Abstract
Multiple sclerosis (MS) is a devastating autoimmune demyelinating disease of the CNS. This study investigated whether expression and activity of the calcium-activated protease calpain correlated with Th1/Th2 dysregulation in MS patients during states of relapse and remission. Calpain expression and activity were significantly increased in peripheral blood mononuclear cells (PBMCs) from MS patients, compared to controls, with the highest expression and activity noted during relapse. Th1 cytokines were highest and Th2 cytokines were lowest in MS patients during relapse. Treatment with calpain inhibitor, calpeptin, decreased Th1 cytokines in PBMCs from MS patients. Calpain inhibitor also reduced degradation of myelin basic protein (MBP) by inhibiting the calpain secreted from MBP-specific T cells. Taken together, these results suggested calpain involvement in Th1/Th2 dysregulation in MS patients.
Keywords: multiple sclerosis, calpain, cytokines, Th1/Th2 dysregulation, T cell activation
1. Introduction
Multiple sclerosis (MS) is a devastating autoimmune/demyelinating disease and only recently has a neurodegenerative component of MS been identified (Bjartmar and Trapp, 2001; Guyton et al., 2005; Pitt et al., 2000; Trapp et al., 1999). Calpain is one of the proteases involved in T cell activation (Schaecher et al., 2002) as well as in axonal damage and cell death (Guyton et al., 2005). Demyelination is believed to be the result of a chronic T cell-mediated, autoimmune response against myelin proteins. There may be a conduction block at the site of the lesion or slower conduction time along the affected nerve. Myelin proteins, e.g., myelin basic protein (MBP) and proteolipid protein (PLP), are degraded in MS, with a resulting destabilization of the myelin membrane. Proteases (acidic and neutral) that degrade these proteins and cytoskeletal proteins have been implicated in myelinolysis and cell death (Ray and Banik, 2003; Shields and Banik, 1998b). Increased activity and expression of the Ca2+-activated protease calpain has been found in tissue of patients with MS as well as in the corresponding animal model, experimental allergic encephalomyelitis (EAE) (Banik, 1992; Cuzner et al., 1975; Guyton et al., 2005; Hassen et al., 2006; Shields and Banik, 1998a; Shields et al., 1998; Smith, 1977).
Ubiquitous calpains exist as micro (μ) and milli calpain (m-calpain) isoforms that require μM and mM levels, respectively, for activation (Murachi, 1984; Suzuki, 1987). It has been implicated in the breakdown of lens proteins in cataract formation, optic nerve degeneration, and in neuronal degeneration in central nervous system (CNS) injury, ischemia, Parkinson’s disease, and Alzheimer’s disease (Fukiage et al., 1997; Nath et al., 1996; Posmantur et al., 1994; Ray and Banik, 2003; Saito et al., 1993; Shields and Banik, 1998a). Calpain activity and expression are increased in infiltrating inflammatory cells in plaque and normal appearing white matter tissue of MS and in CNS tissue of EAE animals (Guyton et al., 2005; Shields et al., 1999b). Therefore, it is likely that calpain, which plays a major role in myelin breakdown and T-cell activation in demyelinating disease, may have its activity and expression altered during the course of the disease process in MS and EAE (Guyton et al., 2005; Schaecher et al., 2002; Shields et al., 1999b). Activated calpain may be released from these cells (e.g. microglia, macrophages, T cells), thereby producing immunogenic peptides from digested myelin proteins for presentation to and sensitization of other T lymphocytes (Deshpande et al., 1995b; Smith et al., 1998).
Increased calpain has been correlated with neuron death and axon degeneration in Lewis rats with EAE (Guyton et al., 2005). We have previously shown that calpain plays an integral role in the activation of T cells, which is blocked by calpain inhibitors (Schaecher et al., 2001). Calpain secreted by activated T cells degrades myelin proteins, degradation of these proteins was inhibited by its endogenous inhibitor calpastatin and by calpeptin, a calpain inhibitor (Deshpande et al., 1995b). Also, calpain is involved in cell migration and other inflammatory cascades that are necessary for development and progression of MS (Carragher and Frame, 2002; Glading et al., 2002). Activated inflammatory cells such as T cells and monocytes have increased levels of calpain expression and activity in vitro (Deshpande et al., 1995a). The finding of increased calpain activity and expression in MS plaque, normal appearing white matter in MS and spinal cords of animals with EAE is attributed to infiltrating inflammatory cells, activated microglia and reactive astrocytes (Guyton et al., 2005; Iwamoto et al., 1991; Schaecher et al., 2002; Shields et al., 1999b).
Calpain expression is increased in MS plaques, EAE splenic cells before onset of disease and in EAE spinal cord after disease onset (Guyton et al., 2005; Hickey, 1991; Schaecher et al., 2002; Shields et al., 2000; Shields et al., 1999a). Calpain is also involved in T cell activation in normal peripheral blood mononuclear cells (PBMCs) stimulated with anti-CD3/CD28, and treatment with calpain inhibitor blocks IL-2 production and CD25 expression (markers for T cell activation) (Schaecher et al., 2001). Further studies demonstrated that treatment with calpain inhibitor blocked the ability of calpain to cleave IκB, thus blocking the translocation of the transcription factor NFκB, a major player in the inflammatory process, to the nucleus (Schaecher et al., 2004).
