Abstract
Dendritic cells (DCs) activate and shape the adaptive immune response by capturing antigens, migrating to peripheral lymphoid organs where naïve T cells reside, expressing high levels of MHC and costimulatory molecules and secreting cytokines and chemokines. DCs are endowed with a high degree of functional plasticity and their functions are tightly regulated. Besides initiating adaptive immune responses, DCs play a key role in maintaining peripheral tolerance toward self-antigens. On the basis of the information gathered from the tissue where they reside, DCs adjust their functional activity to ensure that protective immunity is favoured while unwanted or exaggerated immune responses are prevented. A wide variety of signals from neighbouring cells affecting DC functional activity have been described. Here we will discuss the complex role of extracellular nucleotides in the regulation of DC function and the role of P2 receptors as possible tools to manipulate immune responses.
Key words: antigen presentation, ATP, autoimmunity, chemokine, dendritic cells, immune deviation, immunological tolerance, inflammation, interleukin-12
Introduction
Dendritic cells (DCs) are a heterogeneous bone marrow-derived leukocyte population of specialised antigen-presenting cells functioning as initiators and regulators of T cell responses and influencing the activity of B lymphocytes and natural killer cells [1]. In humans, DC subsets include interstitial DCs found in peripheral tissues, Langerhans cells of the skin and plasmacytoid dendritic cells (pDCs) mostly present in the blood and in lymphoid organs. While interstitial DCs and Langerhans cells derive from a myeloid precursor, pDCs were initially considered of lymphoid origin. Later studies have shown that pDCs can be differentiated from either common lymphoid or common myeloid precursors in both humans and mice. For simplicity we will hereafter refer to interstitial DCs and Langerhans cells as myeloid DCs. In the mouse, at least six different DC subsets have been identified. The existence of diverse DC populations specialised in particular tasks ensures efficient induction of host defense to multiple pathogens and tumor cells as well as the preservation of self-tolerance. In addition, each DC subtype is endowed with a certain degree of functional plasticity making the same cell able to work as a promoter of inflammation or a regulator of immune responses, depending on the nature of the stimulus and on the environmental conditions in which the cell has been activated [2]. Recent studies on the function of P2 receptors on DCs have revealed that the presence of nucleotides (in particular ATP) in the extracellular milieu surrounding DCs can significantly modify DC functions.
P2 receptors
P2 receptors are a class of plasma membrane receptors expressed by virtually all cell types. Their activation elicits diverse responses depending on cell type, receptors expressed and nucleotide concentration. Two P2 receptor subfamilies have been described so far and are named P2Y and P2X. Members of the two groups differ in protein structure, pharmacology and function [3–5].
P2Y receptors are seven membrane-spanning, G-protein-coupled receptors whose activation triggers generation of inositol 1,4,5-trisphosphate and release of Ca2+ from the intracellular stores [6]. Eight P2Y subtypes have been cloned so far and are named P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 [6, 7].
Neurons, heart, skeletal muscle, platelets, liver and digestive tract express P2Y1 mRNA [6]. Stimulation of this subtype has been linked to platelet aggregation and nitric oxide (NO) release [8]. P2Y1 is potently activated by ADP. The P2Y2 receptor is expressed in skeletal muscle, heart, lung, spleen, placenta and kidney [6, 9]. Its function has been linked to ion transport in epithelia [10]. P2Y2 is activated with similar efficiency by ATP and UTP. P2Y4 is present in the intestine, lung and placenta [6]. UTP is a potent agonist at P2Y4. Expression of P2Y6 has been found in many human tissues, including spleen, thymus, placenta, intestine, lung and brain [6, 11, 12]. UDP is very active at P2Y6. The P2Y11 subtype has been found in corneal epithelia, endothelial and pancreatic duct cells, promyelocytic HL-60 cells, dendritic cells and lymphocytes; its activation is associated with increased intracellular concentration of cyclic AMP [13–16]. ATP is the preferred ligand at P2Y11 [17].
CD34+ stem cells, mast cells, vascular smooth muscle cells and platelets express the P2Y12 subtype [18–20]. It is potently activated by ADP and linked to ADP-induced shape changes in platelets [21].
P2Y13 is expressed in bone marrow, spleen, liver, brain, airway epithelial cells, red blood cells, monocytes, dendritic and T cells [18–20, 22, 23]. P2Y13 has recently been linked to the regulation of hepatic high-density lipoprotein (HDL) endocytosis [24]. Its preferred agonist is ADP [25]. The recently identified P2Y14 subtype has been found in hematopoietic cells, monocyte-derived dendritic cells and human airway epithelial cells. The P2Y14 subtype responds to UDP-glucose and related sugar nucleotides [23, 26, 27].
P2X proteins are membrane receptors that form ion channels upon activation by extracellular ATP. Ligation of the agonist induces oligomerization and formation of homo—or in some cases hetero—multimer ion selective channels, being permeable to monovalent and divalent cations [28–30]. The P2X subunit is formed by an extracellular loop, two transmembrane domains and two (amino- and carboxyl-terminal) cytoplasmic domains. Seven P2X subtypes have been cloned so far (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, and P2X7). They were originally identified in mammalian neurons and smooth muscle cells, and subsequently also found in fibroblasts, lymphocytes, macrophages, and dendritic cells [5]. All P2X subtypes are activated by ATP.
