Abstract
There is evidence that elevated tissue concentrations of glutamate may contribute to pain and sensitivity in certain musculoskeletal pain conditions. In the present study the food additive monosodium glutamate (MSG) was injected intravenously into rats to determine whether it could significantly elevate interstitial concentrations of glutamate in the masseter muscle and whether MSG administration could excite and/or sensitize slowly conducting masseter afferent fibers through N-methyl-D-aspartate (NMDA) receptor activation. The interstitial concentration of glutamate after systemic injection of isotonic phosphate-buffered saline (control) or MSG (10 and 50 mg/kg) was measured with a glutamate selective biosensor. The pre-injection baseline interstitial concentration of glutamate in the rat masseter muscle was 24±11 μM. Peak interstitial concentration after injection of 50 mg/kg MSG was 63±18 μM and remained elevated above baseline for ~18 minutes In vivo single unit recording experiments were undertaken to assess the effect of MSG (50 mg/kg) on masseter afferent fibers. Injection of MSG evoked a brief discharge in one afferent fiber, and significantly decreased (~25%) the average afferent mechanical threshold (n=10) during the first 5 min after injection of MSG. Intravenous injection of ketamine (1 mg/kg), 5 minutes prior to MSG, prevented the MSG-induced decreases in the mechanical threshold of masseter afferent fibers. The present results indicate that a 2–3 fold elevation in interstitial glutamate levels in the masseter muscle is sufficient to excite and induce afferent mechanical sensitization through NMDA receptor activation. These findings suggest that modest elevations of interstitial glutamate concentration could alter musculoskeletal pain sensitivity in humans.
Keywords: Afferent Fiber, Glutamate, Muscle pain, Mechanical threshold, Masseter muscle, MSG
1. Introduction
Monosodium glutamate (MSG) is a food additive that is currently considered to be without serious safety concerns (Filer and Stegink, 1994). Initially, elevated consumption of MSG was linked to the symptoms such as headache, burning sensations, facial pressure and chest tightness (Schaumburg et al., 1969). More recent work has failed to evoke these symptoms consistently in healthy subjects after ingestion of large doses of MSG, even in individuals believed to be sensitive to MSG (Geha et al., 2000a,b). However, it is not known whether elevated tissue levels of glutamate alter sensory perception even in subjects who do not report adverse symptoms or overt pain after systemic administration of MSG
Evidence is gradually building which suggests that elevated tissue concentrations of glutamate contribute to pain and sensitivity in certain musculoskeletal pain conditions. For example, tissue concentrations of glutamate in the tendons of sufferers of conditions such as “jumpers knee” and “tennis elbow”, appear to be elevated 3–4 times over those measured in healthy controls (Alfredson et al., 2000, 2001; Alfredson and Lorentzon, 2002). Elevated concentrations of glutamate have also been associated with pain intensity and mechanical sensitivity in some (Rosendal et al., 2004) but not all studies of chronic myalgia sufferers (Ashina et al., 2003). It has been estimated that more than one third of an oral dose of MSG (150 mg/kg) is taken up by skeletal muscle (Graham et al., 2000), which suggests that dietary intake of glutamate may influence levels of glutamate in muscle tissue. Local injection of a “pharmacological dose” of MSG (0.2 ml, 170 mg/ml) into the masseter muscle has been found to induce an intense but short lasting period of muscle pain as well as a more prolonged period of localized mechanical sensitivity in healthy subjects (Svensson et al., 2003; Cairns et al., 2006). However, the concentration of glutamate in the muscle after injection of such high local doses of MSG cannot be considered physiologic (Gambarota et al., 2005). Thus, it remains unknown what minimum change in tissue glutamate concentration is necessary to induce mechanical sensitivity in skeletal muscle.
Some of the actions of glutamate in peripheral tissues such as skin, muscle and viscera can be explained by the activation of peripheral N-methyl-d-aspartate (NMDA) receptors that are expressed by slowly conducting (Aδ and C) afferent fibers which innervate these tissues (McRoberts et al., 2001; Cairns et al., 2002, 2003b; Du et al., 2003). Pain and mechanical sensitivity in humans, and afferent fiber discharge and decreased mechanical threshold in rats, can be induced by injection of glutamate into the masseter muscle and can be attenuated by local administration of NMDA receptor antagonists (Cairns et al., 2003b, 2006), which suggests that peripheral NMDA receptor activation is responsible for these effects of glutamate.
In the present study, MSG was injected intravenously into male and female rats to determine whether it could significantly elevate interstitial concentrations of glutamate in the masseter muscle and whether MSG administration could excite and/or sensitize slowly conducting masseter afferent fibers through NMDA receptor activation.
2. Materials and Methods
2.1 Measurement of Masseter Muscle Glutamate Levels
Adult Sprague-Dawley rats of either sex (n = 8 males, weight: 338±22 g, n = 8 females, weight: 259±5 g) were prepared for acute experiments under gas anesthesia (O2: 0.3–0.4 l/min; isoflurane 2.0–2.5%). A tracheal cannula was inserted and artificial ventilation initiated with a rodent ventilator. The carotid artery was cannulated for monitoring blood pressure and the femoral vein was cannulated to permit delivery of systemic drugs. The rat’s head was placed in a stereotaxic frame and a small incision in the skin overlying the masseter muscle was made to permit insertion of either a glutamate biosensor (n=5 male and 5 female rats) or a microdialysis probe (n=3 male and 3 female rats). Heart rate, mean blood pressure, expired CO2 and body core temperature were continuously monitored throughout the whole experiment. All procedures were performed in adherence with the principles of the Canadian Council on Animal Care and were approved by the University of British Columbia Animal Care Committee.
