Abstract
Elevated levels of extracellular nucleotides are present at sites of inflammation, platelet degranulation and cellular damage or lysis. These extracellular nucleotides can lead to the activation of purinergic (nucleotide) receptors on various leukocytes, including monocytes, macrophages, eosinophils, and neutrophils. In turn, nucleotide receptor activation has been linked to increased cellular production and release of multiple inflammatory mediators, including superoxide anion, nitric oxide and other reactive oxygen species (ROS). In the present review, we will summarize the evidence that extracellular nucleotides can facilitate the generation of multiple ROS by leukocytes. In addition, we will discuss several potential mechanisms by which nucleotide-enhanced ROS production may occur. Delineation of these mechanisms is important for understanding the processes associated with nucleotide-induced antimicrobial activities, cell signalling, apoptosis, and pathology.
Key words: extracellular nucleotides, NADPH oxidases, nitric oxide synthases, purinergic receptors, reactive oxygen species
Overview of the biological roles of ROS
The cellular production of oxygen radicals has been implicated in a wide variety of biological processes including host defense, regulation of cell apoptosis, modulation of cell signalling as well as aging and the generation of pathological conditions [1–9]. Cells produce a variety of oxygen radicals often termed reactive oxygen species (ROS) which include superoxide (O−2), hydrogen peroxide (H2O2), hydroxyl radicals (·OH), nitric oxide (NO), and peroxynitrite (ONOO-). The generation of ROS can be mediated by several enzyme systems including NADPH oxidases, nitric oxide synthases, xanthine oxidase, and the mitochondrial respiratory chain. Furthermore, production of ROS is subject to intricate regulation by a variety of hormones, cytokines, and toxins, thereby illustrating the importance of these processes in mammalian biology.
Numerous studies have revealed that the production of O−2 anions and other ROS by immune cells such as neutrophils, eosinophils, and macrophages plays a fundamental role in the mammalian immune response [3–8]. These ROS act as antimicrobials and facilitate in the killing of invading microorganisms. For example, one functional consequence of ROS production by bacterial lipopolysaccharide- (LPS-) primed macrophages is the formation of ONOO-, which is an extremely reactive intermediate formed by the reaction of O−2 and NO that contributes to microbial killing and cellular injury [7]. Moreover, the importance of ROS-mediated anti-microbial activity in host defense is illustrated by the observation that defects in the function of the major ROS producing enzyme in phagocytes, i.e., NADPH oxidase, results in a severe immunodeficiency disorder termed chronic granulomatous disease [1, 3–5, 10, 11]. This disease has been linked to specific mutations in NADPH oxidase genes and causes the individual to be highly susceptible to frequent and life-threatening infections by bacteria and fungi [10, 11].
Although the antimicrobial activity of ROS is one of the most characterized functions of these agents, there is evidence that ROS are important participants in other biological processes [2, 3, 6, 12–26]. For example, ROS have been proposed to serve as intracellular second messengers [12–18]. In this regard, intracellular ROS generation has been linked to the regulation of a variety of cell signalling events, such as the nuclear translocation of the transcription factor APE1/Ref1 [20] as well as the activation of NF-κB, AP-1, p90Rsk and members of the MAP kinase family [12–18, 20, 21]. Although the relevant mechanisms are not fully resolved, multiple processes have been proposed to account for ROS contributions to the activation of MAP kinases [13, 16, 22, 23]. Cells contain numerous redox-sensitive systems that are modified in response to oxidation, including glutathione, thioredoxin, and cysteine-containing proteins [16, 22, 23]. For example, many protein phosphatases can be reversibly inactivated via the modification of critical cysteines by ROS [22, 24], and this process may lead to the enhanced phosphorylation/activation of enzymes such as p90Rsk and the MAP kinases [21, 24]. In addition, signalling proteins that are upstream of the MAP kinases, such as the small MW G-protein Ras, have also been proposed to function as redox sensors [23] and as such may contribute to the effects of ROS on cell signalling events.
Another key role of ROS involves its capacity to affect cardiovascular function and cellular apoptosis [2]. At low levels, ROS such as O−2 and NO can play an important role in the control of vessel tone, tissue repair and remodeling, and angiogenesis [2, 3, 6, 25, 26]. Conversely, at higher levels, ROS generation has been linked to a variety of pathological conditions, including atherosclerosis, hypertension, heart failure and aging [2, 25, 26]. In addition, there is also evidence that ROS may contribute to the tissue/organ damage associated with ischemic-reperfusion injury and diabetes mellitus [2, 6, 7, 25, 26].
Immunological sources and mechanisms of ROS generation
Cellular sources of ROS
The diverse role of ROS in mammalian biology is consistent with the observation that a diverse array of cell types possess the capacity to generate ROS. With respect to immunological function, the production of O−2 and H2O2 has been documented in macrophages, eosinophils, neutrophils, microglia, mesangial cells, endothelial cells and many other cell types [1–6, 26]. Similarly, the capacity to generate NO is widely distributed and includes by cell types such as macrophages, microglia, mesangial cells, vascular smooth muscle cells, endothelial cells, pancreatic islet cells, and neuronal cells [7–9, 25]. In the present review, we will focus our discussion on how ROS such as O−2, H2O2, and NO are generated by immune cell types that are subject to nucleotide receptor regulation, namely, macrophages, eosinophils, neutrophils, microglia, and mesangial cells.
Mechanisms of O−2 generation
As noted above, the generation of ROS can be mediated by several enzyme systems including NADPH oxidases, nitric oxide synthases, xanthine oxidase, and the mitochondrial respiratory chain [1–6]. With respect to the regulated production of O−2, the principal enzyme systems controlling this endpoint are the NADPH oxidases, which have been characterized in both phagocytic and non-phagocytic cell types [3, 6]. The O−2 generated through this reaction can be rapidly converted inside the cell to H2O2 by dismutation, and in the presence of iron salts, O−2 and H2O2 can interact to form the hydroxyl radical OH. [2, 4]. Altogether, these ROS are potent antimicrobial compounds, and in this section we will focus on several of the major mechanisms controlling phagocytic NADPH oxidase (which shares many similarities to non-phagocytic NADPH oxidase) [3–6], and in subsequent sections we will discuss how nucleotide receptor signalling has been reported to contribute to this regulation.
Numerous studies have revealed that the NADPH oxidase of phagocytic cells is a protein complex of at least six different subunits, including gp91phox (also known as NADPH oxidase 2 or Nox2), p22phox, p67phox, p47phox, p40phox, and Rac1/2 [1–6]. The oxidase-specific components of phagocytic cells are designated by the term phox, which derives from the name phagocyte oxidase. In addition, several other proteins (e.g., the GTPase Rap1A and p29 peroxiredoxin) have also recently been implicated in the control of NADPH oxidase activity [3, 6]. The overall regulation of this system is intricate and involves stimulus-induced spatiotemporal assembly and activation of the complex. The complex is subject to multiple levels of control, including (a) separation of the subunits into different subcellular compartments during the inactive state, (b) reversible protein-protein and protein-phospholipid interactions, and (c) modulation by protein phosphorylation. The catalytic core of NADPH oxidase is composed of two integral membrane proteins, the glycoprotein gp91phox and the protein subunit p22phox, which together constitute a heterodimeric flavocytochrome termed cytochrome b558 [1–6].