The P2X1 subtype is expressed by smooth muscle cells, megakariocytes, platelets, lymphocytes, dendritic cells, epithelial cells, ventricular myocardium, and neurons [31–34]. 2′,3′-(4-benzoyl)benzoyl-ATP (BzATP) and α,β-methylene ATP are good agonists at this subtype.
P2X2 has different functional splice variants. 2meSATP is a better agonist than ATP for this subtype. It is expressed in pancreatic cells and neurons, where along with P2X3, it is involved in nociceptive responses after nerve injury [35]. P2X3 receptor is expressed by neurons and its activation has been linked to nociceptive signaling [35, 36]; mRNA expression of this subtype has also been found in keratinocytes, and CD43+ hematopoietic cell precursors [32]. P2X4 has been found in neurons, hematopoietic cell precursors, macrophages, monocyte-derived dendritic cells and fibroblasts, keratinocytes, and placenta [9, 23, 37]. P2X5 and P2X6 mRNAs have been detected in neurons, keratinocytes and thyrocytes [38–40]. The P2X7 receptor is expressed in macrophages, microglia, dendritic cells and placenta [7, 9]. It is a non-desensitising receptor with the peculiar capacity to undergo a permeability transition from a cationic selective channel to a plasma membrane pore upon stimulation with high or pulsed ATP doses. BzATP is more potent than ATP at this subtype.
What extracellular nucleotides signal to DCs?
Nucleotides are present at relatively high amounts in the cytoplasm of cells where their concentration ranges from 1–10 mM. In the extracellular space their concentration is considerably lower ranging between 1 and 10 nM. Due to the steep concentration gradient, their small size and their high mobility in the extracellular compartment, nucleotides can be rapidly released along with other cellular components following mechanical stress, cell damage or death. Increased nucleotide concentration in the extracellular space is therefore closely associated with tissue stress or damage [41–43]. However non-lytic nucleotide release may occur in many cell types under a variety of conditions. Activated platelets represent a relevant source of ATP released concomitantly with several inflammatory mediators during clot formation [43]. ATP is released from exercising skeletal muscle as well as from vascular endothelial cells and smooth muscle cells in conditions of increased blood flow or upon mechanical stimulus [44–47]. Moreover, ATP secretion from endothelial cells and leukocytes may be induced by pathogen-associated molecules such as LPS [48, 49].
The ability of DCs to sense tissue stress is a cardinal point of the danger theory [50]. In this model, rather than be activated solely by the recognition of foreign pathogens, DCs react to the presence of environmental molecules associated with tissue stress, the so-called danger signals. Danger signals can be classified into endogenous and exogenous. Endogenous danger signals can be further subdivided into constitutive (ATP, adenosine, some heat shock proteins) and inducible (type I interferons, some other heat shock proteins). Exogenous danger signals include microbial-associated molecules recognised by Toll-like receptors expressed on a variety of cells including DCs. The ability to recognise endogenous danger signals allows the immune system to discriminate between harmless (e.g., commensal flora at mucosal surfaces) and pathogenic organisms by assessing their effect (damage) on the host. However, tissue damage might also be secondary to the intrinsic toxicity of sustained inflammation, which can ultimately be as harmful as the infection itself. In order to restore homeostasis, a timely termination of inflammatory processes is required. Extracellular ATP, in fact, has been suggested to be a signal of danger whose function is to activate DCs as well as to limit excessive inflammatory responses and promote tolerance.
Dendritic cells circulate in the bloodstream or reside in peripheral tissues where they are specialised in the uptake of potential antigens. In this life-cycle stage, DCs are considered “immature” and express chemokine receptors for inflammatory chemokines such as CXCR1, CCR1, CCR2 and CCR5 and for other inflammatory factors enabling them to migrate from the blood to inflamed tissues [51–53]. Recent work by Idzko and colleagues shows that ATP released from dying cells can be included in the list of mediators able to recruit dendritic cells. In vitro, exposure to low concentrations (100 nM) of extra-cellular ATP, through the activation of P2Y receptors, induces intracellular calcium mobilisation, actin polymerisation and chemotaxis of immature, but not mature, monocyte-derived DCs [54].
Additional evidence suggesting a role for P2Y receptors in cell trafficking comes from the observation of monocytes/macrophages from CD39-deficient mice that have impaired ability to metabolise extracellular ATP and display P2Y signaling pathway desensitisation associated with reduced chemotactic responses [55]. In addition, exposure to ATP gradients has been reported to inhibit chemokineelicited migration of monocyte-derived DCs and freshly isolated CD1a+ dermal dendritic cells but not circulating peripheral blood CD1c+ DCs or plasmacytoid DCs through a P2Y11-dependent mechanism [16]. While all four DC populations express discrete amounts of mRNA encoding P2Y11, only monocyte-derived DCs and dermal CD1a+ DCs display responsiveness to P2Y11 agonists, consistent with the susceptibility to ATP gradients.