Glutamate biosensor
The glutamate biosensor (Pinnacle Technology, Inc., USA) was calibrated in vitro according to the manufacturer’s instructions. The glutamate biosensor is a platinum-iridium electrode coated with a layer of a passive-selective membrane (Nafion and Cellulose Acetate) followed by a layer of the enzyme glutamate oxidase, which catalyzes the conversion of glutamate and oxygen to α-ketoglutaric acid and hydrogen peroxide. The hydrogen peroxide produced is detected when it diffuses through the passive-selective membrane and is oxidized at the surface of the platinum-iridium electrode (applied potential: 600 mV). The passive-selective membrane limits interference from other substances present in muscle tissue which could be oxidized at the surface of the platinum-iridium electrode at a similar applied potential. The glutamate biosensor was inserted into the masseter muscle and allowed to stabilize for at least 60 min prior to any experiments being undertaken. Once the probe output stabilized, a 5 min baseline was recorded, followed by injection of isotonic phosphate-buffered saline (3.0 ml/kg, control) over 5 seconds. After any change in the output of the probe returned to baseline, a volume of a 17 mg/ml (0.1 M) solution of MSG in distilled water was injected over 5 seconds to provide a total dose of 10 mg/kg of MSG. After the output of the probe again returned to baseline, a volume of a 17 mg/ml solution of MSG in distilled water was injected over 5 seconds to provide a total dose of 50 mg/kg of MSG. Once the output of the probe returned to baseline levels after the 50 mg/kg injection, the experiment was ended and the animal terminated. Intravenous doses of glutamate were chosen based on estimates that about one third of a 150 mg/kg oral (i.e. 50 mg/kg) dose of MSG is deposited in skeletal muscle (Graham et al., 2000).
Microdialysis probe
The microdialyis probe (MAB 1.2.4.PES with 6 kD cutoff, Scientific Products and Equipment Ltd., Canada) was inserted into the masseter muscle, connected to a microinfusion pump (MAB 20 microdialysis pump, WM Altea AB, Sweden) and perfused with at 2 μl/min. An initial stabilization period of 90 min was allowed, followed by a sampling period of 60 min wherein two consecutive 60 μl samples of dialysate were collected. At the end of the sampling period, the experiment was ended and the animal terminated. Glutamate recovery was determined in vitro (n=3) by measuring the concentration of glutamate in the dialysate solution after placing the microdialysis probe in a solution of 100 μM MSG dissolved in buffered isotonic phosphate-buffered saline. Samples were stored in a −20 C freezer over night and analyzed the next day.
After derivatization with phthaldialdehyde reagent (Sigma Aldrich, USA), glutamate concentrations in the dialysate solution were measured by high performance liquid chromatography following a previously published method (Zhang et al., 2001). Calibration curves were constructed by running samples of 0, 0.1, 1, 10 and 100 μM glutamate in isotonic phosphate-buffered saline. The HPLC apparatus consisted of an Apollo c18 reverse phase 5 μm column, (Man Tech, Canada), LC-10AD microdialysis pump, SIL 9A autoinjector and RF-551 fluorescence detector set to excitation and emission wavelengths of 330 and 445 nm, respectively (Shimadzu, Japan). The output of the fluorescence detector was routed through a computer equipped with EZStart 7.3 SP1 chromatography software for recording and analysis (Shimadzu, Japan). Two mobile phases (phase A: tetrahydrofuran–methanol–0.1 mol/L sodium acetate, pH 7, 5:95:900 by volume; phase B: methanol) were applied by gradient at a flow rate of 1.0 ml/min as follows: 0% B, 25% B (5 min), 35% B (10 min), 55% B (15 min), 95% B (19 min – 32 min) (Zhang et al., 2001).
2.2 Afferent fiber recording
Although it would have been ideal to have recorded both interstitial glutamate concentration and afferent mechanical sensitivity in the same rat, this was not feasible due to the potential for tissue damage related to insertion of the probes affecting the response properties of the masseter afferent fibers as well as to the sensitivity of the glutamate biosensor probe to any movement. Therefore, to assess whether systemic administration of MSG could excite and/or sensitize masseter muscle afferent fibers, additional adult Sprague-Dawley rats of either sex (n = 13 males, weight: 394±22 g, n = 13 females, weight: 267±4 g) were prepared for acute in vivo recording of trigeminal afferent activity under gas anesthesia as described above (O2: 0.3–0.4 l/min; isoflurane 2.0–2.5%). The rat’s head was placed in a stereotaxic frame, the skin over the dorsal surface of the skull reflected and a trephination made on the left side of the skull to allow a microelectrode to be lowered through the brain and into the trigeminal ganglion. The skin, muscle and dura overlying the brainstem and upper cervical spinal cord were removed to permit placement of a stimulating electrode in contact with the brainstem for antidromic activation of brainstem projecting masticatory muscle afferent fibers that have been shown to project predominantly to the caudal brainstem (Shigenaga et al., 1988; Capra and Wax, 1989; Cairns et al., 2001, 2002). Physiological parameters were continuously monitored throughout the experiment as described above.
2.3 Stimulation and Recording Techniques
Single trigeminal afferent activity within the trigeminal ganglion was recorded by a parylene-coated tungsten microelectrode (2 MΩ, A-M Systems Inc., Carlsborg, WA, USA). A blunt probe was applied as a mechanical search stimulus to the skin over the masseter muscle while the electrode was slowly lowered in an attempt to identify trigeminal afferent fibers with a masseter muscle mechanoreceptive field. When a unit was found that appeared to respond to mechanical stimulation of the masseter muscle, the skin overlying the mechanoreceptive field was pulled gently away from contact with the muscle, and brush, pinch, and pressure stimuli were applied directly to the skin surface. If the unit did not respond to any of these cutaneous stimuli, then the mechanoreceptive field was considered to lie within the muscle.
To determine if the masseter afferent fiber projected to the caudal brainstem, constant-current electrical stimuli (100 μs biphasic pulse, range 10–90 μA, 0.5 Hz) were applied to a stimulating electrode (2MΩ, parylene-coated tungsten electrode, A-M Systems Inc., Carlsborg, USA) that had been lowered into the ipsilateral caudal brainstem to evoke antidromic action potentials. Antidromic action potentials were collided with orthodromic action potentials evoked by mechanical stimulation of the masseter muscle to confirm the projection of the afferent fiber to the caudal brainstem. At the end of the experiment, the distance between the stimulating and recording electrodes was measured, and divided by the latency of the antidromically-evoked response of an afferent fiber to give an estimation of the conduction velocity (CV) of the recorded afferent fiber.
Solutions of isotonic phosphate-buffered saline (3 ml/kg) or MSG (50 mg/kg) in isotonic phosphate-buffered saline were injected into the femoral vein over 5 seconds. The volume of the MSG solution (17 mg/ml) was adjusted to give the 50 mg/kg dose. The volume of phosphate-buffered saline was equivalent to the volume of MSG solution which would have been required to deliver a 50 mg/kg dose of MSG solution.