In the resting state, a small portion of the cytochrome b558 subunits gp91phox and p22phox are inserted into the plasma membrane with the remaining gp91phox and p22phox being localized to specific cytotoplasmic granules and secretory vesicles [1, 3–6]. Cellular priming with factors such as LPS can lead to an up-regulation of gp91phox and to a lesser degree p22phox [3, 27]. In addition, LPS priming can further recruit gp91phox and p22phox from secretory vesicles to the plasma membrane as well as promote the assembly and activation of the final NADPH complex within specific granules, which is a process likely to be important for the intracellular destruction of bacteria [1, 3–6, 26]. The oxidase activity of cytochrome b558 is dormant until activated by four cytosolic components p67phox, p47phox, p40phox, and Rac, which in turn are subject to multiple levels of regulation, modification or activation in order to reach the final functional state [1, 3–6]. Priming of this system involves the stimulus-induced phosphorylation of p47phox that results in a conformational change that unmasks a protein binding motif (an SH3 domain) and a phosphoinositide- (such as 3-phosphoinositides) binding domain termed a Phox homology or PX domain [3–6]. The phosphorylated and unfolded p47phox is recruited to p22phox at the plasma or granular membrane, which is a process that has been suggested to be facilitated by the PX domain present in p47phox. In addition, it has also been proposed that p40phox also contains a phosphoinositide binding (PX) domain that participates in its capacity to facilitate the interaction of p67phox with gp91phox at the membrane. Overall, phosphorylation of the p47phox/p40phox/p67phox complex allows for its dissociation/structural reorganization and subsequent recruitment to the plasma membrane at sites containing the cytochrome b558 subunits [1, 3–6]. Simultaneous to these events is the stimulus-induced activation of Rac2 by guanine nucleotide exchange factors that promote the activated/GTP loaded form of Rac2 to be recruited to the membrane whereupon it also facilitates p67phox interaction with cytochrome b558.
With respect to the key regulatory step that centers on p47phox phosphorylation, many distinct protein kinases have been implicated in this process. These kinases include several isoforms of protein kinase C (PKC), p38 mitogen-activated protein (MAP) kinases, p21-activated kinase (PAK), as well as other protein kinases [1, 3–6]. Furthermore, it has long been known that Ca++ fluxes are critical for the activation of NADPH oxidase, and it is likely that its participation in this regulation includes its capacity to activate protein kinases such as certain PKC isoforms. Moreover, activation of Rac-dependent pathways may not only play an important role in recruiting phox proteins to the plasma or granular membrane as discussed above, but Rac activation may also contribute to the phosphorylation of phox proteins via its role in initiating the activation of MAP kinase family members such as p38. Final assembly/activation of the NADPH complex requires additional phosphorylation steps resulting in complete assembly of the oxidase complex at the membrane and the transfer of electrons from NADPH across the membrane culminating in the generation of O−2. In sum, stimuli that promote changes in intracellular Ca++ levels, activation of isoforms of PKC, and/or activation of MAP kinase members such as p38 can all cooperate to regulate NADPH oxidase activity. These observations support the concept that ligands for distinct receptor classes, which activate differing signalling pathways, can converge on NADPH oxidase regulation and O−2 generation. This concept is relevant to further discussions in this review regarding the capacity of extracellular nucleotides to regulate NADPH oxidase activity in cooperation with other priming agents such as LPS or chemoattractants.
Mechanisms controlling NO generation
NO is a gaseous free radical that plays a role in a variety of biological functions, including host defense, vasodilatation, cellular apoptosis, and the regulation of receptors (e.g., the cardiac ryanodine receptor), enzymes (e.g., activation of soluble guanylate cyclase) and transcription factors (e.g., the inactivation of zinc finger transcription factors and the S-nitrosylation/inactivation of NF-κB/IκB) [7–9, 25, 26, 28, 29]. At high levels, NO can also have deleterious effects including cellular mutagenesis and necrosis [7–9, 25, 26]. Furthermore, NO can react with O−2 to form ONOO-, which is a powerful oxidizing agent and antimicrobial compound [2, 7, 25, 26].
The synthesis of NO occurs via the enzymatic oxidation of the terminal guanidino-nitrogen of L-arginine, thereby resulting in the formation of NO and L-citrulline [7–9, 25]. The enzymes involved in catalyzing this reaction are termed NO synthases (NOS). Isoforms of these enzymes are produced either constitutively, such as those produced by endothelial cells (eNOS) and neuronal cells (nNOS), or as an inducible form of the enzyme (iNOS) largely expressed by macrophages and related cell types. Numerous studies have focused on establishing the structure of these isoforms and have revealed that all three isoforms are multimeric complexes that have similar structural features, especially with respect to the nature of their catalytic sites. In this regard, it has been reported that each of the isoforms requires multiple cofactors such as FAD, heme, calmodulin, and tetrahydrobiopterin. Whereas eNOS and nNOS are activated by Ca++-calmodulin binding and therefore rapidly controlled in response to Ca++ fluxes, iNOS is primarily regulated at the transcriptional level and is only weakly affected by changes in intracellular Ca++ levels. Accordingly, NO production by eNOS and nNOS is transient and localized, whereas NO production by iNOS is often large and sustained [7–9, 25].
With respect to the induction of iNOS, the promoter of the iNOS gene has binding sites for a wide array of transcription factors, but several of the more well-characterized factors controlling iNOS expression are NF-κB, interferon regulatory factor-1 (IRF-1), and signal transducer and activator of transcription factor-1 (STAT-1) [30–34]. Therefore, factors that promote NF-κB activation (such as TNF-α and IL-1) together with those that promote IRF-1 or STAT-1 activation (such as IFN-γ) cooperate to yield synergistic activation of iNOS expression. In this review, we will focus on the regulation of iNOS expression and its relevance to purinergic receptor signalling.
Extracellular nucleotides and the regulation of ROS production
General extracellular nucleotide action
Despite the rapid metabolism of extracellular nucleotides, numerous studies have revealed that purines (adenosine, ADP, and ATP) and/or pyrimidines (UDP and UTP) can be found at high concentrations (millimolar) in the extracellular milieu following events such as tissue damage or platelet degranulation [35–40]. These agents can act as important mediators in a plethora of physiological responses, including neurotransmission, cardiovascular homeostasis, pulmonary function, smooth muscle contraction, and immune activity [38–42]. With respect to the role of extracellular nucleotides in regulating the generation of ROS, there is considerable evidence that nucleotides play a key role in modulating inflammation, mediator production, cell-mediated killing, and apoptosis [38–42]. The role of nucleotides in immune function is also illustrated by the observation that they can greatly enhance the effects of bacterial LPS on macrophage and monocyte activation by augmenting the production of mediators such as NO and other free radicals, in addition to numerous cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α) [43–48]. The increased production of these mediators, although important for the activation of the immune system and for bactericidal effects, can also contribute to the deleterious effects on tissues and organs observed in sepsis. In this section, we will focus on the reported capacity of extracellular nucleotides to promote the generation of ROS by immune cells, and we will then discuss the potential mechanisms by which nucleotide receptors may mediate this process.
Nucleotide receptor families and signalling
Nucleosides and nucleotides exert their effects by interacting with specific cell surface receptors, which in turn modulate a diverse array of cell signalling and transcriptional events. Receptors for extracellular nucleotides are known as P2 receptors, and these receptors are divided into two subfamilies: P2Y and P2X [38–40, 49–51]. Eight P2Y receptors have been identified in mammals and have been designated P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14 [40]. These receptors are predicted to contain seven-transmembrane domains and they are associated with heterotrimeric G-protein activation [38, 40, 49]. On the other hand, the P2X receptors are a family of seven distinct subunit isoforms (P2X1–) that are thought to act as predominantly homotrimeric ligand-gated, cation-selective ion channels that contain two predicted membrane-spanning domains [38–40, 50–52].
