Abstract
Rgs2 (regulator of G-protein signaling-2)-deficient mice exhibit severe hypertension, and genetic variations of RGS2 occur in hypertensive patients. RGS2 mRNA up-regulation by angiotensin II (Ang II) in vascular smooth muscle cells (VSMC) is a potentially important negative feedback mechanism in blood pressure homeostasis, but how it occurs is unknown. Here we demonstrate that group VIA phospholipase A2 (iPLA2β) plays a pivotal role in Ang II-induced RGS2 mRNA up-regulation in VSMC by three independent approaches, including pharmacologic inhibition with a bromoenol lactone suicide substrate, suppression of iPLA2β expression with antisense oligo-nucleotides, and genetic deletion in iPLA2β-null mice. Selective inhibition of iPLA2β by each of these approaches abolishes Ang II-induced RGS2 mRNA up-regulation. Furthermore, using adenovirus-mediated gene transfer, we demonstrate that restoration of iPLA2β-expression in iPLA2β-null VSMC reconstitutes the ability of Ang II to up-regulate RGS2 mRNA expression. In contrast, Ang II-induced vasodilator-stimulated phosphoprotein phosphorylation and Ang II receptor expression are unaffected. Moreover, in wild-type but not iPLA2β-null VSMC, Ang II stimulates iPLA2 enzymatic activity significantly. Both arachidonic acid and lysophosphatidylcholine, products of iPLA2β action, induce RGS2 mRNA up-regulation. Inhibition of lipoxygenases, particularly 15-lipoxygenase, and cyclooxygenases, but not cytochrome P450-dependent epoxygenases inhibits Ang II- or AA-induced RGS2 mRNA expression. Moreover, RGS2 protein expression is also up-regulated by Ang II, and this is attenuated by bromoenol lactone. Disruption of the Ang II/iPLA2β/RGS2 feedback pathway in iPLA2β-null cells potentiates Ang II-induced vasodilator-stimulated phosphoprotein and Akt phosphorylation in a time-dependent manner. Collectively, our results demonstrate that iPLA2β participates in Ang II-induced transcriptional up-regulation of RGS2 in VSMC.
Regulators of G protein signaling (RGS)2 comprise a family of more than 25 diverse, multifunctional signaling proteins. RGS proteins share a 120-amino acid residue sequence homology domain (RGS domain) that directly binds the activated α-sub-unit of heterotrimetic G-protein, stimulates its GTPase activity, and thereby attenuates G-protein signaling (1). RGS2 has a ubiquitous tissue distribution, and is the most selective and potent inhibitor of Gαq function (2). RGS2 can also inhibit Gαi function under certain circumstances (3) and can interact directly with type III and V adenylyl cyclases to inhibit their activities and attenuate Gαs signaling (4). Consistent with its interaction with multiple G proteins, RGS2 has been implicated as an important modulator in diverse cellular functions (5), including calcium signaling, ion channel activity, cell proliferation, GLU4 translocation, adipocyte differentiation, and anxiety. In particular, Rgs2-null mice exhibit persistent vascular constriction and severe hypertension (6-8), suggesting that Rgs2 deficiency in vascular smooth muscle is responsible for the hypertension. Importantly, genetic variations in the RGS2 gene have recently been identified in hypertensive patients (9, 10).
The importance of RGS2 in diverse cellular functions suggests that it must be regulated by a complex signaling network that could include post-transcriptional phosphorylation by PKC (11) and by PKG (6), but a growing body of evidence suggests that the RGS2 mRNA expression level is rapidly and dynamically regulated by a variety of stimuli in many cells and tissues (13-28). In cultured VSMC, multiple RGS isoform transcripts (RGS2, RGS3S, RGS3L, RGS4, RGS5, RGS10, RGS11, RGS12, and GAIP) are expressed (13, 14, 29), but only RGS2 mRNA is dynamically up-regulated by Ang II in a concentration-dependent manner (13, 14). Ang II is a multifunctional peptide that has multiple and opposing effects on VSMC (30). Ang II rapidly stimulates VSMC contraction, increases vascular tone, and causes a rise in blood pressure, but Ang II also triggers negative feedback pathways to restrain vasoconstrictor effects.
Both positive and negative effects of Ang II on VSMC are equally important for VSMC function, although perhaps more attention has been focused on vasoconstrictor actions. Nonetheless, loss of negative regulation by Ang II could lead to VSMC dysfunction, and it has been demonstrated that hyper-tension in Rgs2-deficient mice is selectively blocked by an AT1 receptor antagonist, but not by nicotinic or α1-adrenegic receptor antagonists (8), suggesting that Ang II-induced RGS2 mRNA up-regulation plays a pivotal role in attenuating Ang II-induced rises in blood pressure. The signaling pathways linking Ang II receptor stimulation to RGS2 mRNA up-regulation in VSMC have not yet been elucidated.