The baseline mechanical threshold (BMT: minimum force required to evoke afferent discharge) of the fiber was measured with a hand held electronic von Frey hair (VF hair; blunt polypropylene tip, diameter 0.5 mm, Model 1601C, IITC Inc., Woodland Hills, CA, USA) which was applied manually every min starting 15 min prior to and ending 5 min prior to injection of glutamate. Baseline primary afferent fiber activity was recorded for 5 min immediately preceding systemic injection of glutamate or isotonic phosphate-buffered saline. Mechanical threshold measurements were reinitiated 1 min after injection of glutamate and continued every min for a total of 20 min post injection. In subsequent experiments where the effect of ketamine was examined, ketamine (1 mg/kg) was injected over 5 seconds, 5 min prior to injection of glutamate. A dose of 1 mg/kg ketamine has been shown to be analgesic in rats (Plesan et al., 1998; Lee and Lee, 2001).
The electronic von Frey hair has been previously employed to assess masseter muscle afferent mechanical threshold in rats (Cairns et al., 2002, 2003a; Mann et al., 2006). Manual application of the von Frey hair to the masseter muscle to determine afferent mechanical threshold results in a log linear application of force over time that can be described by the equation,
where k is the force-time constant and t is time. We have determined that for the majority of masseter afferent fibers recorded in this study, there was no significant correlation between the force-time constant and BMT.
2.4 Data Analysis
Input from the glutamate biosensor was routed through a four-channel potentiostat (Model 3104) and data analyzed with Pinnacle Acquisition Laboratory software (Pinnacle Technology, Inc., USA). Baseline glutamate levels were calculated from a 5 min epoch just prior to the initial injection of isotonic phosphate-buffered saline. Peak values were calculated as the highest concentration of glutamate after injection. The duration was calculated as the period from the time point at which the post-injection glutamate level was greater than two standard deviation units above the pre-injection baseline until the time point where it returned below this value. The AUC was calculated by summing all the concentration-time bins in the defined duration area. For microdialysis, baseline glutamate levels in the dialysate were determined as the mean of two consecutive 30 min epochs obtained at least 120 minutes after inserting the probe into the muscle.
The activity of each identified afferent fiber was amplified (gain: 1000x; bandwidth 30–1,000 Hz) and fed together with the output of the electronic von Frey hair into a computer equipped with a CED 1401 Plus board and analysis software (Spike 2; Cambridge Electronic Design, Cambridge, U.K.). The mechanical threshold was measured offline from the recorded output of the electronic von Frey hair. The mean afferent fiber mechanical threshold (in g) was calculated from the average of each 5 consecutive stimuli (1/min) over each 5-min epoch beginning 15 min prior to and ending 20 min after injection of isotonic phosphate-buffered saline, glutamate or ketamine followed by glutamate. Relative mechanical threshold was calculated by dividing mean afferent fiber mechanical threshold for each 5 min epoch by the mean afferent fiber mechanical threshold calculated for the initial 5 min epoch.
2.5 Statistics
The effect of intravenously administered solutions on peak, duration and AUC of masseter glutamate levels was assessed with one-factor repeated measures ANOVA. Post-hoc comparisons versus saline control experiments were undertaken with the Holm-Sidak method. The effect of isotonic phosphate-buffered saline, glutamate or ketamine and glutamate on relative afferent mechanical threshold as well as the change in the force-time constant for mechanical stimulation was assessed with one-factor repeated measures ANOVA. Post-hoc comparisons versus the initial 5 min baseline period were undertaken with the Holm-Sidak method when appropriate. Pearson product moment correlation was used to assess relationships between BMT and peak MSG-induced changes in mechanical sensitivity.
Given that a relatively small number of males and females were used for data collection in the present study, statistical analysis of sex-related differences in interstitial glutamate concentration or the effect of elevated interstitial glutamate concentrations on mechanical threshold were not undertaken. Data in the text is reported as the mean and standard error of the mean (SEM).
3. Results
3.1 Intramuscular Concentration of Glutamate
Average glutamate concentrations in the masseter muscle of rats were assessed with the glutamate biosensor and compared with the results obtained by microdialysis, which has been employed to measure tissue glutamate concentrations in the past (deGroot et al., 2000; Alfredson et al., 2001; Tegeder et al., 2002; Ashina et al., 2003). As measured by the glutamate biosensor, the baseline concentration of glutamate in the rat masseter muscle was determined to be 24 ± 11 μM (n = 10). When measured using microdialysis, the average concentration of glutamate in the dialysate solution was 3.9 ± 1.5 μM (n=6). The maximum in vitro recovery of glutamate by the microdialysis probes used was estimated to be about 6%. Based on this degree of recovery, the average concentration of glutamate in the muscle would have been about 65 μM.
The effect of injection of isotonic phosphate-buffered saline and MSG (10 and 50 mg/kg) on masseter glutamate levels was assessed with the glutamate biosensor (Figure 1). Injection of isotonic phosphate-buffered saline did not change the concentration of interstitial glutamate. Peak concentration, duration of increased concentration and overall AUC were all significantly increased compared to isotonic phosphate-buffered saline control after a dose of 50 mg/kg MSG was administered (P < 0.05 two way repeated measures ANOVA and post-hoc Holm-Sidak method).
Figure 1.