Whether circulating DCs need some unidentified signal present in peripheral tissues (such as the skin) or in the in vitro cultures to express functional P2Y11 protein on cell membrane is unknown. These findings suggest a complex role for ATP in the regulation of DC trafficking: ATP might work as a chemoattractant for DCs toward the site of tissue damage; on the other hand ATP gradients might prolong the permanence of immature DCs at the site of the antigen encounter (Figure 1).
Figure 1 (a–c).
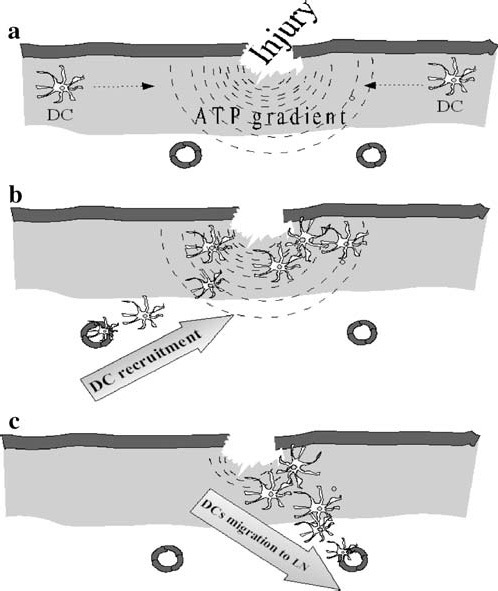
Proposed role of extracellular ATP in the regulation of DC trafficking. Due to cell death, the extracellular space surrounding sites of tissue injury is characterised by increased ATP concentration. Circulating DCs might follow the ATP gradient to traffic to perilesional area where an antigen encounter is more likely to occur (a) and where ATP might reach concentrations in the micromolar range. P2Y11 activation transiently inhibits DCs migration, prolonging their persistence at the site of antigen encounter (b). At later stages, due to the action of ecto-nucleotidases, extracellular ATP levels drop down, and the chemokines CCR7 and CXCR4 are upregulated by P2Y11 signaling. This sets DCs for efficient migration from peripheral tissues toward regional lymph nodes (c).
Dendritic cells exposed to pathogen-associated molecules engaging Toll-like receptors undergo maturation, a complex process turning immature DCs into efficient antigen-presenting cells. Additional stimuli driving DC maturation include inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) or the engagement of CD40 by CD40 ligand expressed on activated T lymphocytes.
Early after activation, DCs produce inflammatory cytokines including TNF-α, IL-1 and IL-6 and downregulate the expression of inflammatory chemokine receptors and of P2Y11 [16], while upregulating lymphoid chemokine receptors CCR7 and CXCR4 [53, 56]. This sets DCs to leave the site where activation occurred, enter into lymphatic circulation and migrate to draining lymph nodes.
Maturing DCs progressively reduce antigen uptake activity, while upregulating the expression of molecules involved in antigen presentation such as major histocompatibility complexes (MHC) I and II, the co-stimulatory molecules CD80 and CD86 providing signals 1 and 2 for T cell activation. Other surface molecules involved in the interaction with T cells, such as CD54 and CD40, and OX40 ligand are also upregulated. In addition chemokines released by DCs at early stages of maturation, such as CCL2, CCL3, CCL4, CCL5, CXCL8 and CXCL10 recruit circulating monocytes, immature DCs, T cells and neutrophils at the site of the antigen encounter [52, 53]. Migrating DCs upregulate the production of lymphoid chemokines including CCL17, CCL19 and CCL22, providing chemotactic signals for naïve T cells and mature DCs in the lymph nodes.
Besides presenting antigens and necessary co-stimulatory signals for the activation of T lymphocytes, DCs instruct T helper cells to differentiate into IFN-γ-(Th 1 phenotype) or IL-4-producing cells (Th 2). Th 1 differentiation is driven by the presence of IL-12 in the microenvironment where antigen presentation occurs [57, 58]. DCs are a major source of IL-12, which is also an important stimulatory factor for NK cells. Activated DCs migrating to the lymph node induce NK recruitment through a CXCR3-dependent mechanism [59]. DCs are a relevant source of the CXCR3 ligands CXCL9, CXCL10 and CXCL11, and both myeloid DC and pDC supernatants have been shown to induce NK cell migration in vitro [60]. NK trafficking into inflamed lymph node has been proven necessary for efficient priming of Th 1 lymphocytes in vivo [59]. In fact, IFN-γ released by NK cells at the site of antigen presentation induces STAT-1 activation, which in turn elicits the expression of the transcription factor t-bet in naïve T lymphocytes. Subsequently t-bet promotes Th 1 differentiation by driving the expression of IL-12 receptor [61]. In this context, IL-12 produced by DCs by triggering its cognate receptor on naïve T cells, induces STAT-4 activation, a key event for Th 1 differentiation. Conversely, the absence of IL-12 and signals leading to t-bet expression favours Th 2 development (Figure 2).
Figure 2 (a, b).