Phospholipase A2 (PLA2) enzymes hydrolyze the sn-2 acyl linkages in phospholipid substrates to yield a free fatty acid, e.g. arachidonic acid (AA), and a 2-lysophospholipid, e.g. lysophosphatidylcholine (LPC). Among the recognized PLA2 families are low molecular weight secretory PLA2 (sPLA2) that require millimolar [Ca2+] for activity and the group IV cytosolic PLA2 (cPLA2), of which the first recognized member is the 85-kDa group IVA PLA2 (cPLA2α) that is induced to associate with its membrane substrates by rises in cytosolic [Ca2+] in the submicromolar range (31). Group VI PLA2 (iPLA2) family members do not require Ca2+ for catalytic activity and are inhibited by a bromoenol lactone (BEL) suicide substrate at concentrations that do not inhibit sPLA2 or cPLA2 enzymes. The group VIA PLA2 (iPLA2β) resides predominantly in the cytosol of resting cells, but the group VIB PLA2 (iPLA2γ) has a peroxisomal targeting sequence and is predominantly membrane-associated. Several other members of this family are now recognized (e.g. δ, ε, ξ, and η) (32).
Among the iPLA2 family members, iPLA2β the first recognized and best characterized, and it is a 84–88-kDa protein that contains a GTSTG lipase consensus sequence and an ATP-binding motif. iPLA2β is expressed in a variety of cell types and tissues and has been implicated in several cellular functions (33-35). In particular, iPLA2β is a major PLA2 family member in VSMC and is responsible for agonist-induced AA release (36, 37). Whereas AA, its metabolites, and LPC have all been implicated in transcriptional regulation (38-40), it has not yet been determined whether iPLA2β is activated when VSMC are stimulated with Ang II or whether iPLA2β reaction products participate in Ang II-induced RGS2 mRNA up-regulation in VSMC. We have examined these questions with a combination of pharmacologic, molecular biologic and genetic approaches and report our findings here.
EXPERIMENTAL PROCEDURES
Materials and Animals
Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN). The iPLA2β−/− mice were generated in the laboratory of Dr. John Turk, as described elsewhere (41), and have been found to exhibit several phenotypic abnormalities (41-43). The animals were fed and grown under the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health. Ang II was purchased from Calbiochem.L-α-Lysophosphatidylcholine (LPC) was purchased from Sigma. BEL, AA, 17-octadecynoic acid, MK886, baicalein, luteolin, and PGF2α (prostaglandin F2α) were purchased from Cayman (Ann Arbor, MI). Nordihydroguaiaretic acid and indomethacin were purchased from Biomol (Plymouth Meeting, PA). A goat polyclonal anti-iPLA2β antibody, a monoclonal anti-cPLA2 antibody, and the rabbit polyclonal anti-AT1R and RGS2 antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA). The rabbit polyclonal anti-vasodilator-stimulated phosphoprotein (VASP), Akt, and β-actin antibodies were purchased from Cell Signaling (Danvers, MA).
Primary Cell Culture
Rat thoracic aortic VSMC were isolated by enzymatic dissociation and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, as described (44). Mouse aortic VSMC were isolated by enzymatic method, as described by Sakata et al. (45), with some modifications. Briefly, the age and gender-matched iPLA2β WT and knock-out mice were euthanized. The aortas were rapidly removed and the adventitia was cleared. The vessels were incised longitudinally and then further digested by collagenase type I (Worthington Biochemical Corp, 1 mg/ml) and elastase type III (Sigma, 0.125 mg/ml) at 37 °C in a CO2 humidified incubator for 40 min. After 40 min incubation, the digested vessels were pipetted vigorously every 10 min for another 40 min. Then, the cell suspension was centrifuged and the cell pellets were resuspended in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Finally, the cell suspension was placed on a 6-well plate and cultured for 3–7 days. All primary cell cultures used in the present study were within 10 passages.
Real-time PCR
After stimulation, the culture medium was aspirated and TRI Reagent was added to cells to stop reactions and stabilize RNA. Total RNA was isolated from cells based on the manufacturer's instructions (Molecular Research Center, Inc., Cincinnati, OH). The cDNAs were synthesized by Moloney murine leukemia virus reverse transcriptase (Invitrogen) using a random hexamer. The PCR primers used were: 5′-TTGGAAGACCCGTTTGAGCTATT-3′ (forward), 5′-GGAGAAGGCTTGATAAAAGTTTGCT-3′ (reverse) for rat and mouse RGS2; 5′-TCAGGATCTCATGCCCATCTCT-3′ (forward), 5′-TGGTCGTGACTCCGCTTCTC-3′ (reverse) for mouse iPLA2β; 5′-AGAAACGGCTACCACATCCAA-3′ (forward), 5′-GGGTCGGGAGTGGGTAATTT-3′ (reverse) for rat and mouse 18 S rRNA. To rule out a potential genomic DNA contamination, primers were designed to crossover the boundary of intron and exon of individual genes. The real-time PCR were performed using a QuantiTect™ SYBR Green PCR kit (Qiagen, Valencia, CA) in an ABI Prism 7700 Sequence Detection System. The specificity of the PCR was verified by dissociation curve analysis and agarose gel electrophoresis. RGS2 and iPLA2β mRNAs were normalized to the 18 S rRNA and quantified by the standard curve analysis. In all experiments, RGS2 mRNA expression level was normalized to the basal level. To ensure the specificity of real-time PCR amplification, two controls were included in each real-time PCR run: one control is without adding template and the other is without adding transcriptase in the reaction.