With respect to the ligand specificity and cell signalling events initiated by these two classes of P2 receptors, there exist many overlapping properties within subfamily groupings. There is extensive literature covering the pharmacology of these receptors and the reader is referred to several recent reviews for more detailed information [38–40, 49–51]. Briefly, within the P2Y receptor subfamily, both adenine nucleotides and/or uridine nucleotides can serve as ligands, depending on the specific receptor. For example, P2Y1, P2Y12 and P2Y13 exhibit a preference for ADP binding, whereas P2Y2 and P2Y11 can be activated by ATP. In addition, P2Y2 and P2Y4 can be stimulated by UTP, whereas P2Y6 exhibits a preference for UDP binding and P2Y14 is activated upon UDP-glucose binding. With respect to cell signalling, P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y13 and P2Y14 can all promote phosphoinositide hydrolysis resulting in increased inositol-1,4,5-trisphosphate (IP3) generation [40 and references therein], which leads to an elevation of cytoplasmic free Ca++ levels, and perhaps diacylglycerol production, which can activate various PKC isoforms. Furthermore, several P2Y receptors have been implicated in the regulation of cAMP formation, i.e., P2Y1 and P2Y12 have been reported to attenuate cAMP levels, and P2Y12-14 have been linked to a Gi protein-like activity. Conversely, P2Y11 activation has been associated with elevations in cAMP-dependent pathways via the action of a heterotrimeric Gs protein complex; whereas P2Y13 has been reported to have biphasic effects on cAMP levels [40 and references therein]. Interestingly, several recent reports have suggested that ligand binding to P2Y1, P2Y2 and P2Y12 can also result in the stimulation of pathways dependent on the activation of the small MW G-protein Rac, including activation of PAK-1 and the p38 MAP kinase [53–58]. Given the importance of Ca++ fluxes and the activation of PAK-1, p38 MAP kinase and various PKC isoforms in the regulation of NADPH oxidase, it is not surprising that various P2Y agonists have been implicated in ROS generation (see below).
In terms of cell signalling via P2X receptors, there is considerable evidence indicating that these receptors act as ATP-gated ion channels that can dramatically elevate intracellular free cytoplasmic Ca++ levels [38–40, 50, 51]. Furthermore, the P2X7 receptor has been shown by several groups to promote protein complex formation, cytoskeletal reorganization and membrane blebbing in macrophages and other cell types via the regulation of Rho and the activation of p38 MAP kinase [59–62]. In addition to inducing cytoskeletal changes, MAP kinase stimulation (p38, Jun kinases (JNKs) and ERKs 1/2) and alterations in ion fluxes (Ca++ and Na+ influx, K+ efflux), several reports have appeared demonstrating that ligand binding to P2X7 receptor can also stimulate IκBα degradation and the activation of the transcription factor NF-κB [63–66]. Therefore, analogous to the P2Y receptors, P2X-mediated elevations in intracellular Ca++ levels and stimulation of p38 MAP kinase may contribute to extracellular nucleotide-induced NADPH oxidase assembly and activation (see Table 1), whereas the capacity of P2X7 stimulation to enhance NF-κB activation is a likely contributing factor to the capacity of this receptor system to potentiate LPS-induced iNOS expression and NO production [39, 65, 66] (see Table 2).
Table 1.
Summary of extracellular nucleotide regulation of ROS production by immune and tumor cells
| Cell type | Treatments* | Nucleotide effect on ROS production* | Receptors proposed | Method for measuring ROS production | References |
|---|---|---|---|---|---|
| Human neutrophils | fMLP + ATP, ADP, or AMP | ATP or ADP: ↑ | Not discussed | SOD-sensitive reduction of ferricytochrome c | [68] |
| AMP: ↑ | |||||
| Immune complex + ATP, ADP, or AMP | ATP, ADP, or AMP: ↑ | ||||
| Rat peritoneal neutrophils | fMLP + ATP, ADP, or AMP | ATP or ADP: ↑ | Not discussed | Ferrithiocyanate formation ± ferricytochrome c ± SOD | [69] |
| Immune complex + ATP, ADP, or AMP | ATP, ADP, or AMP: ↑ | ||||
| Human or rat blood neutrophils | fMLP or immune complex + ATP | ATP: ↑ | |||
| Human neutrophils | fMLP + ATP, UTP, or ITP | ATP, UTP, or ITP: ↑ | None identified | Ferricytochrome c reduction assay | [70] |
| Human HL 60 cells (promyelocytic leukemia cells) | ATP, ADP, dATP, UTP, dUTP, UDP, ITP, CTP, or TTP | ATP or UTP: ↑ | P2 receptors | SOD-sensitive reduction of ferricytochrome c | [72] |
| Human neutrophils | ATP | ATP: ↑ | Not discussed | Ferricytochrome c reduction assay | [71] |
| Rat alveolar macrophages | ATP, ADP, AMP, or ATPγS | ATP, ADP, or ATPγS: ↑ | 2 or more P2 receptor classes | SOD-sensitive reduction of ferricytochrome c | [74] |
| Human eosinophils | ATP, ATPγS, 2-MeS-ATP, UTP, GTP, ADP, BzATP, or CTP | ATP, ATPγS, 2-MeS-ATP, UTP, GTP, or BzATP: ↑ | P2Y and P2X receptors | Lucigenin-dependent chemi-luminescence | [75, 76] |
| oATP or KN62: ↑ | |||||
| Human neutrophils and promyelocytes (HL-60 cells) | BzATP | BzATP: ↑ | P2X7 | DCFDA fluorescence (intracellular ROS) | [73] |
| Human DU145 cells (prostate cancer) | ATP, ADP, UTP, or 2-MeS-ATP | ATP, ADP, UTP, or 2-MeS-ATP: ↑ | P2 receptors | DCFDA fluorescence (intracellular ROS) | [21] |
| Rat mesangial cells | BzATP | BzATP: ↑ | P2X7 | DCFDA fluorescence (intracellular ROS) | [77] |
| Rat microglia | BzATP or ATP | BzATP or ATP: ↑ | P2X7 | SOD-sensitive reduction of ferricytochrome c; tetrazolium dye reduction; translocation of p67phox (NADPH oxidase subunit) | [78] |
| Human ARO cells (thyroid cancer) | ATP | ATP: ↑ | P2Y | DCFDA fluorescence (intracellular ROS) | [20] |
| Murine RAW 264.7 macrophages | BzATP, ATP, UTP, or αβ-Methylene-ATP | BzATP or ATP: ↑ | P2X7 | DCFDA fluorescence (intracellular ROS) | [79] |
* The upward arrow (↑ indicates that the specified treatment enhanced the indicated parameter (ROS production), whereas the downward arrow (↑ designates that the specified treatment or inhibitor (e.g., oATP or KN-62) attenuated ROS production. SOD superoxide dismutase, DCFDA the intracellular ROS-reactive indicator dye- 2–7–dichlorodihydrofluorescein diacetate.
Table 2.