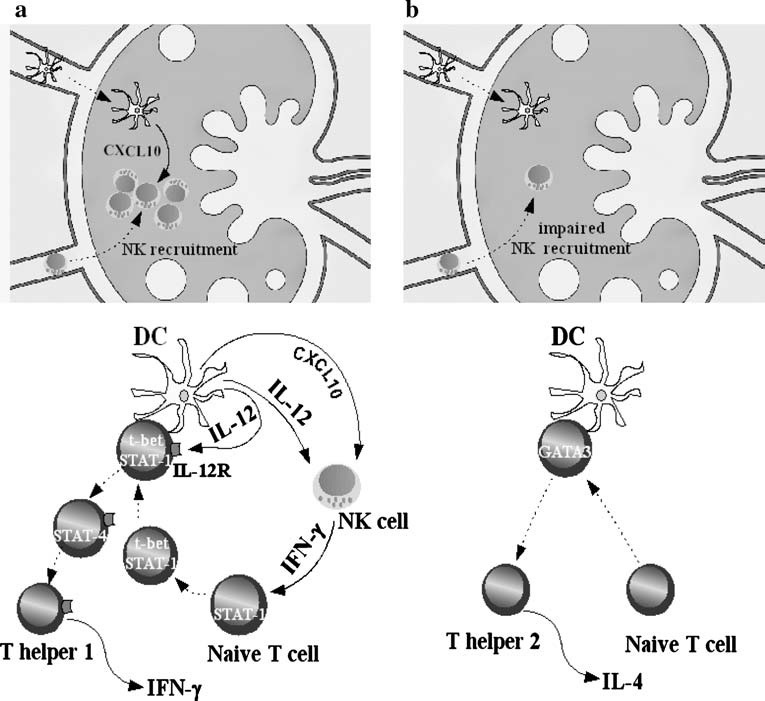
Dendritic cells shape T lymphocyte responses. (a) DCs migrating to the lymph node participate in NK recruitment by producing the CXCR3 ligand CXCL10. NK trafficking into inflamed lymph nodes is necessary for efficient priming of type 1 T lymphocytes. During antigen presentation, IFN-γ released by NK allows naïve T lymphocytes to be activated by IL-12 through the induction of IL-12 receptor. Under these conditions, DCs deliver signal 1 (MHC-peptide complex engaging TCR), signal 2 (DC costimulatory molecules CD80 or CD86 engaging CD28 on T lymphocyte surface) and signal 3 (STAT-4 activation by IL-12). (b) In ATP-conditioned DCs, simultaneous inhibition of IL-12 and CXCL10, but not IL-10 production, impairs the development of type 1 responses: reduced NK recruitment and antigen presentation in the absence of IL-12, but in the presence of IL-10, favours T helper 2 and/or T regulatory 1 differentiation.
Immature monocyte-derived DCs exposed to chronic stimulation with low micromolar concentrations of ATP but not UTP undergo partial membrane maturation and upregulate CD83, CD54, CD86 as well as the lymphoid chemokine receptor CCR7 conferring functional responsiveness to lymphoid chemokines such as CCL19 and CXCL12 [56, 62]. Dendritic cells stimulated with ATP alone fail to produce detectable cytokines [62]. However, when DCs are stimulated with the prototypic stimulus bacterial endotoxin (LPS) in the presence of ATP, profound changes in the maturation program are induced. While LPS-treated DCs produce high amounts of proinflammatory (IL-12, IL-23, TNF-α, IL-1, IL-6) and regulatory (IL-10 and IL-1 receptor antagonist) cytokines, when stimulated with LPS in the presence of low concentrations of ATP, DC production of IL-12, TNF-α, and IL-1 is completely abrogated. Conversely, production of the regulatory cytokine IL-10 is unaffected and IL-1 receptor antagonist is increased. As a result of the blocked IL-12 production, DCs exposed to extracellular ATP have reduced ability to induce Th1 differentiation in vitro [62, 63]. In addition, the production of chemokines preferentially attracting Th 1 polarised lymphocytes such as CXCL10 and CCL5 is also blocked, yet DCs still produce high amounts of the Th 2-attracting chemokines CCL-22 and CCL-17 [56]. The lack of CXCL10 (a ligand for CXCR3) production might also impair DC contribution to the recruitment of NK cells into the lymph node thus further favouring Th 2 development.
Altered DC maturation induced by the presence of extracellular ATP is reminiscent of the effects of treatment with cyclic adenosine monophosphate (cAMP)-inducing agents. Dendritic cells in which intracellular concentration of cAMP is increased following ligand-activation of Gs protein-coupled receptors, such as EP2 prostaglandin receptor [64], H2 histamine receptor [65, 66], β-adrenergic receptors [67], or A2 adenosine receptor [68], display blocked TNF-α and IL-12p70 production but unaffected or even increased IL-10 together with augmented expression of CD83 and CD86. Similar effects were observed by increasing intracellular cAMP by ADP ribosylation of the Gαs subunit of G proteins by cholera toxin [69, 70], by stimulation of adenylyl cyclases through forskolin administration [67] and by treatment with membrane-permeable cAMP analogues such as dibutyryl cAMP [67] or 8-bromocyclic AMP [71]. Extracellular ATP can increase the intracellular level of cAMP through the activation of the P2Y11 receptor that is coupled to the adenylyl cyclase and the phosphoinositide pathways [72]. Moreover P2Y11 has been suggested to be the P2 receptor mediating the ATPinduced DC maturation [13]. The stimulation of the cAMP pathway is also involved in the reported synergy between extracellular ATP and TNF-α or suboptimal doses of LPS in the induction of IL-12p40 by DCs. Either TNF-α or suboptimal doses of LPS are unable to elicit IL-12 production unless this stimulation takes place in the presence of extracellular ATP [13, 73]. However the synergistic effect is limited to the IL-12 p40 chain; no IL-12p70 heterodimer is produced by DCs exposed to extracellular ATP irrespective of the stimulus used [63].
