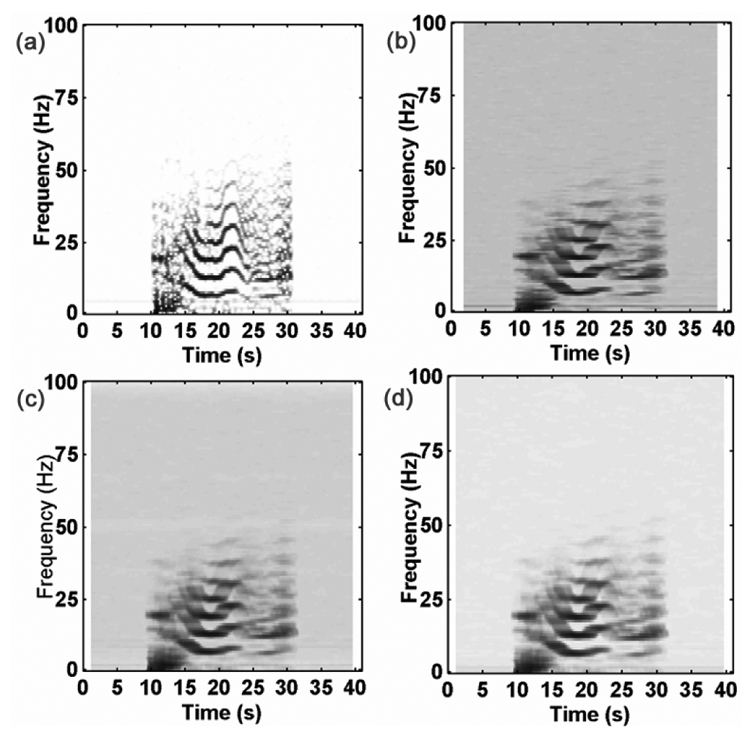
In order to verify if the results obtained on simulations also apply on real SEEG recordings, we chose an example of two SEEG signals recorded from temporal lobe brain structures (see section 2.3.3). These signals are representative of an observation that is often made at seizure onset. They are displayed in Fig. 4-a: after a period of background activity with sporadic spikes, a higher frequency activity (rapid discharge) is observed on both signals. The amplitude of this narrow band activity abruptly decreases and then gradually increases again while its frequency slows down. Both signals are nonstationary as depicted on Fig. 4-b that provides their respective spectrogram.
Fig. 4.
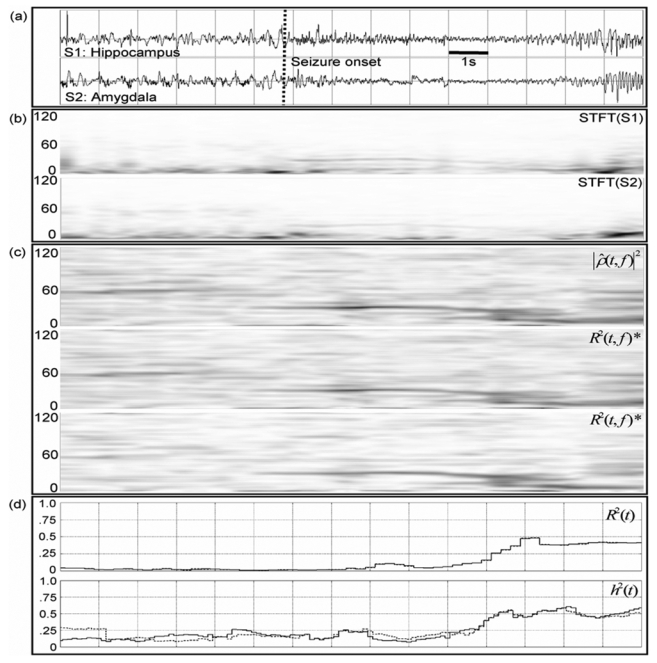
Results obtained on real data, a) Two SEEG signals recorded from hippocampus (top) and amygdala (bottom) in an epileptic patient (TLE) and b) corresponding spectrograms, c) Estimated relationship in the time-frequency plane for both methods. (*) Time-frequency representations of R̂2[t, f] maximized for time delay τ (middle, range −20 to 20 ms) and for fixed τ (bottom, τm = τM = 4 ms). d) Estimated relationship by two frequency-independent methods, one linear (r2[t], top) and the other nonlinear (h2[t], bottom).