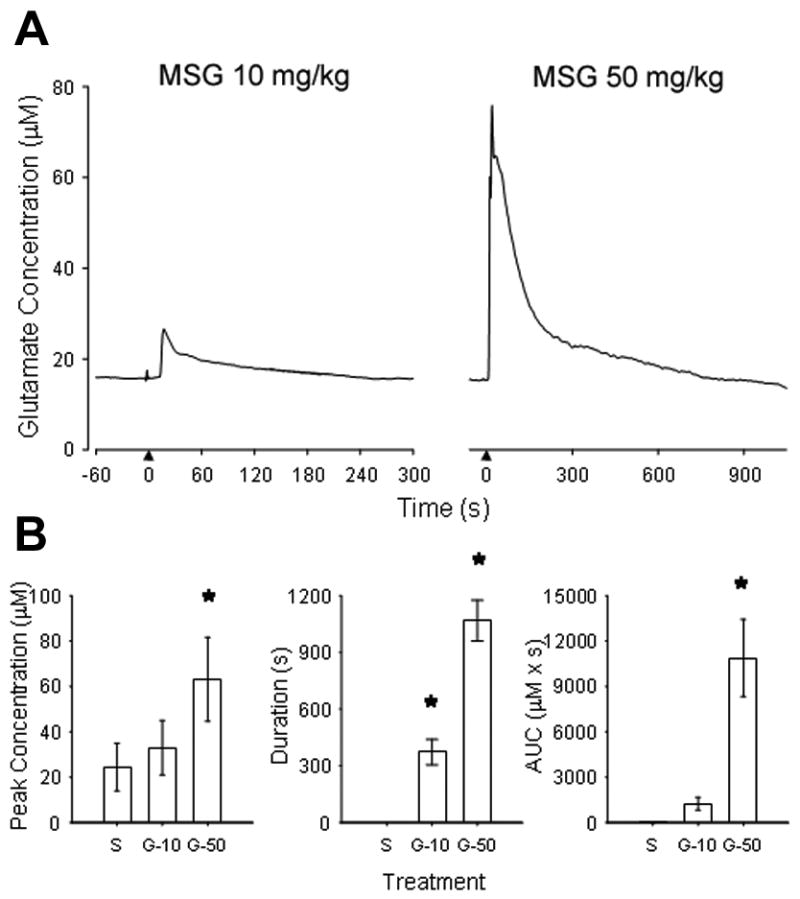
The line drawing in A illustrates an example of the change in glutamate concentration in the masseter muscle after intravenous injection of 10 and 50 mg/kg glutamate into a female rat. Note that after a delay of about 10–15 sec, glutamate concentrations rapidly rose in the muscle and then slowly returned to baseline. The bar graphs in B indicated the mean (± SE) peak, duration and area under the curve (AUC) for masseter muscle glutamate levels after injection of isotonic phosphate-buffered saline (S), glutamate 10 mg/kg (G-10) and 50 mg/kg (G-50) in 5 male and 5 female rats. * P < 0.05 one-way repeated measures ANOVA and post-hoc Holm-Sidak method compared with saline control.
3.2 Afferent Fiber Sensitivity
Control in vivo single unit recording experiments were undertaken to first assess the effect of injection of isotonic phosphate-buffered saline (n= 10 rats) on masseter afferent excitability and mechanical threshold. Six of these 10 fibers exhibited a very low level of spontaneous discharge (mean discharge rate: 0.009±0.005 Hz). Intravenous injection of isotonic phosphate-buffered saline did not evoke afferent discharge or significantly change the mechanical threshold (MT) of the ten Aδ afferent fibers (CV: 7.0±0.9 m/s, BMT: 20±10 g) examined.
In contrast to control experiments, injection of 50 mg/kg MSG evoked a brief discharge in one of the ten Aδ afferent fibers examined (Figure 2). This afferent fiber was recorded in a female rat and had a slow CV (3.4 m/sec) and relatively low BMT (6 g). The discharge consisted of two brief bursts at 10 and 15 s post injection of MSG. Injection of 50 mg/kg MSG also significantly lowered the mechanical threshold of the 10 Aδ fibers (CV: 7.0±0.9 m/s, BMT: 25±6 g) examined, in a time-dependent manner (Figure 3). Four of these 10 fibers exhibited a very low level of spontaneous discharge (mean discharge rate: 0.008±0.004 Hz). Overall, injection of 50 mg/kg of MSG significantly decreased the mean relative threshold by ~25% during the first 5 min after injection (Figure 4). Afferent mechanical thresholds slowly returned to baseline levels over the next 10 minutes. MSG-induced peak decrease in mechanical threshold exhibited a significant inverse relationship with BMT (r = −0.65), which suggests that MSG had a more pronounced effect on afferent fibers with high mechanical thresholds.
Figure 2.
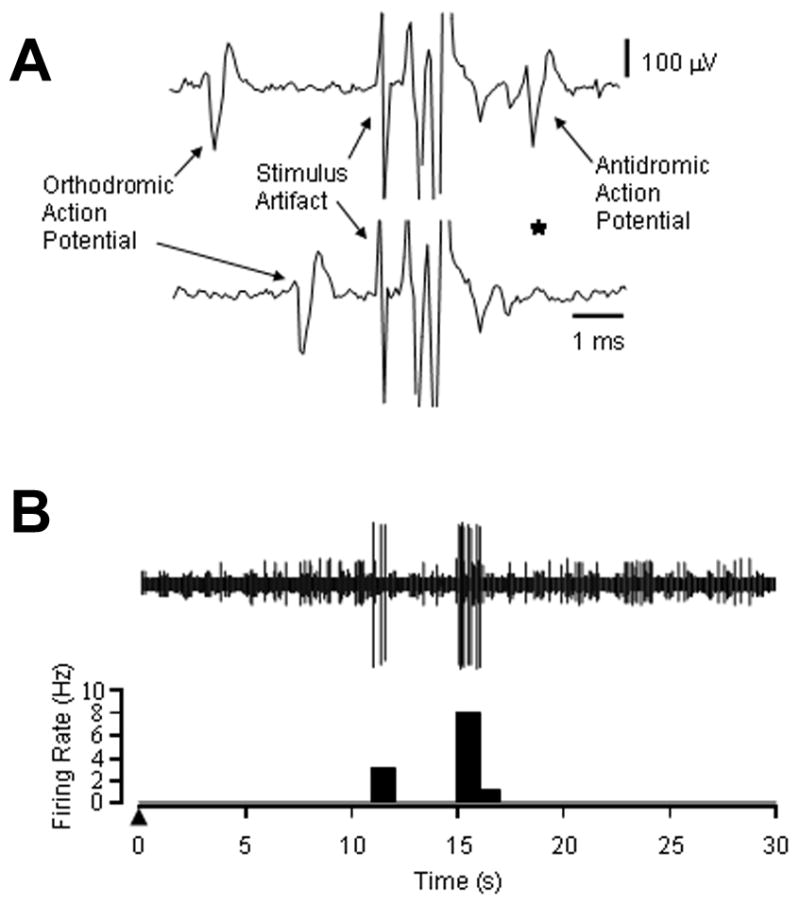
The response of a masseter afferent fiber recorded in a female rat to injection of MSG is shown. In A, the line drawings illustrate an orthodromic action potential evoked by mechanical stimulation of the masseter muscle and an antidromic action potential evoked by electrical stimulation of the caudal brain stem. Shortening the interval between the orthodromic and antidromic action potentials resulted in a collision (*, disappearance of the antidromic action potential), which confirmed that this afferent fiber projected from the masseter muscle to the caudal brain stem. The calculated CV for this afferent fiber was 3.4 m/s and the BMT was 6 g. In B, the line drawing and post stimulus histogram illustrate the firing of this afferent fiber in response to injection of monosodium glutamate into the femoral vein (▲). Two short bursts of action potential discharge occurred at 10 and 15 s post injection.