Recombinant Adenovirus Construction and Infection
The iPLA2β adenovirus were generated by our laboratory and purified by cesium chloride gradient ultracentrifugation, as described previously (46). VSMC were starved in serum-free medium for 24 h and then incubated with the iPLA2β adenovirus (multiplicity of infection of 500) plus the Tet-on (encoding a tetracycline-regulatory transcription factor) viruses for 12 h. Subsequently, the cells were cultured in serum- and adenovirus-free medium for 36 h to allow iPLA2β expression. Different concentrations of doxycycline were present for the total 48-h period. The adenoviral infection conditions were optimized to maximize iPLA2β expression and to minimize the cytopathic effect of the adenovirus. Under the optimal conditions, over 95% of VSMC were found to be infected based on the expression of green fluorescent protein, which was co-expressed with iPLA2β (46).
iPLA2 Assay
iPLA2 enzymatic activity was measured by a commercially available PLA2 assay kit (Cayman, Ann Arbor, MI) under a Ca2+ -free condition that has been well characterized (47- 49). Briefly, after specific treatment as indicated in the text, VSMC were collected by a cell scraper in a 500-μl buffer containing 50 mm HEPES (pH 7.4) and 1 mm EDTA. Cells were sonicated on ice (Branson Sonifier 450, duty cycle 30%, speed 30 s, interval 1 min, 6–10 times). Cell homogenates were cleared by centrifugation at 10,000 × g for 15 min at 4° C. The supernatant was kept on ice, and the concentration of protein was determined. To detect the activity of iPLA2, but not cPLA2, phospholipase activity was assayed by incubating the samples with the substrate, arachidonoyl thio-phosphatidylcholine, for 1 h at 24 °C in a calcium-free buffer (300 mm NaCl, 0.5% Triton X-100, 60% glycerol, 4 mm EGTA, 10 mm HEPES, pH 7.4, and 2 mg/ml bovine serum albumin). The reaction was stopped by adding 5,5′-dithiobis(nitrobenzoic acid) for 5 min, and the absorbance was determined at 414 nm using a standard plate reader. The activity of iPLA2 was expressed in absorbance per mg of protein. As described (47, 48), the BEL-insensitive background iPLA2-independent component of basal lipase activity was determined in control samples when all specific iPLA2 activity was inhibited with BEL (25 μm, 30 min at 37 °C). This background activity was subtracted from all reading absorbance.
Antisense Oligonucleotide Studies
A 20-base long antisense corresponding to conserved nucleotides 59–78 in iPLA2β sequence was utilized (antisense oligonucleotide: 5′-CTCCTTCACCCGGAATGGGT-3′ (50). The sense complement of the antisense oligonucleotide was used as a control (5′-ACCCATTCCGGGTGAAGGAG-3′). Both antisense and sense oligo-nucleotides contained phosphorothioate linkages to limit their degradation and were purchased from Synthegen (Houston, TX). As described by manufacturer, 60–70% confluent rat VSMC were transfected in a 6-well plate by using Lipofectamine PLUS Reagent (Invitrogen). After 48 h transfection, the cells were starved and then either stimulated by Ang II for RGS2 mRNA determination by real-time PCR or harvested for iPLA2β protein determination by Western blot analysis.
Generation and Characterization of an iPLA2β Antibody
A rabbit polyclonal antibody for iPLA2β was produced by Genemed Synthesis (San Francisco, CA) against hamster iPLA2β amino acid 557–576 (PRFNQNINLKPPTQPADQLV). The antiserum was affinity purified as described previously (44). Three lines of evidence suggest this anti-iPLA2β antibody is specific (Fig. 4, A and B): 1) it recognized the exogenously expressed iPLA2β; 2) it recognized a 80–85-kDa protein that was present in iPLA2β wild-type cells but was absent in iPLA2β null cells; 3) preincubation of the iPLA2β antibody with the iPLA2β epitope peptide abolished its binding ability.
FIGURE 4. Reconstitution of iPLA2β expression by adenoviral-mediated gene transfer in iPLA2β-null VSMC dose-dependently restores Ang II-induced RGS2 mRNA up-regulation.