Dysregulation of Th1 and Th2 cytokines in MS is well established (Hollifield et al., 2003). Studies demonstrate that the Th1 subset of CD4+ T cell cytokines (pro-inflammatory IL-2, IFN-γ, IL-12) are increased during disease activity while Th2 cytokines (anti-inflammatory IL-4, IL-10, TGF-β) are increased during reduced disease activity (Gajewski et al., 1989; Murphy and Reiner, 2002). The purpose of the investigation is to examine whether the levels of calpain expression and activity are altered in the PBMCs of MS patients during remission and relapse concomitant with cytokine production. Our results indicate increased calpain expression and activity in activated PBMCs isolated from MS patients are greater in relapse than those of remission and matched controls. An increased calpain activity also correlates with greater production of pro-inflammatory IL-2/IFN-γ cytokines and lower production of anti-inflammatory cytokines IL-10 and IL-4. These findings suggested calpain plays a modulatory role in T cell activation and production of Th1/Th2 type cytokines during the relapsing and remitting phase of the disease. A part of this work has been presented in abstract form (Imam et al., 2004).
2. Materials and Methods
2.1 Study Subjects
The study included patients diagnosed with relapsing-remitting MS that are presently either taking interferon therapy or not on any treatment. The protocol for the study was approved by the Institutional Review Board (IRB), and informed consent was obtained from all participants. Twenty-one MS blood samples were tested. Six samples were tested during a relapse (active disease) and fifteen during remission. All MS patients had been previously diagnosed with clinically definite, MRI-confirmed, relapsing-remitting disease, according to Poser’s criteria (Poser et al., 1983). Control subjects included twenty-one blood samples from healthy age and sex matched donors with no history of autoimmune disease.
2.2 Isolation and stimulation of PBMC
Blood samples (20 ml) from MS patients and their matched controls were collected. Whole blood was mixed with gelatin (2.5% gelatin in 0.9% saline dissolved at 37°C), layered on top of Ficoll-Paque PlusTM, and centrifuged. The upper layer of plasma was carefully removed. The lower cloudy layer containing the PBMCs was transferred to a centrifuge tube and suspended in 8ml of balanced salt solution.
After centrifugation, the supernatant was removed. The pellet was resuspended in 8 ml of balanced salt solution and once again centrifuged. PBMCs in the pellet were counted and diluted in RPMI medium supplemented with 0.5% fetal bovine serum and 1% penicillin-streptomycin to a concentration of 1×106 cells/ml. Cells (2×106 per well) were plated on a 6-well plate. PBMCs were activated with 10μg/ml anti-CD3 and 5μg/ml anti-CD28 or left unactivated and incubated for 24 hours. Cells are treated according to the following protocols: (1) no treatment (control), (2) activated, (3) activated following pre-treatment with calpeptin, (4) activated with calcium added, (5) activated with calpeptin and calcium added.
Cells (2×106) in 2 ml of complete media were placed in each well of a six-well plate. Some wells were pre-treated with 100 μM calpeptin and incubated for 1 hour. Cells were activated with anti-CD3 (5 μg/ml) and anti-CD28 (10 μg/ml) in the presence of 1 mM Ca2+. The cells were incubated for 24 hours and analyzed.
2.3 Isolation of MBP-Specific T Cells
Isolation of MBP-specific T cells was previously described (Zhang et al., 1994). Briefly, PBMCs isolated from MS patients’ blood were plated at a total cell count of 2×105 cells/well in 96-well U-bottom plates (Costar). The MBP fragment consisting of amino acids 83-99 was added at a concentration of 10mg/ml. After seven days in culture, cells were re-stimulated with autologous irradiated PBMCs pre-incubated with the 83-99 MBP peptide. Cells were placed into wells (approximately 1×104 cells/well) and each well was incubated with 1×105 irradiated PBMCs pre-pulsed with the MBP peptide. This culture developed for 72 hours with the last 16 hours consisting of [3H]-thymidine pulsing at a concentration of 1 μCi/well. Wells responding with a count of greater than 1500cpm and was at least 3 to 4 times greater than the wells that received no peptide were considered specific for the MBP peptide. Positive clones were stimulated with 2 μg/ml PHA and 1×105 irradiated PBMCs at a concentration of 3 cells/well (via limiting dilution). Every 3 days, all cultures were placed in new media and 50 U/ml of rIL-2. These cultures were maintained for 11 days and tested for proliferation to MBP 83-99. Those that were designated positive were maintained for further studies. All radioactive counts were done in a Beckman scintillation counter.
2.3 Measurement of Calpain Expression and Activity
Western blots were performed to determine calpain expression at the protein level using polyclonal antibody against the 80 kD m-calpain subunit and used to indirectly measure calpain activity by determining the amount of calpain-specific 145 kD spectrin breakdown product (SBDP). Briefly, 2×106 cells were lyzed for total protein extraction. Equal amounts of protein from each sample were resolved via a 4–20% gradient slab SDS-PAGE (Laemmli, 1970). These were then transferred to membranes and probed with the appropriate primary antibodies (anti-mcalpain at 1:500, anti-SBDP at 1:500) and secondary anti-bodies (goat anti-rabbit peroxidase) (Towbin et al., 1979; Vaessen et al., 1981). The protein bands were detected by ECL (Amersham Pharmacia) and quantified using Quantity One software (Bio-Rad Laboratories).