Interestingly, under optimal stimulation conditions, the concomitant activation of the cAMP pathway by either extracellular ATP or prostaglandin E2, besides inhibiting IL-12p70, results in enhanced production of IL-23, a heterodimeric cytokine, a member of the small IL-12 family, composed of the p19 and IL-12p40 proteins. While IL-12 p70 is essential for the polarisation of naïve T cells toward Th 1 phenotype, IL-23 is thought to act preferentially on memory T lymphocytes stimulating IFN-γ production [74]. Consistently, DCs stimulated with E. coli in the presence of ATP display decreased ability to prime Th 1 responses but are still efficient in promoting IFN-γ production by memory T lymphocytes [75]. This might have important consequences for the regulation of immune responses as follows: in the presence of excessive tissue damage, large amounts of self peptides usually confined in the intracellular compartment become available to surrounding DCs. These DCs are limited in their ability to prime Th 1 responses by the concomitant presence of extracellular ATP, thus reducing the risk of activating potentially self-reactive IFN-γ producing cells. Concurrently, DCs are still able to support IFN-γ production by memory T cells, whose priming likely does not occur in the presence of overwhelming amounts of self-antigens and that therefore might be instrumental for pathogen eradication.
Triggering the P2Y11 receptor, besides inhibiting Th 1 priming, can also confer immunosuppressive activity to DCs. Marteau and colleagues, in a very recent report, showed that extracellular ATP induces DCs to produce large amounts of thrombospondin-1 (TSP-1) and to express indoleamine 2,3 dioxygenase [76]. Thrombospondin-1 exerts immunoregulatory activity by different mechanisms: (1) inhibition of T cell proliferation by binding to its receptor (CD47) on the cell membrane; (2) autocrine inhibition of IL-12 production by activating the phosphoinositol 3 kinase pathway in DCs [77]; (3) autocrine inhibition of DC activation [78]; (4) activation of the potent immunosuppressive cytokine TGF-β1 [79]. In addition Marteau and colleagues showed that ATP synergizes with IFN-γ to induce the expression of indoleamine 2,3 dioxygenase (IDO) [76]. Dendritic cells expressing IDO, an intracellular enzyme involved in the catabolism of the essential amino acid tryptophan, suppress T cell proliferation and promote tolerance [80–83]. In summary, while chronic exposure to very low (nanomolar) concentrations of extracellular ATP induces chemotactic activity of DCs, micromolar concentrations modify DC function to promote less self-harmful type 2 responses and/or tolerance by a variety of mechanisms due to adenylyl cyclase activation.
Another set of studies addressed the effects on DC physiology of high extracellular ATP doses showing that in the millimolar range, ATP causes the opening of P2X7 ion channel across the DC membrane and consequently increases permeability to low molecular weight solutes. Due to the availability of specific antibodies and inhibitors, P2X7 is the best characterised P2 receptor. It is expressed at very high levels on both murine and human DCs [31, 84]. Enhanced DC membrane permeability following treatment with ATP doses suggestive of activation of the P2X7 pore triggers rapid secretion of IL-1β and TNF-α by mature dendritic cells [31]. It has been shown that in mononuclear phagocytes, P2X7-mediated IL-1β release is due to the activation of interleukin-1-converting enzyme/caspase-1, which cleaves pre-stored IL-1 precursor to produce the mature form of IL-1β [85]. High membrane expression of P2X7 by DCs correlates with sensitivity to cytotoxic effects of extracellular ATP inducing apoptosis or necrosis depending on the dose and length of exposure [84, 86, 87]. Moreover it has been suggested that during antigen presentation, macrophages that upon activation have upregulated P2X7 expression might be lysed by ATP released from cytotoxic T lymphocytes [88]. Inhibition of P2X7 activation by oxidised ATP results in the prevention of P2X7-induced cell death as well as down-modulation of LPS-induced signaling. In particular, decreased activation of nuclear factor-κB, and of extracellular signal-regulated kinases 1 and 2 are observed in macrophages stimulated with LPS in the presence of P2X7 antagonist, pointing to a role of P2X7 signaling in cell activation induced by Tolllike receptor engagement [89].