Figure 3.
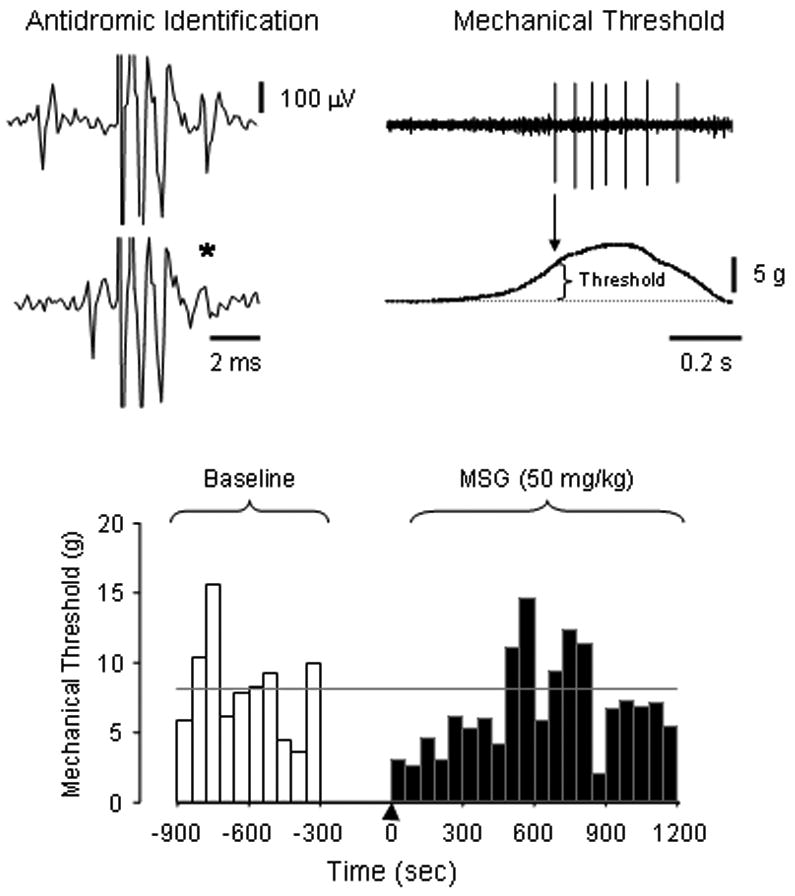
An example of an experiment where the mechanical threshold of an antidromically identified masseter afferent fiber was assessed before and after injection of 50 mg/kg of MSG in a female rat is shown. The line drawings on the upper left and right illustrate that this afferent fiber projected to the caudal brainstem (CV 3.4 m/s) and responded to mechanical stimulation of the masseter muscle with an electronic Von Frey hair. The bar graph below shows the mechanical threshold of this afferent fiber over time prior to and after injection of MSG (▲). The grey line indicates the mean BMT. The mechanical threshold of this fiber was decreased within 60 s of the MSG injection and remained decreased for 8 minutes before returning to baseline levels.
Figure 4.
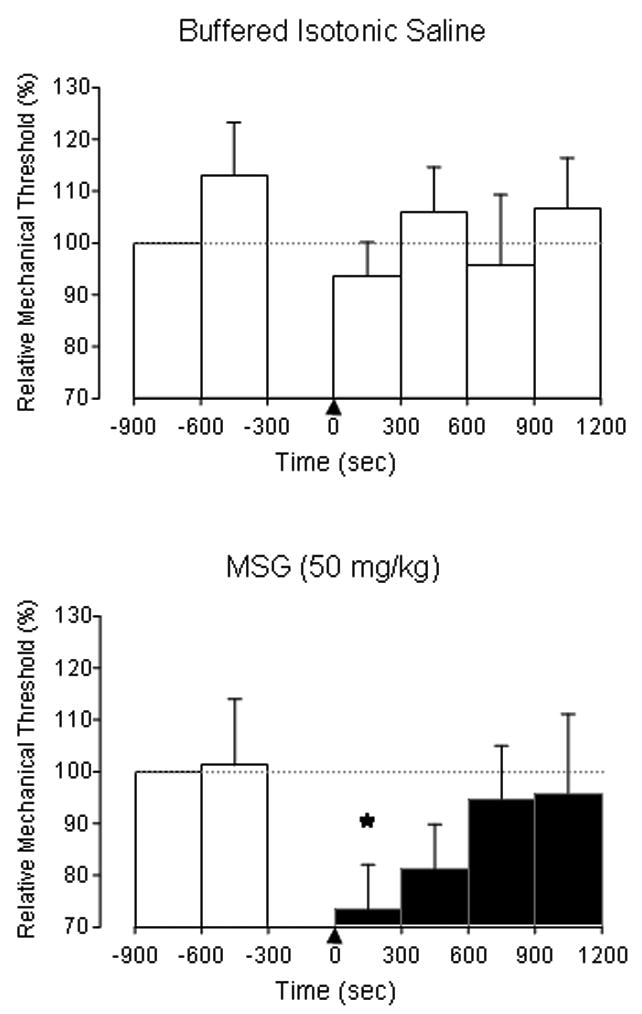
The bar graphs illustrate the population relative mechanical threshold before and after femoral vein injection of buffered isotonic saline (top, n=10) or 50 mg/kg MSG (bottom, n=10). Overall, there was no significant alteration in relative mechanical threshold over time after injection of saline. Following the injection of MSG, there was a significant (*) decrease in mechanical threshold from pre-injection baseline values during the first 5 min epoch. There were no significant sex-related differences in relative mechanical threshold in the saline or glutamate groups and no significant interactions between time and sex. * P < 0.05 one-way repeated measures ANOVA and post-hoc Holm-Sidak method compared with baseline.