The time-frequency characterization of the relationship between signals is illustrated in Fig. 4-c when performed with the estimator based on the coherence (first rectangle) and on the linear correlation coefficient maximized with respect to time delay τ (second rectangle, ranging from −20 ms to 20 ms).
As observed on simulated signals, both estimators lead to similar results: they reveal a similar signature characterized by a strong relationship that takes place over the narrow frequency band activity previously observed on spectrograms (around 30 Hz).
However, for fixed time delay τ (τm = τM =4ms), R̂2[t, f] provides a time-frequency representation of the relationship with enhanced readability when all the other parameters are set to provide identical time and frequency resolution in both methods. This result, displayed in Fig. 4-c (third rectangle), shows that the abrupt transition between SEEG dynamics (from background activity to rapid discharge) is also associated to an abrupt change of the frequency location of the linear relationship. As also noticed in simulations, the lower MSE of R̂2[t, f] with fixed τ leads to a time-frequency representation less noisy (in the sense of “easier to interpret”) than that obtained with the two previous estimators.
We also noticed that it is crucial to take frequency into account in some cases. Indeed, frequency-independent methods may not be able to reveal such a phenomenon of narrow band frequency hypersynchronization at seizure onset. We plotted the curves (Fig. 4-d) obtained with a linear estimator (linear correlation coefficient r2[t] maximized for the time delay, top) and with a nonlinear estimator (nonlinear correlation coefficient h2[t] maximized for the time delay, bottom) on the same analyzed signals. For detailed presentation regarding these two estimators, the reader may refer to Pijn [27]. One can notice that the two curves (Fig. 4-d) do not show significant changes during the transitions in EEG dynamics at seizure onset and are consequently unable to render some relevant information (i.e. the strong relationship between signals located on the narrow frequency band - about 30 Hz - and the abrupt jump of the relationship from the low to the higher frequency when background spiking activity changes into rapid discharge).
IV. Discussion and Conclusion
In the study of brain activity, the natural question about the functional coupling among cerebral structures or regions that generate EEG signals arises in numerous cases. It is addressed here through the characterization of linear interdependencies between SEEG signals in the context of epileptic processes. There are three main results in this study. First, characterizing the relationship in both time and frequency domains may be essential in some situations where this relationship is circumscribed within a particular region of the time-frequency plane (e.g. frequency-independent methods can be blind to relationships establishing on a narrow frequency band). Second, a novel estimator of the linear relationship as function of time and frequency which introduces a frequency dependent time delay has been proposed for nonstationary signals. On various simulations, this estimator has been shown to exhibit better statistical performances (bias and MSE) in comparison with a standard estimator based on the coherence function when a priori information is given about frequency dependent time delay value. Hence the user has the possibility to constrain coherence estimation using this information. Third, the proposed estimator has been used on real data and has also been found to enhance the readability of the time-frequency representation of the relationship for fixed time delay τ. Thus it can improve the interpretation of nonstationary interdependencies between SEEG signals. The choice of the relevant value of τ in a given time interval and in a given frequency band is actually under investigation. The key idea is to make use of a priori anatomical and physiological knowledge (neural pathways and their activations) and statistical results about propagation time delays in a data set built from a large number of patients: if generic reliable information about possible values of delays is available, this information can be used to improve performances of analysis methods of frequency dependent statistical relationship.
Table I.
Mean of the absolute values of bias, mean of the variances, and mean of MSE values computed over the time-frequency (TF) plane, for |ρ̂[t, f]|2 and R̂2[t, f] (with τm = τM = 0; τM = −τm = 5).
| Estimation | Mean of absolute value of bias over TF plane | Mean of variance over TF plane | Mean of MSE over TF plane |
|---|---|---|---|
| |ρ̂[t, f]|2 | 0.19014 | 0.0261 | 0.0702 |
| R̂2[t, f], τm = τM = 0 | 0.09135 | 0.01278 | 0.02627 |
| R̂2[t, f], τM = −τm = 5 | 0.1539 | 0.0178 | 0.04746 |
Acknowledgments
This work is supported by the French Ministry of Research (“Action Concertée Incitative”).