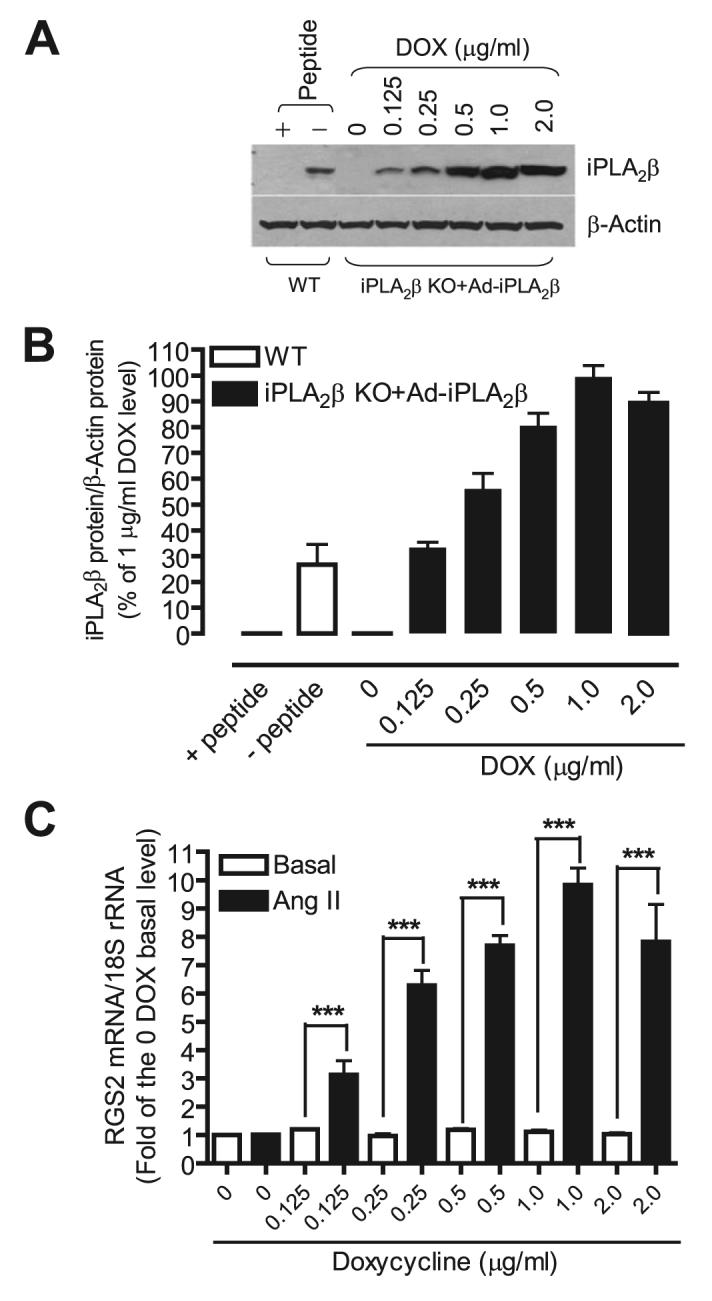
iPLA2β-null cells were infected with a recombinant adenovirus encoding iPLA2β and green fluorescent protein, and a Tet-on adenovirus encoding a transcriptional regulator in the presence of different concentrations of DOX for 12 h. After removal of adenovirus, cells were cultured in the absence of fetal bovine serum for another 36 h. Over 95% of cells were infected by adenovirus based on % of cells that expressed green fluorescent protein. The cells were then stimulated with Ang II (100 nm, 1 h). The iPLA2β and β-actin protein expressions were determined by Western blot. RGS2 mRNA was determined by real-time PCR. A, a representative Western blot. B, summary of the Western blot shown in A. C, RGS2 mRNA from each sample was normalized to 18 S rRNA and then to the sample that has neither Ang II stimulation and nor iPLA2β expression (0 DOX). The summary data are from at least three time independent experiments. ***, p < 0.001.
Western Blot Analysis
After various treatments as indicated in the text, cells were frozen with liquid nitrogen in 10% trichloroacetic acid buffer as previously described (51). Equal amounts of proteins were subjected to SDS-PAGE and Western blot analysis using anti-iPLA2β, and cPLA2,β-actin, VASP Ser157-phospho, Akt Ser473 -phospho antibodies, respectively. The signals were captured by the Kodak Image Station 4000MM and quantified by the Kodak Molecular Image Software.
siRNA Experiment
Rat RGS2 siRNA (On-TARGET plus SMARTpool) and control siRNA (siCONTROL Non-Targeting siRNA) were purchased from Dharmacon (Lafayette, CO). 60% confluent cells were transfected with 20 nm RGS2 and control siRNA using Lipofectamine 2000 (Invitrogen) for 48 h. After harvesting cells, an equal amount of lysate was subjected to Western blot analysis using anti-RGS2 antibody (Santa Cruz).
Statistical Analysis
Each experiment was repeated a minimum of three times. Data were expressed as mean ± S.E. Statistical analysis was performed by using t test for two groups and one-way analysis of variance for multiple groups (GraphPad Prism 4).
RESULTS
iPLA2β Is Required for Ang II-induced RGS2 mRNA Up-regulation in VSMC
To determine whether iPLA2 is involved in Ang II-induced RGS2 mRNA up-regulation in VSMC, we first examined effects of BEL, a suicide substrate that selectively inhibits iPLA2 activity at concentrations that have little effect on sPLA2 or cPLA2 activities (32, 46, 52, 53). Fig. 1, A and B, illustrates that preincubating cells with BEL for 30 min before adding Ang II resulted in a significant and concentration-dependent inhibition of RGS2 mRNA up-regulation compared with control cells.
FIGURE 1. BEL dose dependently inhibits Ang II-induced RGS2 mRNA up-regulation in VSMC.