2.4 Incubation of exogenous MBP with incubation medium from activated MBP-specific T cells
Human MBP (hMBP) lines were selected from PMBCs of an MS patient. The reactivity of these T cells to hMBP was determined by a proliferation assay, and the stimulation index for hMBP was greater than 40×. T cells (1×106) were co-cultured with irradiated PBMC (2×106, 2,500 rad) as antigen presenting cells (APC) and 50μg/ml HMBP in RPMI-1640 with 2 mM L-glutamine, 1 mM sodium pyruvate, 100μg/ml penicillin-G and 100μg/ml streptomycin, but without serum (or 2% AB serum as control) for 24 to 72 hours. The culture containing T cells but no MBP was used as a control. At specified times, cells were centrifuged into pellet and incubation medium (supernatant) fractions. An aliquot (50–70μl) of this medium was incubated with exogenous hBMP (5μg) in 50 mM Tris-HCl buffer, pH 7.6, 2 mM dithiothreitol with or without Ca2+ (5 mM) at 37°C for 1 to 4 hours according to Deshpande et al (Deshpande et al., 1995b). EGTA (5 mM) replaced Ca2+ and served as control. Following termination of the reaction, the samples were analyzed by SDS-PAGE stained with Coomassie blue or immunoblots using an MBP antibody followed by incubation with the ECL system.
2.5 Measurement of Cytokine Secretion via ELISA
Sandwich ELISA protocols were followed according to the kit description given with the ELISA kits (BD Biosciences), as previously described (Haque et al., 2002). Briefly, ELISA micro-well plates were covered with 100 μl per well with capture antibody overnight. After washing, plates were blocked with assay diluent for 1 hour at room temperature. Seven cytokine standards (7.8, 70, 500 pg/ml) and samples were added to the appropriate wells and incubated at room temperature for 2 hours. After washing, 100 μl of detection antibody was added for 1 hour followed by washing and 30-minute incubation with avidin HRP. After appropriate washing, 100 μl of substrate solution was added to each well for 15 minutes at room temperature and then 50 μl of the stop solution was added. Absorbance was read at 450 nm in an EL800 ELISA reader (Bio-Tek).
2.6 Statistical Analyses
Differences in calpain expression and activity and cytokine secretion between the MS patient (during a relapse and remission) and control were analyzed using the rank sums test.
3. Results
3.1 Calpain Expression and Activity in PBMCs from MS Patients
We investigated the role of calpain in T cell activation and measured calpain expression and activity in PBMCs from relapsing or remitting MS patients and age- and sex-matched controls. PBMCs were activated with anti-CD3 (10 μg/ml) and anti-CD28 (5 μg/ml) antibodies (anti-CD3/CD28) to mimic activation of the T cell receptor (TCR) or with media alone and cells were collected 24 hours later for measuring calpain expression and activity. Calpain expression and activity were significantly increased (p≤0.05) following activation with anti-CD3/CD28 in PBMCs from controls, relapse, and remission patients (Figure 1). Higher levels were found in both unactivated and activated PBMCs from MS patients during relapse or remission, as compared to controls; however, no significant increase was noted in relapse as compared to remission patients. These data suggest a disruption in normal calpain expression and activity in MS patients.
Figure 1.
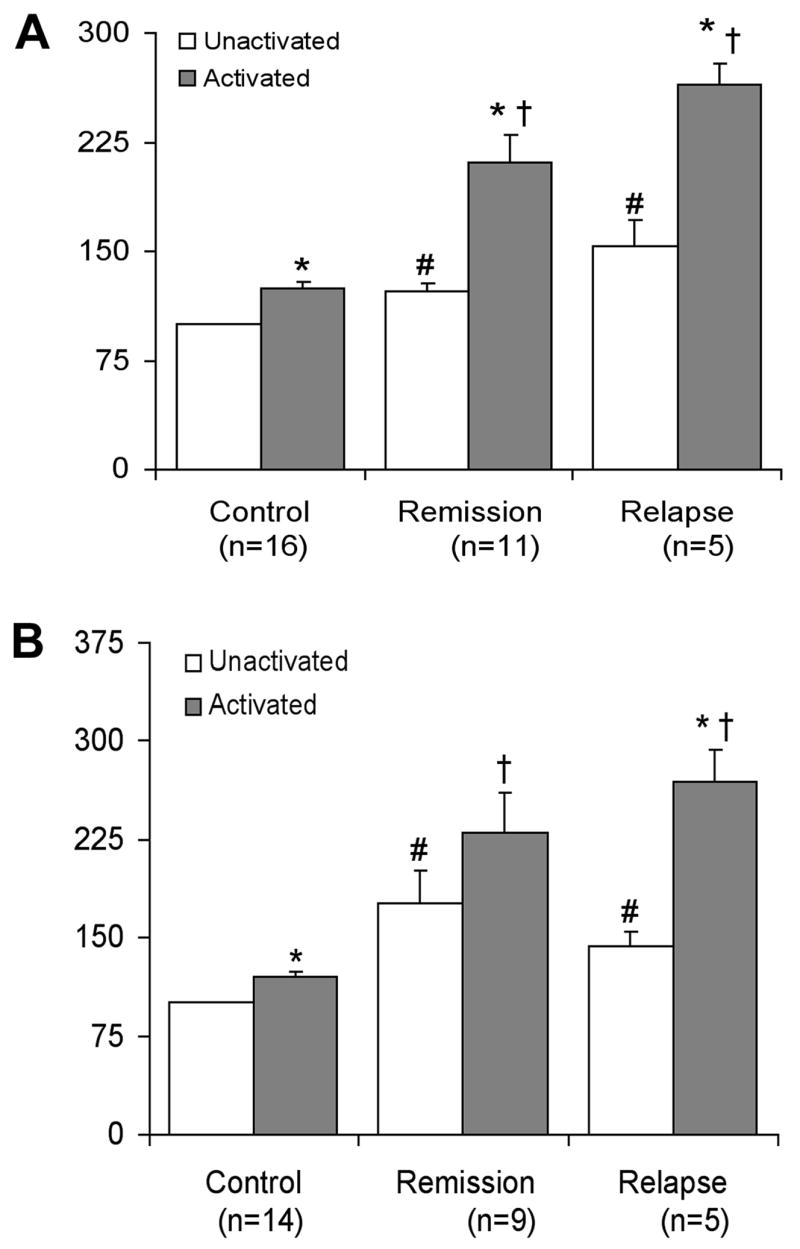
Calpain expression and activity. PBMCs from MS patients during relapse or remission, or age-matched controls were activated for 24h with anti-CD3/CD28. (A) Western blot analysis of calpain expression was performed using an antibody specific for mcalpain. (B) Analysis of calpain activity was performed using an antibody for calpain-specific 145 kD SBDP. The data represent the percent change compared to the unactivated control set at 100%. *p ≤ 0.05 unactivated vs activated. # p≤0.05 compared to unactivated control set at 100%. † p≤0.05 compared to activated control.