To assess whether the mechanical sensitization of masseter afferent fibers induced by injection of glutamate might be due to NMDA receptor mechanisms, 6 additional experiments in male and female rats were undertaken where ketamine (1 mg/kg) was given 5 min prior to the injection of glutamate (50 mg/kg). One of the 6 fibers exhibited spontaneous discharge (rate: 0.027 Hz). Pre-injection of ketamine had no significant effect on mechanical threshold but prevented the subsequent injection of MSG from significantly decreasing the mechanical threshold of these afferent fibers (CV: 7.7±1.6 m/s, BMT: 33±17 g ; Figure 5).
Figure 5.
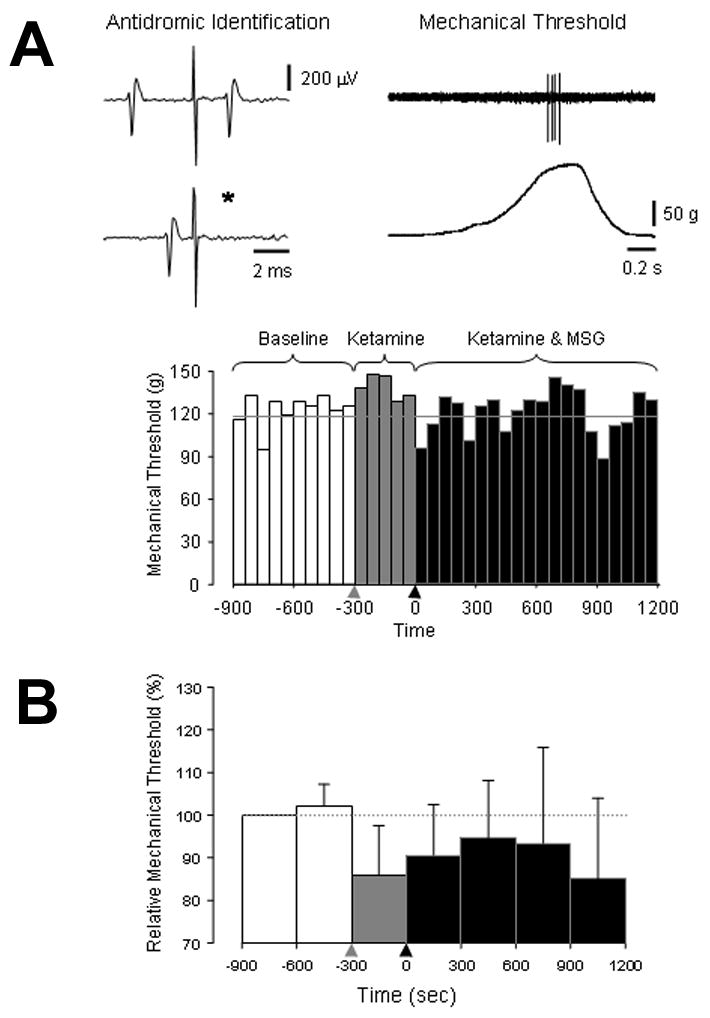
The effect of pre-administration of ketamine on MSG-induced mechanical sensitization is shown. In A, an example of an experiment where the mechanical threshold of an antidromically identified masseter afferent fiber was assessed before and after injection ketamine (1 mg/kg) followed by MSG (50 mg/kg) in a female rat is shown. The line drawings on the upper left and right illustrate that this afferent fiber projected to the caudal brainstem (CV 6.5 m/s) and responded to mechanical stimulation of the masseter muscle with an electronic von Frey hair. The bar graph below shows the mechanical threshold of this afferent fiber over time prior to and after injection of ketamine (
 ) followed by MSG (▲). The grey line in the bar graph indicates the mean BMT. The bar graph in B illustrates the population response (n=6). There was no significant change in mechanical threshold over time in these experiments (P > 0.05 one-way repeated measures ANOVA).
) followed by MSG (▲). The grey line in the bar graph indicates the mean BMT. The bar graph in B illustrates the population response (n=6). There was no significant change in mechanical threshold over time in these experiments (P > 0.05 one-way repeated measures ANOVA).
There was no significant change in the force-time constant for applied mechanical stimulation in the saline control, glutamate or ketamine/glutamate experiments.
4. Discussion
The results of this study indicate that normal mean interstitial concentrations of glutamate in the rat masseter muscle when measured by the glutamate biosensor were about 25 μM. After a single systemic dose of 50 mg/kg MSG, peak interstitial levels of glutamate reached ~65 μM and remained elevated, compared to initial baseline levels, for ~18 minutes. The elevation of glutamate concentrations in the masseter muscle after administration of MSG intravenously was associated with significant mechanical sensitization of slowly conducting masseter afferent fibers during the first 5 min post-systemic injection. Action potential discharge was observed in only one afferent fiber shortly after injection of MSG, at a time when peak glutamate concentrations in the masseter muscle occurred. This finding may, in part, explain why some individuals complain of acute myalgia post ingestion of MSG (Schaumburg et al., 1969; Geha et al., 2000a). The magnitude of MSG-induced mechanical sensitization of masseter afferent fibers decreased over the same time course as the decline in glutamate concentration in the masseter muscle post systemic administration of MSG, which suggests that sensitization was a direct consequence of elevated masseter muscle glutamate levels. Administration of 1 mg/kg ketamine did not significantly alter the mechanical threshold of masseter afferent fibers but did significantly attenuate the ability of MSG to induce mechanical sensitization. This result suggests that glutamate excites and induces mechanical sensitization of masseter afferent fibers in part through an activation of peripheral NMDA receptors and is consistent with previous findings (Cairns et al., 2002, 2003b).
This appears to be the first use of a glutamate biosensor to detect changes in interstitial glutamate concentration in skeletal muscle. The advantage of this biosensor is its time resolution (1 s) for direct measurement of changes in glutamate concentration. In comparison, magnetic resonance spectroscopy, which also allows direct measurement of glutamate concentration in muscle has a time resolution of ~ 60 s and a sensitivity limit of 10 mM (Gambarota et al., 2005). Microdialysis, when used to measure glutamate at fairly high flow rates could have a time resolution of 5–10 min, but the determination of actual tissue concentrations requires an estimate of glutamate recovery. Thus, the glutamate biosensor allowed direct measurement of changes in baseline interstitial glutamate on a time scale not possible with these other techniques. Nevertheless, average baseline glutamate concentrations measured by the glutamate biosensor (24 μM) were more conservative than those determined by microdialysis (65 μM) in the present study, thus peak concentrations of glutamate after administration of MSG as determined with the glutamate biosensor should also be considered conservative.