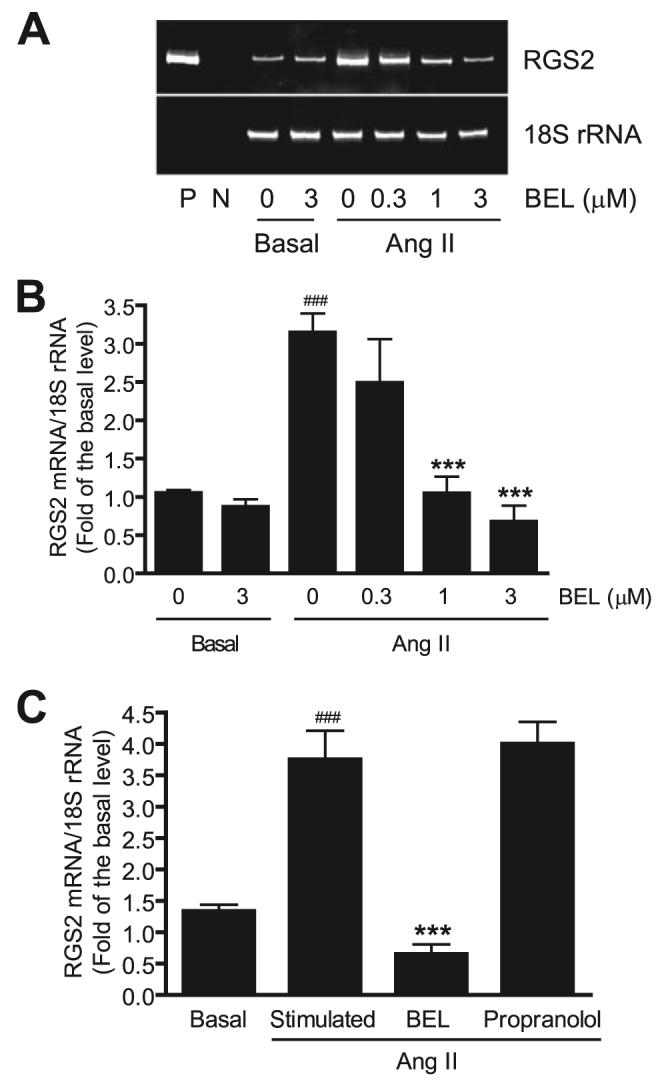
Quiescent VSMC were incubated with different concentrations of BEL, vehicle (Me2SO), or propranolol (10 μm) for 30 min, respectively. Cells were then stimulated with 100 nm Ang II for 1 h. RGS2 mRNA expression was determined by real-time PCR and normalized to 18 S rRNA and to the basal level. A, a representative DNA acrylamide gel of real-time PCR products. P, positive control, using a RGS2 cDNA as PCR template; N, negative control, no PCR template. B, a dose curve of BEL inhibition on RGS2 mRNA expression. C, BEL, not propranolol, inhibits RGS2 mRNA expression. The summary data are from at least four time independent experiments. ###, p < 0.001 versus basal; ***, p < 0.001 versus Ang II stimulation alone.
BEL is also known to inhibit phosphatidate phosphohydrolase-1, as does the compound propranolol, but propranolol does not inhibit iPLA2 activity (54). To determine whether the effects of BEL observed in Fig. 1, A and B, might be attributable to phosphohydrolase-1 inhibition, effects of propranolol on VSMC responses to Ang II were examined, and no suppression of RGS2 mRNA up-regulation was observed (Fig. 1C). This indicates that inhibition of phosphohydrolase-1 activity does not account for suppression of Ang II-induced RGS2 mRNA up-regulation in VSMC and suggests that this effect results from inhibition of iPLA2 activity.
BEL inhibits multiple iPLA2 family members, and its effects cannot a priori be attributed specifically to inhibition of iPLA2β. To address this issue, we examined effects of selectively suppressing iPLA2β expression in VSMC with an antisense oligonucleotide that has previously been shown to be effective in that regard in many cells, including VSMC (48-50, 55-57). Fig. 2, A and B, illustrates that this antisense oligonucleotide (but not the corresponding sense oligonucleotide) suppressed expression of the immunoreactive iPLA2β protein by about 50%. In contrast, the iPLA2β antisense oligonucleotide had no effect on expression of immunoreactive cPLA2 protein, which reflects the specificity of iPLA2β antisense oligonucleotide. Fig. 2C illustrates that the iPLA2β antisense oligonucleotide (but not the sense oligonucleotide) also inhibited Ang II-induced RGS2 mRNA up-regulation in VSMC, which supports the possibility suggested by findings in Fig. 1 that iPLA2β is required for Ang II-induced RGS2 mRNA up-regulation.
FIGURE 2. iPLA2β antisense oligonucleotide down-regulates iPLA2β protein and inhibits Ang II-induced RGS2 mRNA up-regulation in VSMC.