Summary of extracellular nucleotide regulation of iNOS expression and NO production by immune cells
| LPS-stimulated event and system studied | Nucleotide and effect* | References |
|---|---|---|
| Nitric oxide (NO) production | ||
| LPS+ IFNγ-treated murine (CD-1) peritoneal macrophages | 2-MeS-ATP: ↑ | [83] |
| LPS-treated RAW 264.7 murine macrophages | oATP: ↑ | [65, 87] |
| LPS-treated RAW 264.7 murine macrophages | ADP: ↑ | [85] |
| LPS-treated RAW 264.7 murine macrophages | ATP: ↑ | [86] |
| LPS-treated RAW 264.7 murine macrophages | ATP or BzATP: ↑ | [66] |
| LPS-treated rat astrocytes | ATP, ADP, AMP, UTP, BzATP, or 2-MeS-ATP: ↑ | [88] |
| oATP: ↑ | ||
| IFNγ-treated BV-2 murine microglia cells | ATP, ADP, BzATP, or 2-MeS-ATP: ↑ | [89] |
| IL-1β/IFNγ-treated human astrocytes | BzATP: ↑ | [90] |
| iNOS expression | ||
| LPS-treated RAW 264.7 murine macrophages | ATP: ↑ | [84] |
| LPS+ IFNγ-treated murine (CD-1) peritoneal macrophages | 2-MeS-ATP: ↑ | [83] |
| LPS-treated RAW 264.7 murine macrophages | PPADS: ↑oATP: ↑ | [87] |
| LPS-treated RAW 264.7 murine macrophages | BzATP: ↑ | [65, 66] |
| LPS-treated RAW 264.7 murine macrophages | ATP: ↑ | [88] |
| IFNγ-treated murine BV-2 microglia cells | BzATP or ATP: ↑ | [89] |
* The upward arrow (↑ indicates that the specified nucleotide enhanced the indicated parameter (NO production or iNOS expression), whereas the downward arrow (↑ designates that the specified nucleotide or inhibitor attenuated the indicated parameter.
Stimulation of O−2 production by extracellular nucleotides
Extracellular nucleotides and their receptors have an increasingly appreciated role as mediators of inflammatory responses [38–40] and have been implicated in modulating both NO production and the respiratory burst by immune cells, which is the production of O−2, H2O2 and other reactive oxygen intermediates that can contribute to the killing of microorganisms [3–6, 67]. In this regard, various P2 purinergic receptor classes are expressed in immune cells such as macrophages, neutrophils, eosinophils, micorglia, and mesangial cells and, as shown in Table 1, several of these receptor classes have been implicated in the generation of ROS [20, 21, 38–40, 68–79].
As shown in Table 1, one of the earliest reports suggesting that extracellular adenine nucleotides can regulate ROS production came from studies by Ward et al. [68], which revealed that the platelet-induced production of O−2 by human neutrophils appeared to be mediated by an extracellular adenine nucleotide. In these studies, Ward et al. [68] observed that the presence of ATP or ADP enhanced chemoattractant- (formyl-met-leu-phe, fMLP) mediated O−2 production from human neutrophils; whereas AMP and adenosine attenuated fMLP-stimulated O−2 production. Interestingly, when immune complex-stimulated human neutrophils were treated with either ATP, ADP, AMP, or adenosine, the cells exhibited increased O−2 production. Subsequent studies by these investigators using both human and rat neutrophils provided further support that both hydrolyzable and non-hydrolyzable adenine nucleotides potentiated O−2 generation [69]. In these studies by Ward et al. [68, 69], as well as those of Kuhns et al. [70] and Kuroki and Minankami [71], a common observation was that adenine nucleotides stimulated a transient Ca++ flux in neutrophils, but that this was insufficient to promote O−2 production when the cells were treated with these agents alone, i.e., cell exposure to a priming agent such as fMLP, immune complexes or cytochalasin B was necessary in order to detect nucleotide-potentiated O−2 production. Furthermore, when the nucleotide specificity of these effects was analyzed, it was reported for rat neutrophils that only adenine nucleotides enhanced O−2 generation [69]; whereas with human neutrophils, it was observed that ATP, UTP, or ITP all potentiated O−2 generation in primed cells [70]. Similarly, work by Seifert et al. [72] showed that ATP and UTP augmented ROS production in fMLP-primed human neutrophils as well as human promyelocytic HL60 leukemia cells. Although the precise nucleotide receptors involved in these actions were not defined in any of these early studies, the pharmacological data indicating that di- and tri- nucleotides could promote ROS formation are consistent with the involvement of one or more P2 receptors.
More recent investigations by Suh et al. [73] on the nucleotide regulation of O−2 production by human neutrophils and promyelocytic cells revealed that cell treatment with the P2X7 agonist 2–and 3–O-(4-benzoyl) benzoyl-ATP (BzATP) potently promoted sustained Ca++ currents and O−2 production, supporting a role for the nucleotide receptor P2X7 in this process. In these studies, the cells did not appear to require priming by fMLP or other agents for BzATP-induced ROS generation, suggesting that the sustained Ca++ currents and/or other events initiated by BzATP are sufficient for NADPH oxidase priming and activation. The different Ca++ currents induced by BzATP/P2X7 compared to that stimulated by the nucleotides used in earlier studies (ATP and UTP) may account, at least in part, for the differences in nucleotide priming requirements for O−2 production by neutrophils and promyelocytic HL60 cells.
In addition to the influence of extracellular nucleotides on neutrophil-mediated ROS production, other studies have revealed that purinergic receptor ligands can promote ROS generation by other immune cells, including human eosinophils, mouse RAW 264.7 macrophages, as well as rat alveolar macrophages, mesangial cells, and microglia (see Table 1). In the case of rat alveolar macrophages, Murphy et al. [74] found that the addition of ATP, ADP, and ATPγS directly stimulated O−2 generation by these cells; whereas the addition of adenosine and AMP did not promote this activity. These authors determined that ADP exhibited a potency that was greater-than-or-equal-to that of ATPγS, and both were more potent than ATP. Interestingly, the co-addition of optimal concentrations of ADP and ATP yielded an additive effect, thus prompting these investigators to propose the involvement of at least two P2 type receptors. These authors also found that the addition of the nucleotides resulted in sustained Ca++ fluxes and that removal of extracellular Ca++ eliminated the sustained elevation in intracellular Ca++ and greatly attenuated O−2 production. These studies provided additional support for the concept that adenine nucleotides stimulate a Ca++-dependent respiratory burst.
Additional studies characterizing the relationship between nucleotide-stimulated Ca++ fluxes and ROS production focused on the human eosinophil. In the studies by Dichman et al. [75] and Ferrari et al. [76], many nucleotides were found to promote Ca++ mobilization and ROS production, including ATP, 2-methylthio-ATP (2-MeS-ATP), ATPγS, BzATP, and UTP. These authors went on to demonstrate that the P2X7 inhibitors periodate oxidized-adenosine 5–triphosphate (oATP) and KN62 attenuated ATP- and BzATP-stimulated ROS production, suggesting that P2X7 was at least one of the receptor subtypes promoting ROS production in eosinophils. Furthermore, the chelation of extracellular Ca++ was found to attenuate BzATP stimulated ROS production but had little effect on UTP stimulated ROS production. Because BzATP is known to mobilize extracellular Ca++ via the P2X7 receptor, whereas UTP is known to mobilize Ca++ from intracellular stores via P2Y2 and P2Y4 receptors, these data again support the earlier concept that at least two different P2 receptor subtypes can be involved in the process of ROS generation, specifically a purinoceptor (e.g., P2X7) and a pyrimidinoceptor (e.g., P2Y2 or P2Y4).
More recent studies examining nucleotide specificity in mediating ROS production have utilized mesangial, microglial, and murine macrophage-like RAW 264.7 cells, and have also implicated P2X7 in this process. In a study by Harada et al. [77], it was observed that the P2X7 agonist, BzATP promoted ROS generation in a manner that was concentration-dependent and characteristic of NADPH oxidase involvement. Furthermore, these studies also demonstrated that BzATP could induce the production of ONOO-. These results are consistent with the idea that P2X7 promotes the production of O−2 and NO (see below). In the study by Parvathenani et al. [78], it was also observed that primary rat microglia stimulated with either ATP or BzATP released large amounts of O−2. In addition, these authors reported that antagonists of p38 MAP kinase or phosphatidylinositol 3-kinase attenuated O−2 production, which is consistent with the known capacity of p38 MAP kinase and 3-phosphoinositides to enhance NADPH oxidase assembly and activation [1, 3–6].