Although there is a substantial discrepancy between physiological ATP levels detected in the extracellular space and the concentration needed to trigger P2X7in vitro, it is important to consider that the average extracellular nucleotide concentrations might represent significantly different local distributions. In close proximity to leaking plasma membranes of damaged cells, as well as of healthy actively secreting cells, nucleotides might locally reach molar concentration. Moreover, upon activation of pore-forming P2X7, the egress of intracellular nucleotides through the pore can trigger P2X7 on the membrane of adjacent cells, resulting in the amplification of local release of intracellular nucleotides in the extracellular milieu. Furthermore, the microbicidal peptide LL37, representing the C terminus of the cathelicidin family member cationic peptide 18 produced by neutrophils and epithelial cells, has been reported to be an endogenous P2X7 activator that triggers maturation and release of IL-1β from LPS-primed monocytes [90].
The concentration of ATP needed for P2X7 activation in vitro might not reflect the physiological situation. For example, recent evidence provided by Seman and co-workers showed that P2X7 activation can be triggered by nicotinamide-adenine dinucleotide (NAD)-dependent purinoceptor ADP-ribosylation [91]. Although NAD itself is not a ligand for P2X7, NAD released upon tissue injury and inflammation represents the substrate for ecto-ADP ribosyltransferase- 2 (ART-2) catalyzing ADP-ribosylation of P2X7. Interestingly, NAD derived from cell lysates is sufficient to activate P2X7 and in the presence of NAD, P2X7 activation can be triggered by low ATP concentrations. Similarly, we showed that otherwise ineffective concentrations of ATP induce P2X7-mediated cytotoxicity in the presence of the antibiotic polymyxin B [92]. These studies demonstrate that (1) P2X7 and possibly other purinergic receptors might be activated by non-nucleotide agonists, and (2) in vivo, different agonists can cooperate and activate purinoceptors at significantly lower concentrations than those needed in vitro.
To date no study has specifically addressed the modulation of dendritic cell activity by extracellular nucleotides in vivo. However the in vivo administration of the ATP analogue 2-methylthio-ATP inhibits the release of TNF-α and IL-1 and protects mice from endotoxin shock [93, 94]. Although these studies did not specifically address the contribution of DCs to the inflammatory response to LPS, their results are in keeping with what was observed in human monocyte-derived DCs stimulated in vitro with LPS in the presence of extracellular ATP [62, 63].
Mizumoto and colleagues studying contact hypersensitivity to haptens in CD39-deficient mice showed that the impairment of DC ability to metabolise extracellular nucleotides significantly influences their function in vitro and in vivo. CD39-/- Langerhans cells and bone marrow-derived DCs lack ecto-diphosphohydrolase activity with consequent accumulation of extracellular nucleotides in the pericellular space. Under these conditions, DCs and Langerhans cells are unresponsive to ATP due to P2 receptor desensitisation and display impaired antigen-presenting capacity. Moreover T lymphocytes increase pericellular ATP concentration upon activation, suggesting that nucleotides have an important role in DC-T cell communication during antigen presentation [55].
Conclusions
Nucleotides, in particular ATP, have been proposed as endogenous signals of tissue stress. In addition, ATP actively secreted by T lymphocytes might work as an important mediator for cell-cell communication during antigen presentation. In fact extracellular nucleotides, by stimulating P2 receptors, can profoundly influence DC functions and have a great impact on the outcome of immune response. Chronic exposure to low (micromolar) concentrations of extracellular ATP might work as negative feedback to limit DC contribution to exacerbated inflammation, mainly through the activation of P2Y11 and the following rise in the intracellular cAMP concentration. Concentration gradients of extracellular ATP attract immature DCs into injured tissues and prolong their permanence at the site of antigen encounter. In the proximity of damaged cells, where the concentration may be in the micromolar range, ATP blocks the synthesis by DCs of proinflammatory cytokines and chemokines for the recruitment of NK cells and type 1 polarised T lymphocytes and limits DCs’ capacity to promote type 1 responses. As a result, the development of less self-harmful type 2 responses is favoured. In addition, expression of regulatory molecules such as IDO and thrombospondin- 1 might turn DCs into active regulatory cells promoting tolerance rather than immunity.
On the other hand, high concentrations of extracellular ATP causes DC death through P2X7 activation and might represent a mechanism for the elimination of antigen-presenting cells into the lymph node by activated T lymphocytes.
Although further in vivo studies are required to confirm the observations made on monocyte DCs in vitro, targeting P2 receptor function might represent a promising approach to enhance immune responses for increased vaccination efficacy or conversely, to promote the tolerogenic functions of DCs for the treatment of inflammatory and autoimmune diseases.