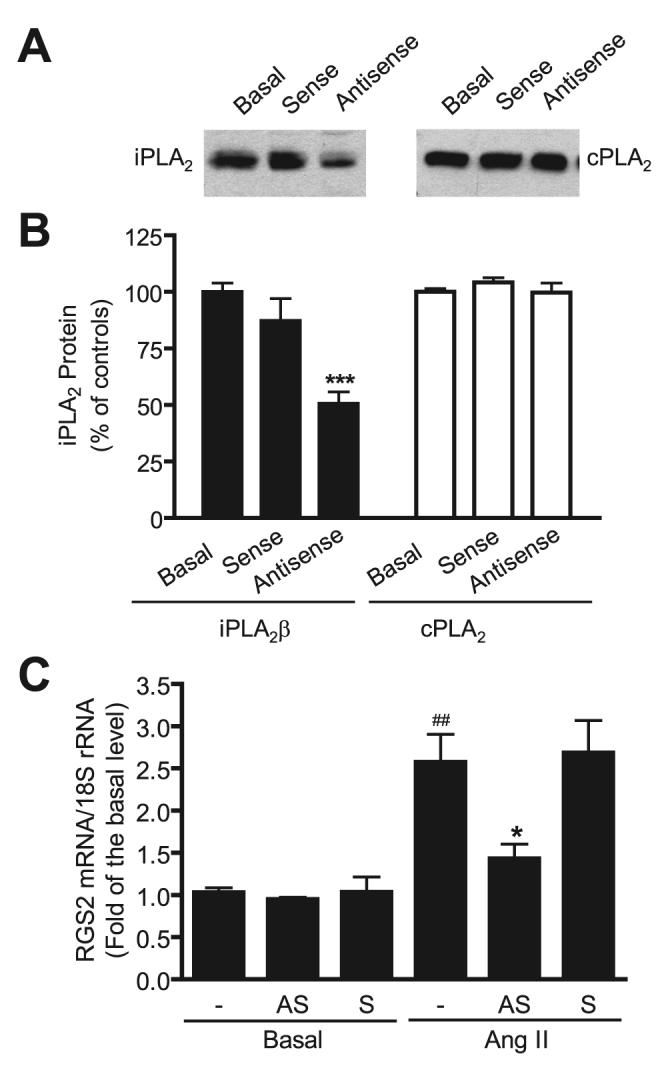
Cells were transfected with 2 μg of iPLA2β antisense or sense oligonucleotide. The cells were cultured in serum-free medium for 48 h for quiescence, and then stimulated with 100 nm Ang II for 1 h. The iPLA2β and cPLA2 proteins were determined by Western blot using anti-iPLA2β antibody (Santa Cruz) and anti-cPLA2 antibody, respectively. RGS2 mRNA was determined by real-time PCR. A, a representative iPLA2β and cPLA2 Western blot. B, summary of the Western blot shown in A. C, antisense, not sense, inhibits RGS2 mRNA expression. The summary data are from at least three time independent experiments. ***, p < 0.001 versus the basal sample (non-transfected cells); ##, p < 0.01 versus the basal sample (without Ang II stimulation); *, p < 0.05 versus Ang II stimulation alone.
In addition, we isolated aortic VSMC from wild-type and iPLA2β-null mice. Real-time PCR (Fig. 3, A and B) and immunoblotting (Fig. 4A) analyses indicated that iPLA2β mRNA and protein, respectively, observed in wild-type VSMC were not detectable in iPLA2β-null VSMC. Mouse VSMC, like rat VSMC, were found to respond to Ang II by increasing the expression level of RGS2 mRNA (Fig. 3C). In contrast, the level of RGS2 mRNA expression was unaffected by incubating iPLA2β-null VSMC with Ang II. An unanticipated observation is that the basal level of RGS2 mRNA was also significantly lower in iPLA2β-null VSMC than in wild-type VSMC.
FIGURE 3. Ang II fails to stimulate RGS2 mRNA expression in iPLA2β-null VSMC.
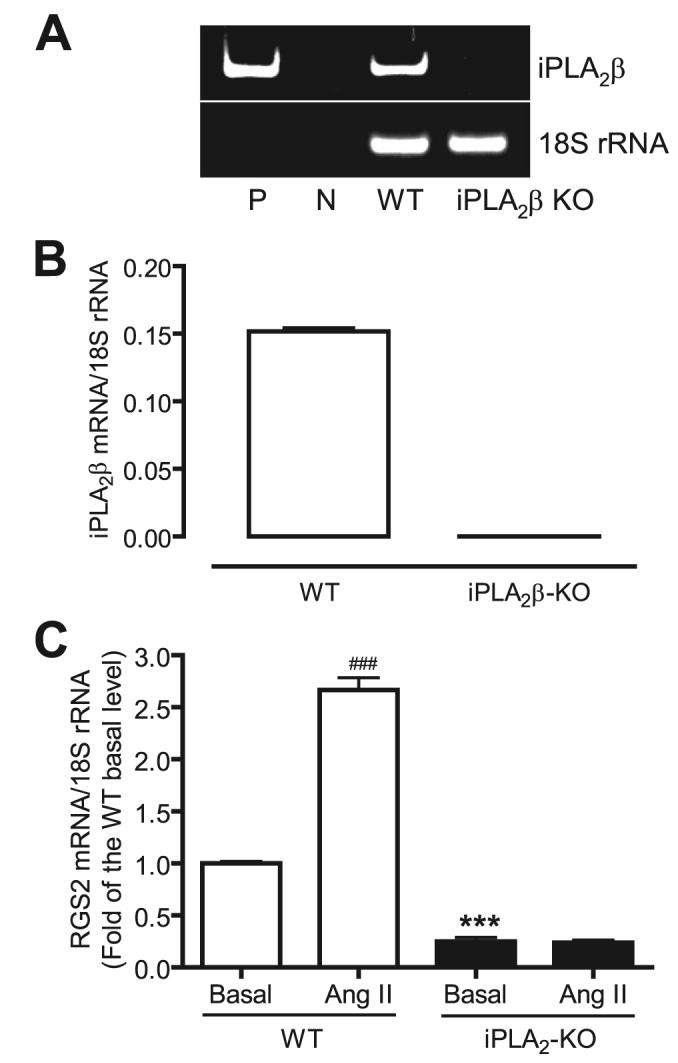
Aortic vascular smooth muscle cells were isolated from wild-type and iPLA2β-null mice (iPLA2β KO). iPLA2β and RGS2 mRNA expressions were determined by real-time PCR. A, a representative DNA acrylamide gel of real-time PCR products. P, positive control, using an iPLA2β cDNA as PCR template; N, negative control, no PCR template. B, summary of the real-time PCR shown in A. C, Ang II fails to stimulate RGS2 mRNA expression in iPLA2β-null VSMC. The summary data are from at least three time independent experiments. ###, p < 0.001 versus basal; ***, p < 0.001 versus basal.