Analogous to the aforementioned studies, recent studies from our laboratory support the idea that the P2X7 receptor is involved in ROS generation by murine RAW 264.7 macrophages and demonstrate that this process is augmented by priming the cells with bacterial LPS [79]. In these studies, treatment of murine RAW 264.7 macrophages with 250 μM BzATP or 3 mM ATP for 30 min resulted in a −?- to 4-fold increase in intracellular ROS production as measured by the fluorescence of the indicator dye 2–7–dichlorodihydrofluorescein diacetate (DCFDA). Moreover, only high ATP doses (3 mM) stimulated detectable ROS production; whereas low ATP doses (250 μM) did not. This pharmacology is consistent with that of P2X7-mediated responses. To assess whether macrophage exposure to bacterial products could prime nucleotide-mediated ROS production, cells were treated with 1 μg/ml LPS for 18 hr and then treated with either buffer, 250 μM BzATP or 3 mM ATP, for 30 min. In these studies, LPS priming alone caused approximately a 3-fold increase in ROS production; whereas the treatment of LPS-primed cells with 250 μM BzATP or 3 mM ATP for 30 min resulted in an additional −?-fold increase in DCFDA fluorescence above that detected following incubation with either LPS or nucleotide alone [79]. These data suggest that oxidative stress is further increased in LPS-primed macrophages following stimulation of P2X7. In this regard, LPS has been shown to promote P2X7 function [80], which may account for the enhanced macrophage responsiveness to P2X7 agonists. In addition, it is possible that LPS potentiation of P2X7-induced events are mediated by the induction and/or activation of enzymes or phox proteins (e.g., gp91phox) involved in ROS production [3, 27].
Besides nucleotide regulation of O−2 production by immune cells, several reports have appeared describing the capacity of extracellular nucleotides to promote O−2 generation by certain tumor cells (see Table 1). For example, Sauer et al. [21] showed that the activation of the protein kinase p90Rsk and the enhanced growth of multicellular human tumor spheroids (DU145 prostate cancer cells) was dependent on ROS generated following purinergic receptor stimulation by ATP. In these studies, it was observed that the generation of ROS was associated with ATP-induced intracellular Ca++ fluxes. In addition, exogenous ATP was found to activate p90Rsk and the ERK1/2 MAP kinases. Interestingly, the radical scavengers vitamin E, dimethyl thiourea, and N-acetyl-cysteine did not inhibit ATP-stimulated ERK 1/2 activation but attenuated p90Rsk activation, suggesting that ROS production may be involved in mediating extracellular ATP-dependent p90Rsk activation. Similarly, Pines et al. [20] also observed that extracellular ATP stimulated ROS generation in human ARO (thyroid cancer) cells, and that this process augmented the translocation and activation of the transcription factor apurinic apyrimidinic endonuclease redox effector factor-1 (APE1/Ref-1).
Nitric oxide production and iNOS expression
Although extracellular nucleotides alone have been shown to be capable of inducing O−2 generation in some cell types such as macrophages, changes in iNOS expression and NO production have not been shown to be strongly affected by cell exposure to nucleotides alone [39, 65, 66]. However, several reports have appeared regarding the capacity of extracellular nucleotides to modulate iNOS expression and NO production in LPS-primed macrophages (see Table 2). In support of the concept that LPS and nucleotide receptors cooperate to control macrophage activation, previous studies have found that extracellular adenine nucleotides can influence multiple LPS effects. For example, previous studies have indicated that a macrophage membrane-associated, LPS-stimulated GTPase activity was potentiated by ATP, ADP, and ATPγS [81] but not by 2-MeS-ATP. Subsequent investigations revealed that co-administration of 2-MeS-ATP to mice reduced LPS-stimulated increases in serum levels of TNF-α and IL-1α and protected the animals from endotoxic death [82]. Moreover, studies by Denlinger et al. [83] revealed that 2-MeS-ATP attenuated LPS-stimulated NO release from elicited murine peritoneal macrophages. Conversely, studies from Tonnetti et al. [84] revealed that cotreatment of RAW 264.7 macrophages with ATP enhanced LPS-stimulated TNF-α mRNA and NO production. Similarly, studies from Denlinger et al. [85], Sperlagh et al. [86], and Aga et al. [66] observed that ADP, ATP, or BzATP potentiated NO release from RAW 264.7 macrophages stimulated by toxic species of LPS. These investigators proposed that the nucleotide receptor P2X7 is critical for influencing LPS signalling, including the modulation of iNOS expression and NO release. Furthermore, because LPS-primed RAW 264.7 cells also have increased O−2 production (see Table 1), it is likely that P2X7-dependent NO production by these cells would contribute to increased ONOO- formation and thus enhanced antimicrobial activity.
Additional evidence suggesting that P2 receptors are involved in LPS-mediated macrophage activation comes from the work of Hu et al. [87] wherein it was shown that pretreatment of RAW 264.7 macrophages with P2 receptor antagonists, oATP or pyridoxal-phosphate-6-azophenyl-2–4–disulfonic acid (PPADS), inhibited LPS-stimulated NO production and/or iNOS expression (see Table 2) and attenuated LPS activation of NF-κB and ERK-1/2. In addition, besides modulating macrophage NO production, extracellular nucleotides have also been reported to influence iNOS expression and NO production in rat and human astrocytes treated with either LPS or IL-1 (see Table 2). For example, Murakami et al. [88] found that stimulating LPS-primed rat astrocytes with either ATP, ADP, AMP, UTP, BzATP, or 2-MeS-ATP augmented NO generation and shifted the LPS dose response curve by approximately one log unit to the left. In addition the P2 antagonist, oATP, was observed to block this effect. These investigators also demonstrated that the magnitude of LPS-induced expression of iNOS by these cells was increased several-fold following the addition of ATP.
Besides the capacity of extracellular nucleotides to enhance LPS-induced iNOS expression or NO production by immune cells, several reports have appeared demonstrating that nucleotide receptor action is likely important for potentiating the action of other cytokines that are known to regulate NO generation. For example, Gendron et al. [89] observed that extracellular nucleotides augmented IFNγ-induced NO release from murine BV-2 microglial cells, but that extracellular nucleotides alone were without effect. These investigators also found that BzATP, and to a lesser degree ATP, enhanced IFN-γ-induced iNOS expression, and that ATP, ADP, BzATP, and 2-MeS-ATP, but not UTP, could potentiate IFNγ-induced NO production, suggesting that the uridine nucleotide receptors P2Y2 and P2Y6 are not involved in this response. However, the broadly-reactive P2X7 receptor antagonist oATP, and suramin, a non-selective P2 receptor antagonist, attenuated the effect of ATP or BzATP on IFNγ-induced NO production, and the authors noted that this outcome is consistent with an involvement of the P2X7 receptor. Similarly, Narcisse et al. [90] reported that BzATP stimulation of IL-1β/IFNγ-treated human astrocytes resulted in enhanced iNOS expression and NO production, and these authors also proposed a role for P2X7 in this process.
In sum, P2 receptors have been implicated in controlling the action of LPS and other cytokines with respect to the induction of iNOS and the production of NO by immune cells. Although many studies support a role for P2X7 in this process, the observation that P2Y receptor ligands (e.g., UTP, ADP, and 2-MeS-ATP) can also modulate LPS-induced signalling and NO/ROS production, together with the fact that NO production by Mycobacterium tuberculosis infection in P2X7 knockout mice is inhibited by P2 blockers, supports the idea that multiple P2X and P2Y receptors participate in NO generation and LPS/cytokine-initiated immune responses [39, 82, 91].