3.2 Th1/Th2 Cytokine Production in Activated PBMCs from MS Patients During Remission or Relapse
In order to compare pro-inflammatory Th1 cytokine (IL-2) and anti-inflammatory Th2 cytokine (IL-4, IL-10) levels in PBMCs from MS patients during remission or relapse, PBMCs were activated for 24 hours with anti-CD3/CD28 antibodies as described above. IL-2 production was significantly (p≤0.05) increased in PBMCs from both relapse and remission MS patients following activation, as compared to unactivated cells. In addition, IL-2 production was significantly higher in activated PBMCs from relapse patients, as compared to remission (Figure 2).
Figure 2.
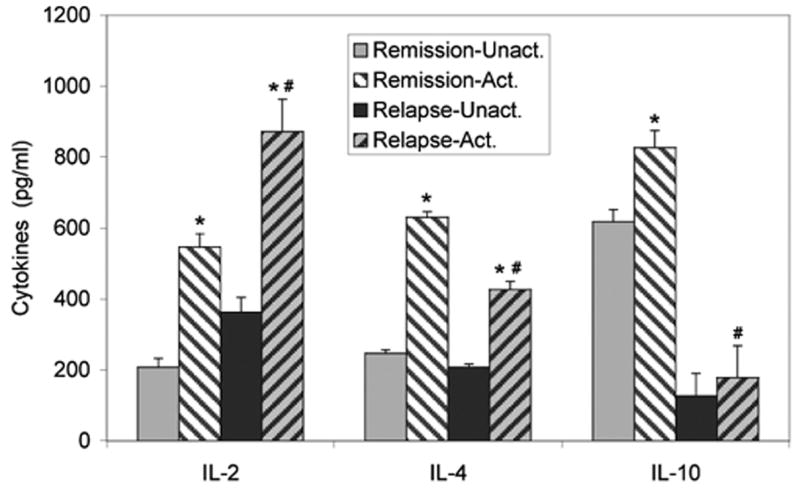
Th1/Th2 cytokine profiles differ between MS patients during remission and relapse. PBMCs, isolated from MS patients during remission or relapse, were activated with anti-CD3/CD28 antibodies or media (unactivated). Supernatants were collected after 24 hours for analysis of IL-2, IL-4 and IL-10 using IL-specific ELISA kits. *p≤0.05 unactivated vs activated; # p≤0.05 remission-activated vs relapse-activated. IL-2: Remission, n=11; Relapse, n=5. IL-4: Remission, n=10; Relapse, n=4. IL-10: Remission, n=9; Relapse, n=4.
IL-4 production was increased in activated PBMCs from remission and relapse MS patients compared to unactivated, while IL-10 was only significantly increased in remission patients. In contrast to the increased levels of IL-2, IL-4, and IL-10 production were significantly decreased in activated PBMCs from relapse patients, as compared to remission patients (Figure 2). The high levels of IL-10 production in unactivated PBMCs from remission patients was unexpected, but may indicate that Th2 cells isolated from remission patients are already induced to produce IL-10 during the remission stage even before isolation. Increased IL-10 production may be necessary to continue a remission state.