We next examined whether restoring iPLA2β expression to iPLA2β-null VSMC would reconstitute the responses to Ang II observed with wild-type VSMC. Restoration of iPLA2β expression in iPLA2β-null VSMC was achieved with tetracycline-inducible, adenovirus-mediated gene transfer (46). Fig. 4, A and B, illustrates that the stable tetracycline analog doxycycline (DOX) induced iPLA2β protein expression in iPLA2β null VSMC in a concentration-dependent manner. Moreover, iPLA2β-null cells in which iPLA2β expression was restored in this way recovered the ability observed in wild-type VSMC to increase expression of RGS2 mRNA upon incubation with Ang II in a concentration-dependent manner (Fig. 4C).
It is noteworthy that a DOX concentration of 1 μg/ml induced maximal increases in expression of iPLA2β protein and RGS2 mRNA, but a DOX concentration of 2 μg/ml failed to cause a further increase in expression of either iPLA2β protein or RGS2 mRNA in response to Ang II (Fig. 4). Second, without transfer of the iPLA2β gene, DOX alone (2 μg/ml) affected neither basal nor Ang II-stimulated RGS2 mRNA expression levels (data not shown). Third, because all samples were incubated with the same titer of adenovirus, restoration of Ang II responsiveness by gene transfer must be attributed to expression of iPLA2β rather than to adenoviral protein(s). Fourth, restoration of iPLA2β expression in iPLA2β-null VSMC did not affect the basal level of RGS2 mRNA expression, even though the response to Ang II was restored. Finally, the level of iPLA2β protein expression achieved at a DOX concentration of 0.125 μg/ml was approximately equivalent to that in wild-type VSMC (Fig. 4, A and B), and under those conditions the magnitude of RGS2 mRNA up-regulation in response to Ang II was similar in wild-type and iPLA2β-null cells (Fig. 3C versus Fig. 4C). Taken together, our results strongly suggest that iPLA2β is essential for Ang II-induced RGS2 mRNA up-regulation in VSMC.
iPLA2β Is Downstream of Ang II Receptor Occupancy in the Signaling Pathway That Leads to Increased Expression of RGS2 mRNA
The participation of iPLA2β in the VSMC response to Ang II could in principle involve effects of the enzyme or its products to increase expression of Ang II receptors or to facilitate Ang II binding to them. An alternative possibility is that iPLA2β may participate in signaling events subsequent to receptor occupancy. To distinguish among these possibilities, we compared other responses to Ang II in wild-type VSMC with or without BEL and in iPLA2β-null VSMC.
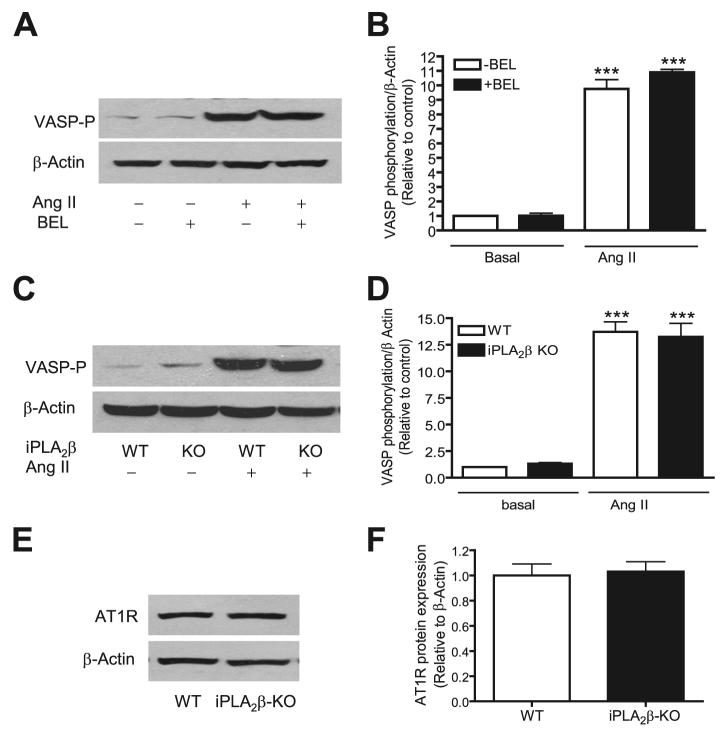
One signaling molecule that is activated by phosphorylation in an Ang II-responsive pathway is VASP. In agreement with reported observations (58), we found that Ang II (100 nm, 5 min) markedly stimulated VASP phosphorylation in wild-type VSMC, but this response was not inhibited by BEL (Fig. 5, A and B) and was also observed with iPLA2β-null cells (Fig. 5, C and D) under conditions where Ang II fails to induce increased expression of RGS2 mRNA. Moreover, Fig. 5, E and F, demonstrate that there is no difference between the iPLA2β-null and wild-type cells in regard with the Ang II receptor expression levels. These results suggest that participation of iPLA2β in response to Ang II occurs downstream of Ang II receptor occupancy.