References
- 1.Kapsenberg ML. Dendritic cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ, Kanzler V, Soumelis M, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 4.Apasov S, Koshiba M, Redgeld F, Sitkovsky MV. Role of extracellular ATP and P1 and P2 classes of purinergic receptors in T cell development and cytoltoxic T lymphocyte effector functions. Immunol Rev. 1995;146:5–19. doi: 10.1111/j.1600-065X.1995.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 5.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 6.Von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2000;62:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 7.Di Virgilio F, Chiozzi P, Ferrari D, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 8.Leon C, Freund M, Ravanat C, Baurand A, Cazenave JP, Gachet C. Key role of the P2Y(1) receptor in tissue factor-induced thrombin-dependent acute thromboembolism: studies in P2Y(1)-knockout mice and mice treated with a P2Y(1) antagonist. Circulation. 2001;103:718–723. doi: 10.1161/01.cir.103.5.718. [DOI] [PubMed] [Google Scholar]
- 9.Roberts VH, Greenwood SL, Elliott AC, Sibley CP, Waters LH. Purinergic receptors in human placenta; evidence for functionally active P2X4, P2X7, P2Y2 and P2Y6. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1374–1386. doi: 10.1152/ajpregu.00612.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(−) transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 11.Southey MC, Hammet F, Hutchins AM, Paidhungat M, Somers GR, Venter DJ. Molecular cloning and sequencing of a novel human P2 nucleotide receptor. Biochim Biophys Acta. 1996;1309:77–80. doi: 10.1016/s0167-4781(96)00148-0. [DOI] [PubMed] [Google Scholar]
- 12.Maier R, Glatz A, Mosbacher J, Bilbe G. Cloning of P2Y6 cDNAs and identification of a pseudogene: comparison of P2Y receptor subtype expression in bone and brain tissues. Biochem Biophys Res Commun. 1997;240:298–302. doi: 10.1006/bbrc.1997.7653. [DOI] [PubMed] [Google Scholar]
- 13.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 14.Conigrave AD, Fernando KC, Gu B, et al. P2Y(11) receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol. 2001;426:157–163. doi: 10.1016/S0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen TD, Meichle S, Kim US, Wong T, Moody MW. P2Y(11), a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G795–G804. doi: 10.1152/ajpgi.2001.280.5.G795. [DOI] [PubMed] [Google Scholar]
- 16.Schnurr M, Toy T, Stoitzner P, et al. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 17.van der Weyden L, Adams DJ, Luttrell BM, Conigrave AD, Morris MB. Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol Cell Biochem. 2000;213:75–81. doi: 10.1023/A:1007168215748. [DOI] [PubMed] [Google Scholar]
- 18.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wihlborg AK, Wang L, Braun OO, et al. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- 20.Falker K, Lange D, Presek P. ADP secretion and subsequent P2Y12 receptor signalling play a crucial role in thrombin-induced ERK2 activation in human platelets. Thromb Haemost. 2004;92:114–123. doi: 10.1160/TH03-12-0729. [DOI] [PubMed] [Google Scholar]
- 21.Hardy AR, Hill DJ, Poole AW. Evidence that the purinergic receptor P2Y12 potentiates platelet shape change by a rho kinase-dependent mechanism. Platelets. 2005;16:415–429. doi: 10.1080/09537100500163424. [DOI] [PubMed] [Google Scholar]
- 22.Communi D, Gonzalez NS, Detheux M, et al. Identification of a novel human ADP receptor coupled to G(i) J Biol Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacquet S, Malaval C, Martinez LO, et al. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci. 2005;62:2508–2515. doi: 10.1007/s00018-005-5194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BC, Cheng T, Adams GB, et al. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- 28.Valera S, Hussy N, Evans RJ, et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- 29.North RA, Suprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 30.Di Virgilio F, Chiozzi P, Falzoni S, et al. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 31.Ferrari D, La Sala A, Chiozzi P, et al. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. Faseb J. 2000;14:2466–2476. doi: 10.1096/fj.00-0031com. [DOI] [PubMed] [Google Scholar]
- 32.Lemoli RM, Ferrari D, Fogli M, et al. Extracellular nucleotides are potent stimulators of human hematopoietic stem cells in vitro and in vivo. Blood. 2004;104:1662–1670. doi: 10.1182/blood-2004-03-0834. [DOI] [PubMed] [Google Scholar]
- 33.Seyffert C, Schmalzing G, Markwardt F. Dissecting individual current components of co-expressed human P2X1 and P2X7 receptors. Curr Top Med Chem. 2004;4:1719–1730. doi: 10.2174/1568026043387160. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Bardini M, Keogh A, dos Remedios CG, Burnstock G. P2X1 receptors are closely associated with connexin 43 in human ventricular myocardium. Int J Cardiol. 2005;98:291–297. doi: 10.1016/j.ijcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 35.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 36.North RA. The P2X3 subunit: a molecular target in pain therapeutics. Curr Opin Invest Drugs. 2003;4:833–840. [PubMed] [Google Scholar]
- 37.Bo X, Zhang Y, Nassar M, Burnstock G, Schoepfer R. A P2X purinoceptor cDNA conferring a novel pharmacological profile. FEBS Lett. 1995;375:129–133. doi: 10.1016/0014-5793(95)01203-Q. [DOI] [PubMed] [Google Scholar]
- 38.Collo G, North RA, Kawashima E, et al. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003;121:1145–1149. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- 40.Caraccio N, Monzani F, Santini E, et al. Extracellular ATP modulates interleukin-6 production by human thyrocytes through functional purinergic P2 receptors. Endocrinology. 2005;146:3172–3178. doi: 10.1210/en.2004-1527. [DOI] [PubMed] [Google Scholar]
- 41.Kratzer MA. Is primary haemostasis controlled by a ‘platelet delay time’ Formulation of a new hypothesis. Platelets. 2003;14:437–443. doi: 10.1080/09537100310001632612. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Arcuino G, Takano T, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- 43.Di Virgilio F, Solini A. P2 receptors: new potential players in atherosclerosis. Br J Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson J, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979;281:384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 45.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelia cells butnot smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Cheek D, Westfall D, Buxton I. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- 47.Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in Raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/S0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 50.Gallucci S., Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 51.Sozzani S, Allavena P, D’Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–1086. [PubMed] [Google Scholar]