3.3 Calpain Inhibition of IL-2/IFN-γ in PBMCs of MS Patients Activated with anti-CD3/CD28 antibodies
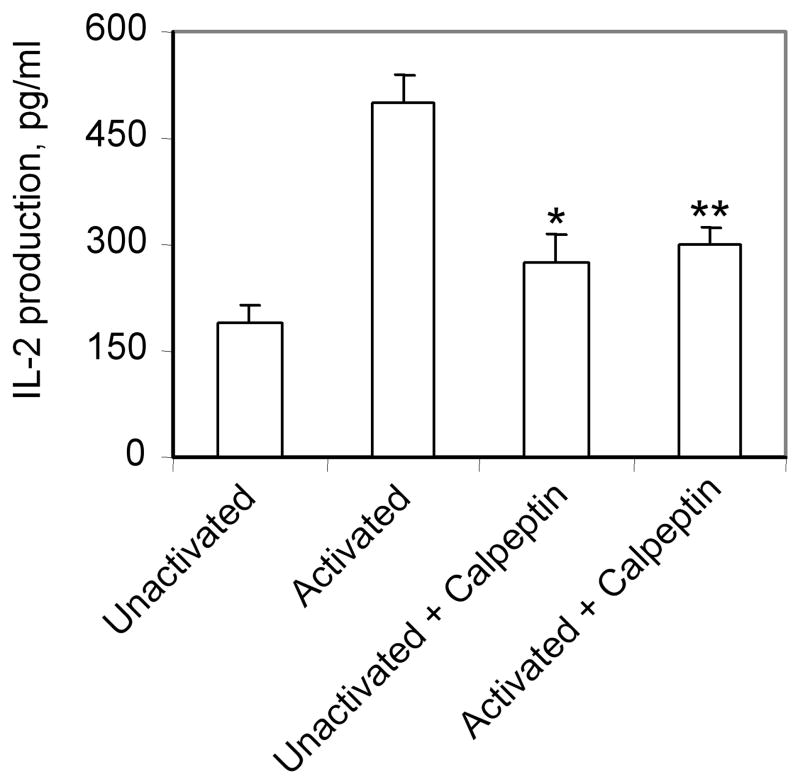
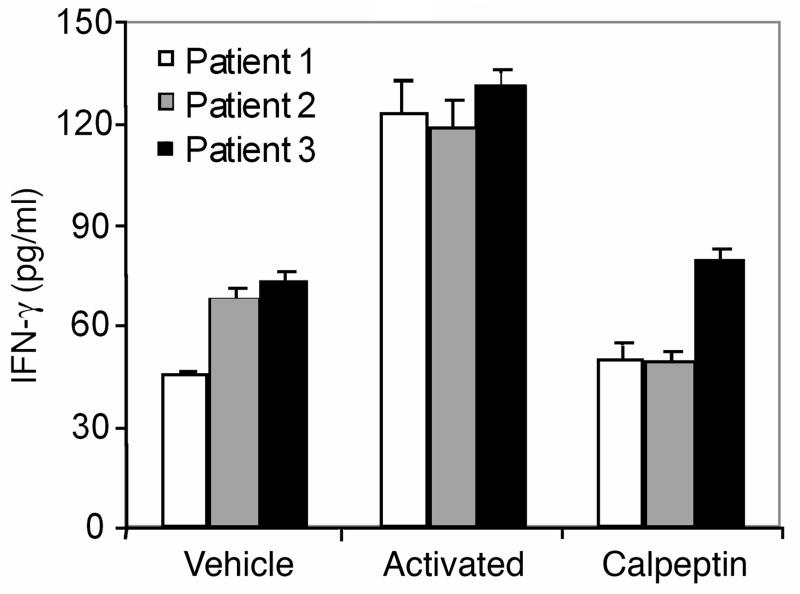
We examined the effect of calpain inhibitors on IL-2/IFN-γ production in PBMCs from MS patients (Figures 3 and 4). Our data indicate significant inhibition of IL-2 production (40%) in activated PBMCs from MS remission by the calpain inhibitor calpeptin (Figure 3). The IFN-γ production from activated PBMCs was also significantly inhibited (more than 50%) by calpeptin (Figure 4). The difference in IL-2 production between unactivated cells and cells plus calpeptin was not statistically significant. These data suggest that calpain inhibitors may reduce the production of inflammatory cytokines, ameliorating the disease.
Figure 3.
Effects of calpain inhibition on IL-2 production in PBMCs from patients activated with anti-CD3/CD28 antibodies. PBMCs, isolated from MS patients (remission, n=3) were activated with anti-CD3/CD28 plus or minus calpeptin (100 μM). PBMCs treated with media alone were used as controls (unactivated). Supernatants were collected after 24 hours for analysis of IL-2 using the IL-2 specific ELISA kit. *p<0.05 unactivated vs activated; **p<0.05 activated vs activated + calpeptin.
Figure 4.
Measurement of IFN-γ in activated remitting patient samples in the presence or absence of calpain inhibitor, calpeptin. PBMCs, isolated from MS patients (n=3) were activated with anti-CD3/CD28 plus or minus calpeptin (100μM). PBMCs treated with media alone were used as control (vehicle). Supernatants were collected after 24 hours for analysis of IFN-γ using IFN-γ-specific ELISA kit. It is important to note that the patient samples without activation produced significant amount of IFN-γ (vehicle).
3.4 Release of Calpain from myelin basic protein (MBP)-Specific T Cells Degrades MBP
To examine whether activated MBP-specific T cells secrete calpain, the incubation medium was incubated with exogenous hMBP as substrate (Figure 5). The exogenous MBP was substantially more degraded by the MBP-specific T cell medium (lane 3, Figure 5) compared to that by control medium released from MBP-specific T cells (lanes 6 and 7, Figure 5). The medium from MBP-specific T cells was more active than that of control medium. EGTA inhibited the MBP degradation by the MBP-specific T cell supernatant (lane 4, Figure 5) indicating breakdown of MBP is largely calpain-dependent.
Figure 5.