Postulated mechanisms of nucleotide regulation of ROS production
Although extracellular nucleotides have been shown to regulate O−2 production and LPS/cytokine-induced NO generation, the exact molecular mechanisms by which these processes occur are currently unclear. However, based on known nucleotide receptor signalling events, together with the well recognized events that control iNOS expression and NADPH oxidase assembly and activation, there are several plausible mechanisms that can be proposed and these will be discussed below.
NADPH oxidase
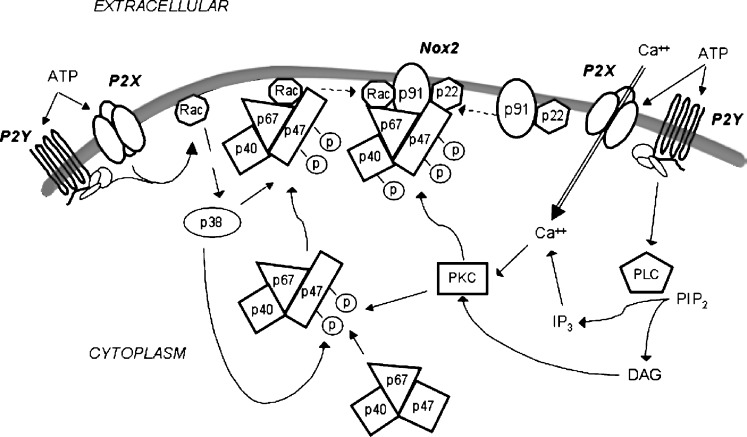
As noted above, NADPH oxidase activity can be regulated by various signalling events such as Ca++ fluxes, as well as activation of various isoforms of PKC, p38, and Rac. Interestingly, there is evidence for both P2X and P2Y receptor mediated activation of NADPH oxidase activity (see Table 1 and Fig. 1). In the case of the P2X receptors, such as P2X7, there is clear evidence that the activation of these receptors allow for the mobilization of extracellular Ca++, and it has been shown that chelation of extracellular Ca++ can block ROS generation by P2X agonists. It is likely that increases in intracellular Ca++ induced by P2X agonists results in the activation of certain kinases, such as PKC isoforms, that are essential for the phosphorylation/activation of NADPH oxidase subunits such as p47phox. Furthermore, activation of P2X7 has been linked to the stimulation of p38 MAP kinase [66], which would also be expected to facilitate the phosphorylation/activation of NADPH oxidase subunits. In the case of P2Y receptors, these receptors are largely linked to the activation of phospholipase C with the subsequent generation of IP3 and diacylglycerol (DAG), which would lead to the elevation of cytoplasmic free Ca++ and the activation of PKC isoforms, respectively. These events would also be expected to promote the phosphorylation/activation of NADPH oxidase subunits. Furthermore, certain P2Y receptors, such as P2Y2, have also been reported to lead to the activation of the small MW G-protein Rac, which in turn would also be predicted to facilitate the phosphorylation, activation and assembly of NADPH oxidase as discussed in the preceding sections.
Fig. 1.
Working model for the involvement of P2X and P2Y receptors in regulating the phagocytic NADPH oxidase complex. In the resting state, a small portion of gp91phox (Nox2) and p22phox are located at the plasma membrane (although priming with factors such as LPS or chemoattractants can recruit additional gp91phox and p22phox to the membrane and promote the assembly of the NADPH complex (see text)). The oxidase function of the gp91phox/p22phox is dormant until it is complexed with the cytosolic components p67phox, p47phox, p40phox, and Rac1/2. Stimulus-induced phosphorylation of p47phox results in a conformational change that allows it to be recruited to the membrane and additional phosphorylation of the p47phox/p40phox/p67phox complex allows for its structural reorganization and assembly with gp91phox/p22phox at the membrane. Also, stimulus-induced recruitment of activated (GTP-loaded) Rac2 to the membrane facilitates the assembly of the functional NADPH oxidase complex. With respect to phox protein phosphorylation, several kinases are postulated to be important, including PKC isoforms and p38 MAP kinase. Also, Ca++ fluxes can promote NADPH oxidase assembly, in part via Ca++-dependent activation of kinases (e.g., PKC isoforms). Moreover, Rac activation may also contribute to phox protein phosphorylation via initiating p38 MAP kinase activation. Final assembly/activation of the NADPH complex requires additional phosphorylation steps. In terms of P2X and P2Y receptor-associated activation of NADPH oxidase activity, it is hypothesized that increases in intracellular Ca++ induced by P2X agonists result in the activation of protein kinases, such as PKC isoforms, that are essential for the phosphorylation/activation of NADPH oxidase subunits including p47phox. Furthermore, activation of P2X7 has been linked to the stimulation of p38 MAP kinase, which would also be expected to facilitate the phosphorylation/activation of NADPH oxidase subunits. In the case of P2Y receptors, many of these receptors can regulate certain phospholipase C (PLC) isoforms with the subsequent conversion of phosphoinositide-4,5-bisphosphate (PIP2) to IP3 and diacylglycerol (DAG), which in turn would lead to the elevation of cytoplasmic free Ca++ and the activation of PKC isoforms, respectively. These events would also be expected to promote the phosphorylation/activation of NADPH oxidase subunits. Furthermore, certain P2Y receptors have also been reported to lead to Rac activation, which in turn would be predicted to facilitate the phosphorylation, activation and assembly of NADPH oxidase
iNOS expression
As noted earlier, iNOS expression is largely induced by the action of the transcription factors NF-κB, IRF-1, and STAT-1 [30–34]. Although extracellular nucleotides alone have not been shown to promote iNOS expression, they have been shown to potentiate the capacity of LPS, IL-1 or IFNγ to induce iNOS expression in macrophages, astrocytes and microglia (see Table 2). In this regard, the cell signalling events associated with the co-presentation of nucleotides and LPS/cytokines in NO generation and iNOS expression remain to be clearly elucidated [26, 32, 54]. However, current evidence supports a role for nucleotide receptor-mediated activation of NF-κB and certain MAP kinases such as p38 MAP kinase. For example, P2X7 activation has been associated with increased degradation of the NF-κB inhibitor IκBα and enhanced NF-κB DNA binding activity [63–66, 87]. Similarly, activation of MAP kinases such as p38 can trigger the nuclear accumulation and activity of various transcription factors, including NF-κB, NFAT, ATF2, Ets, and c-Jun [66, 92–95]. In fact, recent data suggest that the p38 MAP kinase is critical for LPS-stimulated iNOS expression, NO production, and the activation of NF-κB DNA-binding activity in macrophages [96, 97]. Although this increased activation of NF-κB in response to extracellular nucleotides would be expected to enhance iNOS expression in the presence of factors that also promote IRF-1 and STAT-1 activation (e.g., IFNγ/LPS), this nucleotide-induced NF-κB response in the absence of IRF-1 and STAT-1 activation is likely to be insufficient for iNOS induction and would thus explain why nucleotides alone do not promote iNOS expression and NO generation.
Conclusion
The current observations suggest that multiple P2 receptors can contribute to ROS production in many cell types, and that this process is likely important for a diverse array of inflammatory and anti-microbial activities. These findings expand the range of biological processes that appear critically regulated by extracellular nucleotide-mediated events.
Footnotes
This work was supported by National Institutes of Health Grants HL56396 and AI50500.