Appendix A
Let x1 and x2 be two real zero mean wide sense stationary stochastic process with covariance function: Cxi, xj (τ) = E(xi(t)xj(t–τ)), τ∈ℝ; i,j ∈ {l,2} and corresponding power spectral density: γxi,xj (f) = FT(Cxi,xj)(f), f ∈ℝ where FT stands for the Fourier Transform.
The Coherence function between x1 and x2 is defined as:
Property
Let us consider the real band-pass filter defined by:
, where if A is true and otherwise , whose output is z1 or z2 when input is respectively x1 or x2 Let:
which is the absolute value of the correlation coefficient between the two zero mean real variables z1 (t) and z1,(t - τ), maximized relatively to the delay τ (the maximum is reached when τ = τ*). Then if Δf is small and γxixj is a continuous function at f = f0 we have:
Proof
For small values of Δf:
since Cx1,x2 (τ) ∈ℝ ⇒ {γx1,x2 (f0) = γ*x1,x2 (−f0)} and since γxi,xj is continuous at f = f0.
For a complex number z and θ real (it is sufficient to equalize θ with −arg(z)) and hence:
Appendix B
The continuous filter bank used is defined in the time domain as follows:
where h(t) has the characteristics of a low-pass filter and is even, ν0 an arbitrary frequency, and hν0(t) is the real part of h̃ν0(t) = h(t).ei2πν0t. Thus for any real signal x(t), x*hν0 = Re(x*h̃ν0); hence:
As h is even:
where STFTx is the short term Fourier transform of the x while using h for windowing.
References
- 1.Chapman CA, Xu Y, Haykin S, Racine RJ. Beta-frequency (15–35 Hz) electroencephalogram activities elicited by toluene and electrical stimulation in the behaving rat. Neuroscience. 1998;86:1307–1319. doi: 10.1016/s0306-4522(98)00092-x. [DOI] [PubMed] [Google Scholar]
- 2.Brazier MAB. Studies of the EEG activity of limbic structures in man. Electroencephalography and Clinical Neurophysiology. 1968;25:309–318. doi: 10.1016/0013-4694(68)90171-5. [DOI] [PubMed] [Google Scholar]
- 3.Gotman J. Interhemispheric interactions in seizures of focal onset: data from human intracranial recordings. Electroencephalogr Clin Neurophysiol. 1987;67:120–33. doi: 10.1016/0013-4694(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 4.Duckrow RB, Spencer SS. Regional coherence and the transfer of ictal activity during seizure onset in the medial temporal lobe. Electroencephalogr Clin Neurophysiol. 1992;82:415–22. doi: 10.1016/0013-4694(92)90046-k. [DOI] [PubMed] [Google Scholar]
- 5.Franaszczuk PJ, Bergey GK. Application of the directed transfer function method to mesial and lateral onset temporal lobe seizures. Brain Topogr. 1998;11:13–21. doi: 10.1023/a:1022262318579. [DOI] [PubMed] [Google Scholar]
- 6.Franaszczuk PJ, Bergey GK. An autoregressive method for the measurement of synchronization of interictal and ictal EEG signals. Biol Cybern. 1999;81:3–9. doi: 10.1007/s004220050540. [DOI] [PubMed] [Google Scholar]
- 7.Pikovsky A, Rosenblum M, Kurths J. Synchronization : a universal concept in nonlinear sciences. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 8.Mars NJ, Lopes da Silva FH. Propagation of seizure activity in kindled dogs. Electroencephalogr Clin Neurophysiol. 1983;56:194–209. doi: 10.1016/0013-4694(83)90074-3. [DOI] [PubMed] [Google Scholar]
- 9.Pijn JP, Lopes da Silva F. Propagation of electrical activity: nonlinear associations and time delays between EEG signals. In: Zschocke S, Speckmann EJ, editors. Basic Mechanisms of the Eeg, Brain Dynamics. Boston: Birkhauser; 1993. pp. 41–61. [Google Scholar]