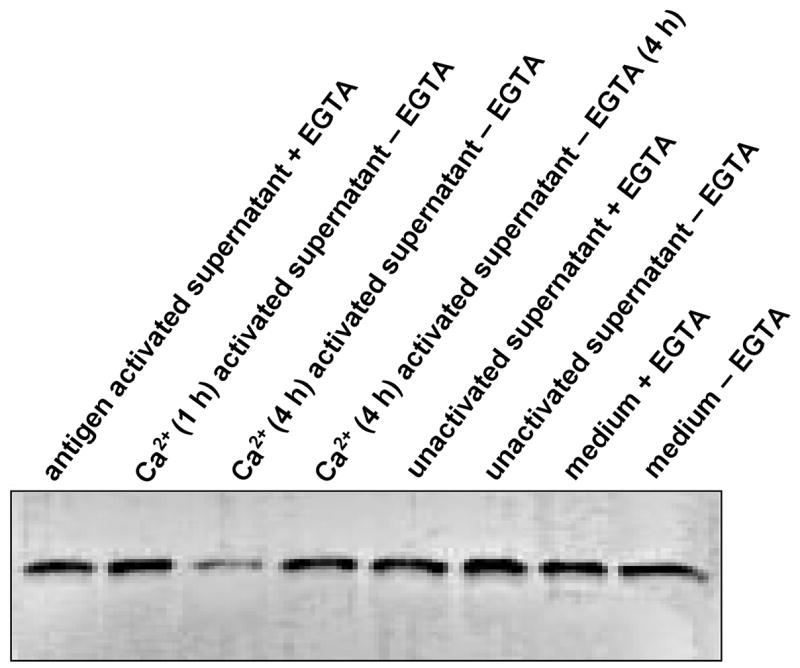
Degradation of MBP by calpain released from activated human MBP-specific T cells. Incubation conditions are described in text. The activated MBP-specific T cells were incubated in the presence of exogenous MBP and with other factors as follows: lane 1, activated MBP-specific T cells + MBP + EGTA (4 h); lane 2, as lane 1 with Ca2+ for 1h but without EGTA; lane 3, as lane 2 for 4 h; lane 4, as lane 3 + EGTA for 4 h; lanes 5–8: unactivated MBP-specific T cells in the presence of exogenous MBP and other factors. Conditions for incubation in lanes 5–8 are the same as lanes 1–4. The MBP degradation was analyzed by SDS-PAGE and immunoblotting.
4. Discussion
This study determined whether changes in cytokine secretion patterns occur in correlation with calpain activation and disease process in MS patients during relapse and remission. Recent findings of increased calpain activity and expression in glial and inflammatory cells in autoimmune disease of MS and its animal model EAE as well as demonstration of calpain-mediated activation of T cells implied its crucial role in demyelinating diseases (Schaecher et al., 2002; Shields and Banik, 1998b; Shields et al., 1999a). In addition to demyelination, MS has been characterized as having a neurodegenerative component with loss of axons and neurons (Guyton et al., 2005). Although the mechanisms of axonal and neuronal degeneration in demyelinating and other neurodegenerative diseases are unclear, recent studies have suggested involvement of calpain in this process (Guyton et al., 2005). The current study indicates that calpain activity and expression, concomitant with increased IL-2 production (Th1), are upregulated in PBMCs of MS patients during relapse, compared to remission patients and controls. In contrast, IL-4 and IL-10 (Th2) levels were higher in PBMCs of MS remission patients compared to relapse (Figure 2).
Calpain activity was significantly increased in PBMCs of MS patients during relapse and remission, compared with normal controls. Calpain expression was also significantly greater in the MS patients than in healthy controls. The translational expression of the 80kD mcalpain level was consistent with the levels of increased calpain activity found in PBMCs. Some of the increased calpain activity may be due to increased translational expression of the enzyme since generation of increased active 76kD calpain fragment was previously observed in MS plaque (Shields and Banik, 1999). Also, our previous immunohistochemical studies showed markedly increased number of glial/inflammatory cells with elevated calpain expression in the white matter of MS patients (Shields and Banik, 1999), suggesting that increased calpain activity in MS is due not only to neural and inflammatory cells, but also to the migratory immune cells. And, calpain activation is known to be involved in the migration of macrophages and T cells (Huttenlocher et al., 1997; Shields and Banik, 1999). Although calpain is activated by increased calcium, the mechanism of calpain activation in diseases (e.g., MS) remains unclear. One recent study has demonstrated increased levels of intracellular calcium in spinal cord of animals with EAE and suggested a role for elevated calcium in the activation of calpain (Guyton et al., 2005). Further, the level of IFN-γ, a pro-inflammatory cytokine produced by activated T cells (CD4+), is increased in MS, causing elevation of intracellular calcium and subsequent calpain activation in C6 glial cells and neurons in vitro (Sribnick et al., 2004; Sur et al., 2003). An increased level of calpain also has been reported in samples from patients with MS (Shields et al., 1998).
Previous findings indicate calpain plays multiple roles in the pathophysiological events in MS, including involvement in T cell activation and IL-2 production (Th1), and possibly of other cytokines, in T cell migration (Diaz-Sanchez et al., 2006; Nakanishi, 2003; Schaecher et al., 2002; Schaecher et al., 2001) and axonal and neuronal damage. Inflammatory components may play a critical role in the disease process as well as in neurodegeneration in MS. The data indicated that calpain expression and activity was significantly elevated in the activated PBMCs collected from MS patients when compared to control subjects. The data also indicated that higher levels of IL-2 (Th1) and lower levels of IL-10 (Th2) are seen in the relapsed patient (Figure 2). Collectively, these data suggest that an overall increase in pro-inflammatory cytokines and a decrease in anti-inflammatory cytokines may carry an additional risk for onset and relapse that occurs in MS. Thus, increases in IL-10 may serve as a regulatory marker of natural or treatment-induced improvement in MS. It would be important to determine whether other IL-10-secreting cell types are involved in the alterations of the inflammatory process in MS.