The first author was supported by the Hematology Training Program NIH 5 T32 HL07899 at the University of Wisconsin.
References
- 1.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J-M, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 3.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 4.DeCoursey TE, Ligeti E. Regulation and termination of NADPH oxidase activity. Cell Mol Life Sci. 2005;62:2173–2193. doi: 10.1007/s00018-005-5177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheppard FR, Kelher MR, Moore EE, et al. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 9.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/S0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 11.Roos D, Winterbourn CC. Immunology. Lethal weapons. Science. 2002;296:669–671. doi: 10.1126/science.1071271. [DOI] [PubMed] [Google Scholar]
- 12.Kaul N, Forman HJ. Activation of NF-kappa B by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 13.Guyton KZ, Liu Y, Gorospe M, et al. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.7.3604. [DOI] [PubMed] [Google Scholar]
- 14.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.36.21906. [DOI] [PubMed] [Google Scholar]
- 15.Lander HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 16.Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- 17.Forman HJ, Torres M. Signaling by the respiratory burst in macrophages. IUBMB Life. 2001;51:365–371. doi: 10.1080/152165401753366122. [DOI] [PubMed] [Google Scholar]
- 18.Iles KE, Dickinson DA, Watanabe N, et al. AP-1 activation through endogenous H(2)O(2) generation by alveolar macrophages. Free Radic Biol Med. 2002;32:1304–1313. doi: 10.1016/S0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 19.Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265:49–72. doi: 10.1016/S0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.Pines A, Perrone L, Bivi N, et al. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nuc Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer H, Klimm B, Hescheler J, Wartenberg M. Activation of p90RSK and growth stimulation of multicellular tumor spheroids are dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. FASEB J. 2001;15:U256–U281. doi: 10.1096/fj.01-0360fje. [DOI] [PubMed] [Google Scholar]
- 22.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 23.Lander HM, Ogiste JS, Teng KK, Novogrodsky A. p21ras as a common signaling target of reactive free radicals and cellular redox stress. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.35.20677. [DOI] [PubMed] [Google Scholar]
- 24.Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med. 2002;33:1121–1132. doi: 10.1016/S0891-5849(02)01000-6. [DOI] [PubMed] [Google Scholar]
- 25.Zaki MH, Akuta T, Akaike T. Nitric oxide-induced nitrative stress involved in microbial pathogenesis. J Pharmacol Sci. 2005;98:117–129. doi: 10.1254/jphs.CRJ05004X. [DOI] [PubMed] [Google Scholar]
- 26.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 27.Cassatella MA, Bazzoni F, Flynn RM, et al. Molecular basis of interferon-γ and lipopoly-saccharide enhancement of phagocyte respiratory burst capability: Studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990;265:20241–20246. [PubMed] [Google Scholar]
- 28.Zahradnikova A, Minarovic I, Venema RC, et al. Inactivation of the cardiac ryanodine receptor calcium release channel by nitric oxide. Cell Calcium. 1997;22:447–453. doi: 10.1016/S0143-4160(97)90072-5. [DOI] [PubMed] [Google Scholar]
- 29.Kroncke KD. Nitrosative stress and transcription. Biol Chem. 2003;384:1365–1377. doi: 10.1515/BC.2003.153. [DOI] [PubMed] [Google Scholar]
- 30.Kamijo R, Harada H, Matsuyama T, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 31.Martin E, Nathan C, Xie QW. Role of interferon regulatory factor-1 in induction of nitric oxide synthase. J Exp Med. 1994;180:977–984. doi: 10.1084/jem.180.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao JJ, Morrison DC, Parmely TJ, et al. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 33.Ganster RW, Taylor BS, Shao LF, et al. Complex regulation of human inducible nitric oxide synthase gene transcription by STAT 1 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:8638–8643. doi: 10.1073/pnas.151239498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Stefano D, Maiuri MC, Iovine B, et al. The role of NF-kappa B, IRF-1, and STAT-1 alpha transcription factors in the iNOS gene induction by gliadin and IFN-gamma in RAW 264.7 macrophages. J Mol Med-JMM. 2006;84:65–74. doi: 10.1007/s00109-005-0713-x. [DOI] [PubMed] [Google Scholar]
- 35.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- 38.Di Virgilio F, Chiozzi P, Ferrari D, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 39.Watters JJ, Sommer JA, Fisette PL, et al. P2X7 nucleotide receptor: modulation of LPS-induced macrophage signaling and mediator production. Drug Develop Res. 2001;53:91–104. doi: 10.1002/ddr.1176. [DOI] [Google Scholar]
- 40.Di Virgilio F, Baricordi OR, Romagnoli R, Baraldi PG. Leukocyte P2 receptors: a novel target for anti-inflammatory and antitumor therapy. Curr Drug Targets. 2005;5:85–99. doi: 10.2174/1568006053004967. [DOI] [PubMed] [Google Scholar]
- 41.Dubyak GR. Purinergic signaling at immunological synapses. J Auton Nerv Syst. 2000;81:64–68. doi: 10.1016/S0165-1838(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 42.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 43.Grahames CB, Michel AD, Chessell IP, Humphrey PP. Pharmacological characterization of ATP- and LPS-induced IL-1 release in human monocytes. Br J Pharmacol. 1999;127:1915–1921. doi: 10.1038/sj.bjp.0702732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffiths RJ, Stam EJ, Downs JT, Otterness IG. ATP induces the release of IL-1 from LPS-primed cells in vivo. J Immunol. 1995;54:2821–2828. [PubMed] [Google Scholar]
- 45.Ferrari D, Chiozzi P, Falzoni S, et al. Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 46.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1 and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 47.Solle M, Labasi J, Perregaux DG, et al. Altered cytokine production in mice lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 48.Watters JJ, Sommer JA, Pfeiffer ZA, et al. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1 production. J Biol Chem. 2002;277:9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- 49.Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002;397:131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- 50.Khakh BS, Burnstock G, Kennedy C, et al. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 51.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 52.Denlinger LC, Fisette PL, Sommer JA, et al. Cutting edge: the nucleotide receptor P2X7 contains multiple protein- and lipid-interaction motifs including a potential binding site for bacterial lipopolysaccharide. J Immunol. 2001;167:1871–1876. doi: 10.4049/jimmunol.167.4.1871. [DOI] [PubMed] [Google Scholar]
- 53.Bagchi S, Liao ZJ, Gonzalez FA, et al. The P2Y2 nucleotide receptor interacts with alpha(v) integrins to activate G(o) and induce cell migration. J Biol Chem. 2005;280:39050–39057. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- 54.Soulet C, Hechler B, Gratacap MP, et al. A differential role of the platelet ADP receptors P2Y(1) and P2Y(12) in Rac activation. J Thromb Haemostasis. 2005;3:2296–2306. doi: 10.1111/j.1538-7836.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- 55.Neary JT, Lenz G, Kang Y, et al. Role of mitogen-activated protein kinase cascades in P2Y receptor-mediated trophic activation of astroglial cells. Drug Develop Res. 2001;53:158–165. doi: 10.1002/ddr.1183. [DOI] [Google Scholar]
- 56.Berenbaum F, Humbert L, Bereziat G, et al. Concomitant recruitment of ERK1/2 and p38 MAPK signalling pathway is required for activation of cytoplasmic phospholipase A2 via ATP in articular chondrocytes. J Biol Chem. 2003;278:13680–13687. doi: 10.1074/jbc.M211570200. [DOI] [PubMed] [Google Scholar]