- 10.Wendling F, Bartolomei F, Bellanger JJ, Chauvel P. Interpretation of interdependencies in epileptic signals using a macroscopic physiological model of the EEG. Clin Neurophysiol. 2001;112:1201–18. doi: 10.1016/s1388-2457(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 11.Iasemidis LD. Epileptic seizure prediction and control. IEEE Trans Biomed Eng. 2003;50:549–58. doi: 10.1109/tbme.2003.810705. [DOI] [PubMed] [Google Scholar]
- 12.Lehnertz K. Non-linear time series analysis of intracranial EEG recordings in patients with epilepsy--an overview. Int J Psychophysiol. 1999;34:45–52. doi: 10.1016/s0167-8760(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 13.Quiroga RQ, Kraskov A, Kreuz T, Grassberger P. Performance of different synchronization measures in real data: A case study on electroencephalographic signals. Physical Review E. 2002;65:041903. doi: 10.1103/PhysRevE.65.041903. [DOI] [PubMed] [Google Scholar]
- 14.Zaveri HP, Williams WJ, Sackellares JC, Beydoun A, Duckrow RB, Spencer SS. Measuring the coherence of intracranial electroencephalograms. Clin Neurophysiol. 1999;110:1717–25. doi: 10.1016/s1388-2457(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 15.Razoumnikova OM. Functional organization of different brain areas during convergent and divergent thinking: an EEG investigation. Cognitive Brain Research. 2000;10:11–18. doi: 10.1016/s0926-6410(00)00017-3. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaev AR, Ivanitsky GA, Ivanitsky AM, Posner MI, Abdullaev YG. Correlation of brain rhythms between frontal and left temporal (Wernicke’s) cortical areas during verbal thinking. Neuroscience Letters. 2001;298:107–110. doi: 10.1016/s0304-3940(00)01740-7. [DOI] [PubMed] [Google Scholar]
- 17.Wendling F, Bartolomei F, Bellanger JJ, Bourien J, Chauvel P. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain. 2003;126:1449–1459. doi: 10.1093/brain/awg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haykin S, Racine RJ, Xu Y, Chapman CA. Monitoring neural oscillation and signal transmission between cortical regions using time-frequency analysis of electroencephalographic activity. Proceedings of IEEE. 1996;84:1295–1301. [Google Scholar]
- 19.Bendat JS, Piersol AG. Random data : analysis and measurement procedures. 3. New York: Wiley; 2000. [Google Scholar]
- 20.Martin JH. The collective electrical behavior of cortical neurons: The electroencephalogram and the mechanisms of epilepsy. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 3. chapter 50. Prentice-Hall; London: 1991. pp. 777–791. [Google Scholar]
- 21.Nunez PL, Cutillo BA. Neocortical dynamics and human EEG rhythms. New York: Oxford University Press; 1995. [Google Scholar]
- 22.Kantz H, Schreiber T. Nonlinear time series analysis. Cambridge; New York: Cambridge University Press; 1997. [Google Scholar]
- 23.Maldonado HM, Delgado-Escueta AV, Walsh GO, Swartz BE, Rand RW. Complex partial seizures of hippocampal and amygdalar origin. Epilepsia. 1998;29:420–433. doi: 10.1111/j.1528-1157.1988.tb03741.x. [DOI] [PubMed] [Google Scholar]
- 24.Talairach J, Bancaud J, Szikla G, Bonis A, Geier S, Vedrenne C. New approach to the neurosurgery of epilepsy. Stereotaxic methodology and therapeutic results. 1. Introduction and history. Neurochirurgie. 1974;20:1–240. [PubMed] [Google Scholar]
- 25.Carter GC. Bias in magnitude-squared coherence estimation due to misalignment. Proceedings of IEEE. 1987;75:236–255. [Google Scholar]
- 26.Schreiber T, Schmitz A. Surrogate time series. Physica D: Nonlinear Phenomena. 2000;142:346–382. [Google Scholar]
- 27.Pijn JP. Quantitative evaluation of EEG signals in epilepsy, nonlinear associations, time delays and nonlinear dynamics. Amsterdam: University of Amsterdam; 1990. [Google Scholar]