CD4+ T helper cells (Th1), key immune cells in MS, are pro-inflammatory and produce IL-2, IFN-γ, IL-12, and TNF-β (Segal, 2003). In contrast, Th-2 cells are anti-inflammatory and produce IL-4, IL-10, and transforming growth factor beta (TGF-β) (Murphy and Reiner, 2002). The decision for a T cell to commit to Th1 or Th2 profile depends on many factors, including cellular environment, antigen concentration, types of antigen presenting cells, and others. Because anti-CD3/CD28 antibodies stimulated naïve T cells with IFN-γ resulted in increased Ca2+ level, and a Th1 profile suggested calpain involvement in the subsequent activation of T cells (Th1) producing IL-2 (Gajewski et al., 1989). Dysregulation of cytokine profiles with increased IL-2 production and decreased IL-10 production in PBMCs of MS patients during relapse and remission, as shown in this study, is consistent with previous report (Hollifield et al., 2003). The Th1/Th2 dysregulation of cytokine profiles was also correlated with increased calpain activity and expression. Transcription factor STAT-6, a substrate degraded by calpain, is important for Th2 cytokine production (Zamorano et al., 2005). This suggests that increased calpain activity in T cells from MS patients may be responsible for the lower levels of Th2 cytokines in patients with MS. Thus, inhibition of calpain may block STAT-6 degradation and restore Th2 cytokine levels, suggesting a possible role for calpain in Th1/Th2 dysregulation. We have previously shown that calpain inhibition blocks IL-2 synthesis in normal PBMCs following stimulation with anit-CD3/CD28 antibodies (Schaecher et al., 2001). This finding is confirmed in the present study demonstrating that the IL-2 production in PBMCs of MS patients during relapse and remission is also suppressed by the calpain inhibitor calpeptin.
The MBP fragments generated by calpain may be immunogenic and involved in epitope spreading. These peptides may sensitize other T cell specificities and promote perpetuation of the demyelinating process. Release of other proteases from activated T cells has been previously demonstrated (Deshpande et al., 1995b). Also, calpain (and other proteases) or other factors, e.g., cytokines, released from activated PBMCs (T cells) may be detrimental to cell survival causing neuronal and oligodendrocyte death. Such effect of activated T cell in neuronal death has been recently found (Giuliani et al., 2003).
To our knowledge, this is the first report of its kind associating calpain activation with cytokine production in PBMCs of MS patients. This study describes conditions that can indeed be used for more detailed assessment of immune function associated with the state of the MS patient in a larger number of patients. Finally, the findings are compelling, in that they reflect changes in immune responsiveness with calpain’s involvement in disease activity. In conclusion, these data offer a clear rationale for continued and more thorough study of immune function, calpain expression and activity and cytokine secretion patterns associated with the state of the MS patient. The data suggests that the study of MS during the course of the disease has potential to lead to insights concerning the mechanisms by which relapse and remission occur in MS. Further, the introduction of calpain inhibitors may have the potential to control the extent of disease progression and intensity of a relapse or alleviate the inflammatory responses associated with progression of the disease as well as axonal and neuronal damage.
Acknowledgments
This work has been supported by grants (NS23221, NS31662, NS41088, NS56176, and C06RR015455) from the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banik NL. Pathogenesis of myelin breakdown in demyelinating diseases: role of proteolytic enzymes. Crit Rev Neurobiol. 1992;6:257–271. [PubMed] [Google Scholar]
- Bjartmar C, Trapp BD. Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol. 2001;14:271–278. doi: 10.1097/00019052-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- Cuzner ML, McDonald WI, Rudge P, Smith M, Borshell N, Davison AN. Leukocyte proteinase activity and acute multiple sclerosis. J Neurol Sci. 1975;26:107–111. doi: 10.1016/0022-510x(75)90118-5. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Goust JM, Chakrabarti AK, Barbosa E, Hogan EL, Banik NL. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J Biol Chem. 1995a;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- Deshpande RV, Goust JM, Hogan EL, Banik NL. Calpain secreted by activated human lymphoid cells degrades myelin. J Neurosci Res. 1995b;42:259–265. doi: 10.1002/jnr.490420214. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol (Berl) 2006;111:289–299. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- Fukiage C, Azuma M, Nakamura Y, Tamada Y, Nakamura M, Shearer TR. SJA6017, a newly synthesized peptide aldehyde inhibitor of calpain: amelioration of cataract in cultured rat lenses. Biochim Biophys Acta. 1997;1361:304–312. doi: 10.1016/s0925-4439(97)00043-4. [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Schell SR, Nau G, Fitch FW. Regulation of T-cell activation: differences among T-cell subsets. Immunol Rev. 1989;111:79–110. doi: 10.1111/j.1600-065x.1989.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Giuliani F, Goodyer CG, Antel JP, Yong VW. Vulnerability of human neurons to T cell-mediated cytotoxicity. J Immunol. 2003;171:368–379. doi: 10.4049/jimmunol.171.1.368. [DOI] [PubMed] [Google Scholar]
- Glading A, Lauffenburger DA, Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 2002;12:46–54. doi: 10.1016/s0962-8924(01)02179-1. [DOI] [PubMed] [Google Scholar]
- Guyton MK, Wingrave JM, Yallapragada AV, Wilford GG, Sribnick EA, Matzelle DD, Tyor WR, Ray SK, Banik NL. Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res. 2005;81:53–61. doi: 10.1002/jnr.20470. [DOI] [PubMed] [Google Scholar]
- Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, Woods K, Smith GN, Cummings OW, Heidler KM, Blum JS, Wilkes DS. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002;169:1542–1549. doi: 10.4049/jimmunol.169.3.1542. [DOI] [PubMed] [Google Scholar]