- 57.Chen L, He HY, Li HM, et al. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Lett. 2004;215:239–247. doi: 10.1016/j.canlet.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez FA, Weisman GA, Erb L, et al. Mechanisms for inhibition of P2 receptors signaling in neural cells. Mol Neurobiol. 2005;31:65–79. doi: 10.1385/MN:31:1-3:065. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Jiang LH, Wilson HL, et al. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verhoef PA, Estacion M, Schilling W, Dubyak GR. P2X7 receptor-dependent blebbing and the activation of Rho-effector kinases, caspases, and IL-1 release. J Immunol. 2003;170:5728–5738. doi: 10.4049/jimmunol.170.11.5728. [DOI] [PubMed] [Google Scholar]
- 61.Morelli A, Chiozzi P, Chiesa A, et al. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeiffer ZA, Aga M, Prabhu U, et al. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol. 2004;75:1173–1182. doi: 10.1189/jlb.1203648. [DOI] [PubMed] [Google Scholar]
- 63.Aga M, Johnson CJ, Hart AP, et al. Modulation of monocyte signaling and pore formation in response to agonists of the nucleotide receptor P2X7. J Leukoc Biol. 2002;72:222–232. [PubMed] [Google Scholar]
- 64.Budagian V, Bulanova E, Brovko L, et al. Signaling through P2X7 receptor in human T cells involves p56(lck), kinases, and transcription factors AP-1 and NF-kappa B. J Biol Chem. 2003;278:1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- 65.Guerra AN, Fisette PL, Pfeiffer ZA, et al. Purinergic receptor regulation of LPS-induced signaling and pathophysiology. J Endotoxin Res. 2003;9:256–263. doi: 10.1179/096805103225001468. [DOI] [PubMed] [Google Scholar]
- 66.Aga M, Watters JJ, Pfeiffer ZA, et al. Evidence for nucleotide receptor modulation of cross talk between MAP kinase and NF-kappa B signaling pathways in murine RAW 264.7 macrophages. Am J Physiol. 2004;286:C923–C930. doi: 10.1152/ajpcell.00417.2003. [DOI] [PubMed] [Google Scholar]
- 67.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 68.Ward PA, Cunningham TW, McCulloch KK, et al. Platelet enhancement of O2−. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Invest. 1988;58:37–47. [PubMed] [Google Scholar]
- 69.Ward PA, Cunningham TW, McCukkoch KK, et al. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest. 1988;58:438–447. [PubMed] [Google Scholar]
- 70.Kuhns DB, Wright DG, Nath J, et al. ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2− generation. Lab Invest. 1988;58:448–453. [PubMed] [Google Scholar]
- 71.Kuroki M, Minakami S. Extracellular ATP triggers superoxide production in human-neutrophils. Biochem Biophys Res Comm. 1989;162:377–380. doi: 10.1016/0006-291X(89)92007-X. [DOI] [PubMed] [Google Scholar]
- 72.Seifert R, Burde R, Schultz G. Activation of NADPH oxidase by purine and pyrimidine nucleotides involves G proteins and is potentiated by chemotactic peptides. Biochem J. 1989;259:813–819. doi: 10.1042/bj2590813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suh BC, Kim JS, Namgung U, et al. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 74.Murphy JK, Livingston FR, Gozal E, et al. Stimulation of the rat alveolar macrophage respiratory burst by extracellular adenine nucleotides. Am J Resp Cell Mol Biol. 1993;9:505–510. doi: 10.1165/ajrcmb/9.5.505. [DOI] [PubMed] [Google Scholar]
- 75.Dichmann S, Idzko M, Zimpfer U, et al. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca++ mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–978. [PubMed] [Google Scholar]
- 76.Ferrari D, Idzko M, Dichmann S, et al. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486:217–224. doi: 10.1016/S0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- 77.Harada H, Tsukimoto A, Ikari A, et al. P2X7 receptor-induced generation of reactive oxygen species in rat mesangial cells. Drug Dev Res. 2003;59:112–117. doi: 10.1002/ddr.10204. [DOI] [Google Scholar]
- 78.Parvathenani LK, Tertyshnikova S, Greco CR, et al. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- 79.Pfeiffer ZA (2003) A role for MAP kinases in the modulation of the macrophage cytoskeleton and inflammatory responses by the nucleotide receptor P2X7. PhD Thesis, University of Wisconsin-Madison, USA
- 80.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 81.Tanke T, van de Loo JW, Rhim H, et al. Bacterial lipopolysaccharide-stimulated GTPase activity in RAW 264.7 macrophage membranes. Biochem J. 1991;277:379–385. doi: 10.1042/bj2770379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Proctor RA, Denlinger LC, Leventhal PS, et al. Protection of mice from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci USA. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denlinger LC, Fisette PL, Garis KA, et al. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- 84.Tonetti M, Sturla L, Giovine M, et al. Extracellular ATP enhances messenger-RNA levels of nitric-oxide synthase and TNF-alpha in lipopolysaccharide-treated RAW-264.7 murine macrophages. Biochem Biophys Res Comm. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- 85.Denlinger LC, Garis KA, Sommer JA, et al. Nuclear translocation of NF-κB in lipopoly-saccharide-treated macrophages fails to correspond to endotoxicity: evidence suggesting a requirement for a gamma interferon-like signal. Infect Immun. 1998;66:1638–1647. doi: 10.1128/iai.66.4.1638-1647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sperlágh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in RAW 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/S0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 87.Hu Y, Fisette PL, Denlinger LC, et al. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitricoxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 88.Murakami K, Nakamura Y, Yoneda Y. Potentiation by ATP of lipopolysaccharide-stimulated nitric oxide production in cultured astrocytes. Neuroscience. 2003;117:37–42. doi: 10.1016/S0306-4522(02)00804-7. [DOI] [PubMed] [Google Scholar]
- 89.Gendron FP, Chalimoniuk M, Strosznajder J, et al. P2X7 nucleotide receptor activation enhances IFNγ-induced type II nitric oxide synthase activity in BV-2 microglial cells. J Neurochem. 2003;87:344–352. doi: 10.1046/j.1471-4159.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 90.Narcisse L, Scemes E, Zhao Y, et al. The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia. 2005;49:245–258. doi: 10.1002/glia.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sikora A, Liu J, Brosnan C, et al. Purinergic signaling regulates radical-mediate bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- 92.Bhat NR, Feinstein DL, Shen Q, Bhat AN. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells: roles of nuclear factors, nuclear factor-κB, cAMP response element-binding protein, CCAAT/enhancer-binding protein-β, and activating transcription factor-2. J Biol Chem. 2002;277:29584–29592. doi: 10.1074/jbc.M204994200. [DOI] [PubMed] [Google Scholar]
- 93.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression: the role of TATA-binding protein (TBP) J Biol Chem. 1999;274:30858–308563. doi: 10.1074/jbc.274.43.30858. [DOI] [PubMed] [Google Scholar]
- 94.Carter AB, Monick MM, Hunninghake GW. Lipopolysaccharide-induced NF-κB activation and cytokine release in human alveolar macrophages is PKC-independent and TK- and PC-PLC-dependent. Am J Respir Cell Mol Biol. 1998;18:384–391. doi: 10.1165/ajrcmb.18.3.2972. [DOI] [PubMed] [Google Scholar]
- 95.Monick MM, Carter AB, Robeff PK, et al. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of β-catenin. J Immunol. 2001;166:4713–4720. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- 96.Chen C, Chen YH, Lin WW. Involvement of p38 mitogen-activated protein kinase in lipo-polysaccharide-induced iNOS and COX-2 expression in J774 macrophages. Immunology. 1999;97:124–129. doi: 10.1046/j.1365-2567.1999.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–488. [PubMed] [Google Scholar]