Interstitial glutamate concentrations have been previously measured with microdialysis probes in a variety of tissues in both humans and animals. In the rat skin, it has been reported that the estimated baseline interstitial glutamate concentration is ~14 μM, or about half that measured in the masseter muscle in this study (deGroot et al., 2000). In healthy humans, the concentration of glutamate in tendons and muscles has been estimated to be between 20 and 70 μM (Alfredson et al., 2000, 2001; Alfredson and Lorentzon, 2002; Tegeder et al., 2002; Ashina et al., 2003; Rosendal et al., 2004). These values in deeper tissues appear consistent with the baseline values determined with the glutamate biosensor and microdialysis probes for the masseter muscle in the present study and suggest that normal interstitial glutamate concentrations in rats and humans are similar.
It was initially proposed that peripheral mechanisms were responsible for the symptoms associated with ingestion of large doses of MSG (Schaumburg et al., 1969). Injection of a pharmacological dose of MSG into the masseter muscle has been shown to decrease the mechanical threshold of slowly conducting masseter afferent fibers by as much as 50% for as long as 3 hours or more (Cairns et al., 2002; Mann et al., 2006). This sensitizing effect of locally injected MSG appears to be mediated through activation of peripheral glutamate receptors, as it can be attenuated by co-administration of the glutamate receptor antagonist kynurenic acid (Cairns et al., 2002). In comparison, in the current study, administration of a 50 mg/kg dose of glutamate significantly decreased the mechanical threshold of slowly conducting masseter afferent fibers by 25% for the first 5 min after injection. During this same time period, our biosensor data indicates that the average glutamate concentration was 45 μM, or roughly twice the measured baseline concentrations, which suggests that this may be the minimum concentration change needed to induce significant mechanical sensitization. In comparison, employing magnetic resonance spectroscopy, it has been reported that intramuscular injection of a pharmacological dose of MSG (1 M, 100 μl) into the masseter muscle yielded initial peak values of 215 mM glutamate (~ 1000 times higher than baseline levels) at the site of injection and concentrations remained above 10 mM (limit of detection) for 10 min (Gambarota et al., 2005). Although the initial clearance of glutamate after intramuscular injection of MSG appears rapid (t½ = 108 s) as it does after systemic injection in the present study (see Figure 1), the results of the current experiments suggest a more prolonged terminal clearance of glutamate from the muscle. Thus, it is possible that after intramuscular injections of high concentrations of MSG, interstitial levels remain elevated for long periods and this alone accounts for the prolonged afferent mechanical sensitization observed, although other mechanisms, such as release of neuropeptides may also contribute (Spigelman and Puil, 1990; Heppelmann and Pawlak, 1997; Hu et al., 1997; Jackson and Hargreaves, 1999).
Elevated tissue concentrations of glutamate have been reported in some human pain conditions involving muscles and tendons. In the extensor carpi radialis brevis tendon of patients with tennis elbow and the patellar tendon of patients with “jumpers knee”, glutamate levels of greater than 200 μM as compared to 50–70 μM for healthy controls have been found (Alfredson et al., 2000, 2001; Alfredson and Lorentzon, 2002). Further, immunohistochemical evidence for peripheral NMDA receptors associated with tendons has been provided (Alfredson et al., 2000, 2001). In addition, women with chronic work-related trapezius myalgia (n = 19) had baseline levels of 47 μM versus 36 μM for healthy controls (n = 20) and baseline pain pressure thresholds showed a significant negative correlation with muscle glutamate levels (Rosendal et al., 2004). During low force exercise, glutamate levels in the trapezius exceeded 40 μM in healthy controls and 60 μM in myalgia patients and were positively correlated to the magnitude of muscle pain reported by subjects (Rosendal et al., 2004). The association of glutamate levels with both ongoing pain magnitude and pressure pain thresholds is consistent with the results of the present and previous studies which indicate that increased interstitial levels of glutamate excite and sensitize muscle afferent fibers through peripheral NMDA receptor activation (Cairns et al., 2002, 2003b).
It has been previously speculated that ingestion of gram quantities of MSG could potentially alter the sensitivity of muscle nociceptors to affect pain sensitivity in chronic pain patients (Cairns et al., 2002). The median daily consumption of glutamate in the Western diet from all sources is around 12 g, with up to 1 g/day of MSG consumed (Geha et al., 2000b; Nelson et al., 2000). This equals about 170 mg/kg/day of glutamate for an individual weighing 70 kg. A single oral dose of 150 mg/kg in adults has been reported to increase serum levels of glutamate 30 min post ingestion by 7–8 times, and more than one third of the total MSG dose was taken up into skeletal muscle tissue (Graham et al., 2000). A range of symptoms that include general weakness, tightness, flushing, sweating, headache/migraine, numbness/tingling, paresthesias, arrhythmias and tachycardia have been reported after ingestion of MSG doses in this range (Schaumburg et al., 1969; Thomassen et al., 1991; Geha et al., 2000a, b). However, blinded, placebo controlled studies have failed to consistently evoke these symptoms in healthy subjects after administration of MSG, even in subjects who appear “MSG sensitive” (Geha et al., 2000a, b). It is now thought that failure to adequately mask the unique taste of MSG in early studies was likely responsible for the high incidence of reports of adverse effects upon oral administration of high doses of MSG (Geha et al., 2000a, b). Nevertheless, as more than 50% of patients who received glutamate intravenously (5 mg/kg ) reported burning sensations, facial pressure or chest tightness in association with peak serum levels of glutamate, it is likely that in at least some individuals elevated concentrations of glutamate are responsible for reported symptoms (Thomassen et al., 1991). The present results indicate that there is a relationship between MSG dose, interstitial levels of glutamate in muscle tissue and afferent mechanical sensitivity. Indeed, the present study suggests that a modest increase in interstitial glutamate concentration in the masseter muscle as a result of systemic administration of MSG is associated with significant mechanical sensitization of slowly conducting masseter muscle afferent fibers. How dietary intake of glutamate may affect healthy individuals or chronic muscle pain patients to alter their sensitivity to muscle pain remains to be determined.