- Hassen GW, Feliberti J, Kesner L, Stracher A, Mokhtarian F. A novel calpain inhibitor for the treatment of acute experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180:135–146. doi: 10.1016/j.jneuroim.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Hickey WF. Migration of hematogenous cells through the blood-brain barrier and the initiation of CNS inflammation. Brain Pathol. 1991;1:97–105. doi: 10.1111/j.1750-3639.1991.tb00646.x. [DOI] [PubMed] [Google Scholar]
- Hollifield RD, Harbige LS, Pham-Dinh D, Sharief MK. Evidence for cytokine dysregulation in multiple sclerosis: peripheral blood mononuclear cell production of pro-inflammatory and anti-inflammatory cytokines during relapse and remission. Autoimmunity. 2003;36:133–141. doi: 10.1080/0891693031000089427. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Palecek SP, Lu Q, Zhang W, Mellgren RL, Lauffenburger DA, Ginsberg MH, Horwitz AF. Regulation of cell migration by the calcium-dependent protease calpain. J Biol Chem. 1997;272:32719–32722. doi: 10.1074/jbc.272.52.32719. [DOI] [PubMed] [Google Scholar]
- Imam SA, Terry EC, Tyor WR, Ray SK, Banik NL. Changes in calpain expression in peripheral blood mononuclear cells from multiple sclerosis patients. 35th Annual American Society for Neurochemistry Meeting; New York, NY. 2004. [Google Scholar]
- Iwamoto N, Thangnipon W, Crawford C, Emson PC. Localization of calpain immunoreactivity in senile plaques and in neurones undergoing neurofibrillary degeneration in Alzheimer’s disease. Brain Res. 1991;561:177–180. doi: 10.1016/0006-8993(91)90766-o. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murachi T. Calcium-dependent proteinases and specific inhibitors: calpain and calpastatin. Biochem Soc Symp. 1984;49:149–167. [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nakanishi H. Microglial functions and proteases. Mol Neurobiol. 2003;27:163–176. doi: 10.1385/MN:27:2:163. [DOI] [PubMed] [Google Scholar]
- Nath R, Raser KJ, Stafford D, Hajimohammadreza I, Posner A, Allen H, Talanian RV, Yuen P, Gilbertsen RB, Wang KK. Non-erythroid alpha-spectrin breakdown by calpain and interleukin 1 beta-converting-enzyme-like protease(s) in apoptotic cells: contributory roles of both protease families in neuronal apoptosis. Biochem J. 1996;319(Pt 3):683–690. doi: 10.1042/bj3190683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Hayes RL, Dixon CE, Taft WC. Neurofilament 68 and neurofilament 200 protein levels decrease after traumatic brain injury. J Neurotrauma. 1994;11:533–545. doi: 10.1089/neu.1994.11.533. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Saito K, Elce JS, Hamos JE, Nixon RA. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993;90:2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkB alpha degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res. 2004;29:1443–1451. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- Schaecher K, Rocchini A, Dinkins J, Matzelle DD, Banik NL. Calpain expression and infiltration of activated T cells in experimental allergic encephalomyelitis over time: increased calpain activity begins with onset of disease. J Neuroimmunol. 2002;129:1–9. doi: 10.1016/s0165-5728(02)00142-x. [DOI] [PubMed] [Google Scholar]
- Schaecher KE, Goust JM, Banik NL. The effects of calpain inhibition upon IL-2 and CD25 expression in human peripheral blood mononuclear cells. J Neuroimmunol. 2001;119:333–342. doi: 10.1016/s0165-5728(01)00367-8. [DOI] [PubMed] [Google Scholar]
- Segal BM. Experimental autoimmune encephalomyelitis: cytokines, effector T cells, and antigen-presenting cells in a prototypical Th1-mediated autoimmune disease. Curr Allergy Asthma Rep. 2003;3:86–93. doi: 10.1007/s11882-003-0017-6. [DOI] [PubMed] [Google Scholar]
- Shields DC, Avgeropoulos NG, Banik NL, Tyor WR. Acute multiple sclerosis characterized by extensive mononuclear phagocyte infiltration. Neurochem Res. 2000;25:1517–1520. doi: 10.1023/a:1007636427861. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Putative role of calpain in the pathophysiology of experimental optic neuritis. Exp Eye Res. 1998a;67:403–410. doi: 10.1006/exer.1998.0537. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998b;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Pathophysiological role of calpain in experimental demyelination. J Neurosci Res. 1999;55:533–541. doi: 10.1002/(SICI)1097-4547(19990301)55:5<533::AID-JNR1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Goust JM, Banik NL. Calpain activity and expression are increased in splenic inflammatory cells associated with experimental allergic encephalomyelitis. J Neuroimmunol. 1999a;99:1–12. doi: 10.1016/s0165-5728(99)00043-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999b;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL. Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A. 1998;95:5768–5772. doi: 10.1073/pnas.95.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, van der Maesen K, Somera FP. Macrophage and microglial responses to cytokines in vitro: Phagocytic activity, proteolytic enzyme release, and free-radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Smith ME. The role of proteolytic enzymes in experiental allergic encephalomyelitis. Neurochem Res. 1977;2:223–246. doi: 10.1007/BF00969354. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004;76:688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Wingrave JM, Nowak MW, Ray SK, Banik NL. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Domain structure and activity regulation. Trends Biochem Sci. 1987;12:103–105. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Vaessen RT, Kreike J, Groot GS. Protein transfer to nitrocellulose filters. A simple method for quantitation of single proteins in complex mixtures. FEBS Lett. 1981;124:193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Zamorano J, Rivas MD, Setien F, Perez GM. Proteolytic regulation of activated STAT6 by calpains. J Immunol. 2005;174:2843–2848. doi: 10.4049/jimmunol.174.5.2843. [DOI] [PubMed] [Google Scholar]
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]