- 52.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/S0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 54.Idzko M, Dichmann S, Ferrari D, et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100:925–932. doi: 10.1182/blood.V100.3.925. [DOI] [PubMed] [Google Scholar]
- 55.Mizumoto N, Kumamoto T, Robson SC, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 56.la Sala A, Sebastiani S, Ferrari D, et al. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715–1722. doi: 10.1182/blood.V99.5.1715. [DOI] [PubMed] [Google Scholar]
- 57.Gately MK, Renzetti LM, Magram J, et al. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 58.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 59.Martin-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 60.Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure myeloid and plasmacytoid dendritic cells produce three waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613–2618. doi: 10.1182/blood-2005-07-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 62.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 63.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 64.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Idzko M, la Sala A, Ferrari D, et al. Expression and function of histamine receptors in human monocyte-derived dendritic cells. J Allergy Clin Immunol. 2002;109:839–846. doi: 10.1067/mai.2002.124044. [DOI] [PubMed] [Google Scholar]
- 66.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panina-Bordignon P, Mazzeo D, Lucia P, et al. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J Clin Invest. 199;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panther E, Idzko M, Herouy Y. Expression and function of adenosine receptors in human dendritic cells. Faseb J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 69.Gagliardi MC, Sallusto F, Marinaro M, Langenkamp A, Lanzavecchia A, De Magistris MT. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol. 2000;30:2394–2403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 70.Braun MC, He J, Wu CY, Kelsall BL. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J Exp Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galgani M, De Rosa V, De Simone S, et al. Cyclic AMP modulates the functional plasticity of immature dendritic cells by inhibiting Src-like kinases through protein kinase A-mediated-signaling. J Biol Chem. 2004;279:32507–32514. doi: 10.1074/jbc.M403355200. [DOI] [PubMed] [Google Scholar]
- 72.Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schnurr M, Then F, Galambos P, et al. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–4709. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 74.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages Il-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 75.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, Maraskovsky E. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105:1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 76.Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- 77.Armant M, Avice M, Hermann P, et al. CD47 ligation selectively downregulates human interleukin-12 production. J Exp Med. 1999;190:1175–1182. doi: 10.1084/jem.190.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Doyen V, Rubio M, Braun D, et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J Exp Med. 2003;198:1277–1283. doi: 10.1084/jem.20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crawford S, Stellmach V, Murphy-Ullrich J, et al. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/S0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 80.Hwu P, Du M, Lapointe R, Do M, Taylor M, Young H. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 81.Mellor A, Keskin D, Johnson T, Chandler P, Munn D. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 82.Terness P, Bauer T, Rose L, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediaton of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munn D, Sharma M, Lee J, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3, dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 84.Mutini C, Falzoni S, Ferrari D, et al. Mouse dendritic cells express the P2X7 purinergic receptor: characterization and possible participation in antigen presentation. J Immunol. 1999;163:1958–1965. [PubMed] [Google Scholar]
- 85.Perregaux D, Gabel C. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1 alpha and Il-1 beta occurs via a similar mechanism. J Immunol. 1998;160:2469–2477. [PubMed] [Google Scholar]
- 86.Coutinho-Silva R, Persechini PM, Bisaggio RD, et al. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276:C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- 87.Nihei OK, de Carvalho AC, Savino W, Alves LA. Pharmacologic properties of P(2Z)/P2X(7)receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000;96:996–1005. [PubMed] [Google Scholar]
- 88.Blanchard D, Wei S, Duan C, Pericle F, Diaz J, Djeu J. Role of extracellular adenosine triphosphate in the cytotoxic T-lymphocyte-mediated lysis of antigen presenting cells. Blood. 1995;85:3173–3182. [PubMed] [Google Scholar]
- 89.Hu Y, Fisette P, Denlinger L, et al. Purinergic receptor modulation of lipopolisaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 90.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 91.Seman M, Adriouch S, Scheuplein F, et al. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART-2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–582. doi: 10.1016/S1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 92.Ferrari D, Pizzirani C, Adinolfi E, et al. The antibiotic polymyxin B modulates P2X7 function. J Immunol. 2004;173:4652–4660. doi: 10.4049/jimmunol.173.7.4652. [DOI] [PubMed] [Google Scholar]
- 93.Proctor R, Denlinger L, Leventhal P, et al. Protection from endotoxic death by 2-methylthio-ATP. Proc Natl Acad Sci USA. 1994;91:6017–6020. doi: 10.1073/pnas.91.13.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Denlinger L, Fisette P, Garis K, et al. Regulation of inducible nitric oxide synthase expression by macrophage purinoceptors and calcium. J Biol Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]