Acknowledgments
Support for this research was provided by grant DE 015420 from the National Institute of Dental and Craniofacial Research (PI: BJ Sessle). BEC and BJS are recipients of Canada Research Chairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfredson H, Forsgren S, Thorsen K, Lorentzon RJ. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper's knee. Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Ljung BO, Thorsen K, Lorentzon RJ. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71:475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- Alfredson H, Lorentzon RJ. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr Drug Targets. 2002;3:43–54. doi: 10.2174/1389450023348028. [DOI] [PubMed] [Google Scholar]
- Ashina M, Stallknecht B, Bendtsen L, Pedersen JF, Schifter S, Galbo H, Olesen J. Tender points are not sites of ongoing inflammation -in vivo evidence in patients with chronic tension-type headache. Cephalalgia. 2003;23:109–116. doi: 10.1046/j.1468-2982.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Gambarota G, Dunning PS, Mulkern RV, Berde CB. Activation of peripheral excitatory amino acid receptors decreases the duration of local anesthesia. Anesthesiology. 2003a;98:521–529. doi: 10.1097/00000542-200302000-00035. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB. Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience. 2002;109:389–399. doi: 10.1016/s0306-4522(01)00489-4. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P. Sex-related differences in human pain perception and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2001;86:782–791. doi: 10.1152/jn.2001.86.2.782. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006:169. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen l. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003b;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Capra NF, Wax TD. Distribution and central projections of primary afferent neurons that innervated the masseter muscle and mandibular periodontium: a double-label study. J Comp Neurol. 1989;279:341–352. doi: 10.1002/cne.902790302. [DOI] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Filer LJJ, Stegink LD. A report of the proceedings of an MSG workshop held August 1991. Crit Rev Food Sci Nutr. 1994;34:159–174. doi: 10.1080/10408399409527655. [DOI] [PubMed] [Google Scholar]
- Gambarota G, Philippens M, Cairns BE, Dong XD, Renema WK, Heerschap A. MRS assessment of glutamate clearance in a novel masticatory muscle pain model. NMR Biomed. 2005;18:345–351. doi: 10.1002/nbm.962. [DOI] [PubMed] [Google Scholar]
- Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, Ditto AM, Harris KE, Shaughnessy MA, Yarnold PR, Corren J, Saxon A. Multicenter, double-blind, placebo-controlled, multiple-challenge evaluation of reported reactions to monosodium glutamate. J Allergy Clin Immunol. 2000a;106:973–980. doi: 10.1067/mai.2000.110794. [DOI] [PubMed] [Google Scholar]
- Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, Ditto AM, Harris KE, Shaughnessy MA, Yarnold PR, Corren J, Saxon A. Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. J Nutr. 2000b;130(4S Suppl):58S–62S. doi: 10.1093/jn/130.4.1058S. [DOI] [PubMed] [Google Scholar]
- Graham TE, Sgro V, Friars D, Gibala MJ. Glutamate ingestion: the plasma and muscle free amino acid pools of resting humans. Am J Physiol Endocrinol Metab. 2000;278:E83–E89. doi: 10.1152/ajpendo.2000.278.1.E83. [DOI] [PubMed] [Google Scholar]
- Heppelmann B, Pawlak M. Sensitization of articular afferents in normal and inflamed knee joints by substance P in the rat. Neurosci Lett. 1997;223:97–100. doi: 10.1016/s0304-3940(97)13408-5. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Li ZW, Si JQ. Evidence for the existence of substance P autoreceptor in the membrane of rat dorsal root ganglion neurons. Neurosci. 1997;77:535–541. doi: 10.1016/s0306-4522(96)00451-4. [DOI] [PubMed] [Google Scholar]
- Jackson DL, Hargreaves KM. Activation of excitatory amino acid receptors in bovine dental pulp evokes the release of iCGRP. J Dent Res. 1999;78:54–60. doi: 10.1177/00220345990780010801. [DOI] [PubMed] [Google Scholar]
- Lee IO, Lee IH. Systemic, but not intrathecal, ketamine produces preemptive analgesia in the rat formalin model. Acta Anaesthesiol Sin. 2001;39:123–127. [PubMed] [Google Scholar]
- Mann MK, Dong XD, Svensson P, Cairns BE. Influence of intramuscular nerve growth factor injection on the response properties of rat masseter muscle afferent fibers. J Orofac Pain. 2006 in press. [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Nelson LM, Matkin C, Longstreth WTJ, McGuire V. Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. II. Diet Am J Epidemiol. 2000;151:164–173. doi: 10.1093/oxfordjournals.aje.a010184. [DOI] [PubMed] [Google Scholar]
- Plesan A, Hedman U, Xu XJ, Wiesenfeld-Hallin Z. Comparison of ketamine and dextromethorphan in potentiating the antinociceptive effect of morphine in rats. Anesth Analg. 1998;86:825–829. doi: 10.1097/00000539-199804000-00027. [DOI] [PubMed] [Google Scholar]
- Rosendal L, Larsson B, Kristiansen J, Peolsson M, Søgaard K, Kjær M, Sørensen J, Gerdle B. Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain. 2004;112:324–334. doi: 10.1016/j.pain.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Schaumburg HH, Byck R, Gerstl R, Mashman JH. Monosodium L-glutamate: its pharmacology and role in the Chinese restaurant syndrome. Science. 1969;163:826–828. doi: 10.1126/science.163.3869.826. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Puil E. Ionic mechanism of substance P actions on neurons in trigeminal root ganglia. J Neurophysiol. 1990;64:273–281. doi: 10.1152/jn.1990.64.1.273. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Tegeder L, Zimmermann J, Meller ST, Geisslinger G. Release of algesic substances in human experimental muscle pain. Inflamm Res. 2002;51:393–402. doi: 10.1007/pl00000320. [DOI] [PubMed] [Google Scholar]
- Thomassen A, Nielsen TT, Bagger JP, Henningsen P. Effects of intravenous glutamate on substrate availability and utilization across the human heart and leg. Metabolism. 1991;40:378–384. doi: 10.1016/0026-0495(91)90148-p. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang X, Zhang T, Chen L. Excitatory amino acids in cerebrospinal fluid of patients with acute head injuries. Clin Chem. 2001;47:1458–1462. [PubMed] [Google Scholar]