FIGURE 5. Inhibition of iPLA2β by BEL or genetic deletion nether affects Ang II-induced VASP protein phosphorylation nor Ang II receptor (AT1R) protein expression.
Prior to Ang II stimulation (100 nm, 5 min), wild-type cells were pretreated with BEL (3 μm, 30 min). The VASP phosphorylation levels in both wild-type (A and B) and iPLA2β-null cells (C and D) were determined using an anti-VASP Ser-157 phospho-specific antibody and normalized to β-actin and then to the basal level. The AT1R protein expression was determined by Western blot using an anti-AT1R antibody (E and F). The summary data are from at least three time independent experiments. ***, p < 0.001 versus basal. KO, knock-out.
Other agonists of G-protein-coupled receptors, such as PGF2α, also induce increased expression of RGS2 in VSMC (Fig. 6). This response was inhibited by BEL in a concentration-dependent manner (Fig. 6A) and did not occur in the iPLA2β-null cells (Fig. 6B). Similarly, increased expression of RGS2 mRNA induced by Ang II was inhibited by BEL (Fig. 1) or by iPLA2β deletion (Fig. 3C). These results suggest that iPLA2β might be a common effector that lies downstream of occupancy of G-protein-coupled receptors that cause increased VSMC expression of RGS2 mRNA.
FIGURE 6. BEL or genetic deletion of iPLA2β abolishes PGF2α-induced RGS2 mRNA up-regulation in VSMC.
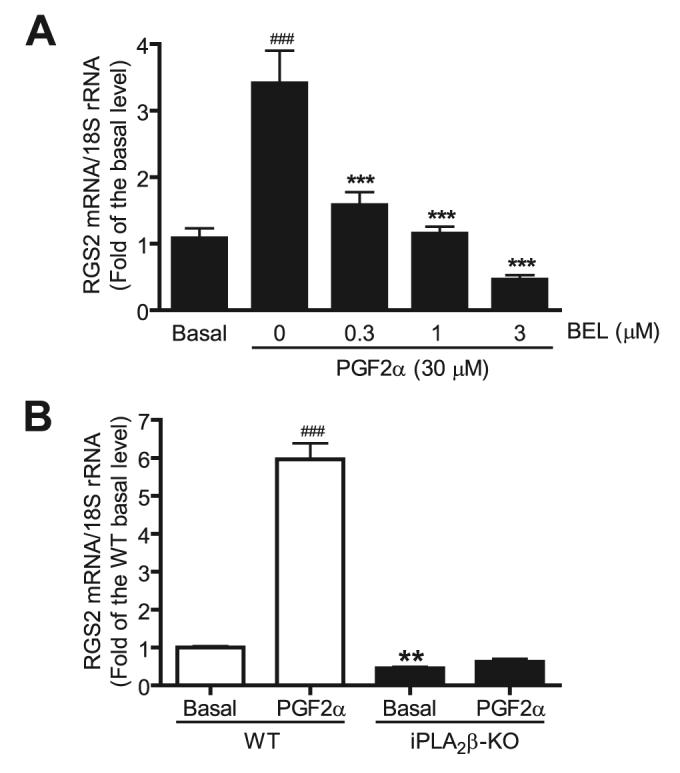
Prior to PGF2α stimulation (30μm, 1 h), wild-type cells (A), but not iPLA2β-null cells (B), were preincubated with different concentrations of BEL. The RGS2 mRNA expression was determined by real-time PCR. The summary data are from at least seven independent experiments. ###, p < 0.001 versus basal; ***, p < 0.001 versus PGF2α stimulation alone; **, p < 0.01 versus basal.
Activation of iPLA2β Is Critical for Ang II-induced RGS2 mRNA Up-regulation
One means by which iPLA2β might participate in signaling is through its enzymatic activity to hydrolyze phospholipids. To examine this possibility, we measured rat VSMC iPLA2 activity in the absence and presence of Ang II using standard assay conditions (47-49). Ang II significantly stimulated iPLA2 activity in wild-type rat VSMC (Fig. 7A), and, as expected, this response was prevented when cells were pretreated with BEL (3 μm, 30 min).
FIGURE 7. Ang II stimulates iPLA2β enzymatic activity in wild-type VSMC, but in BEL-pretreated or iPLA2β-null VSMC.
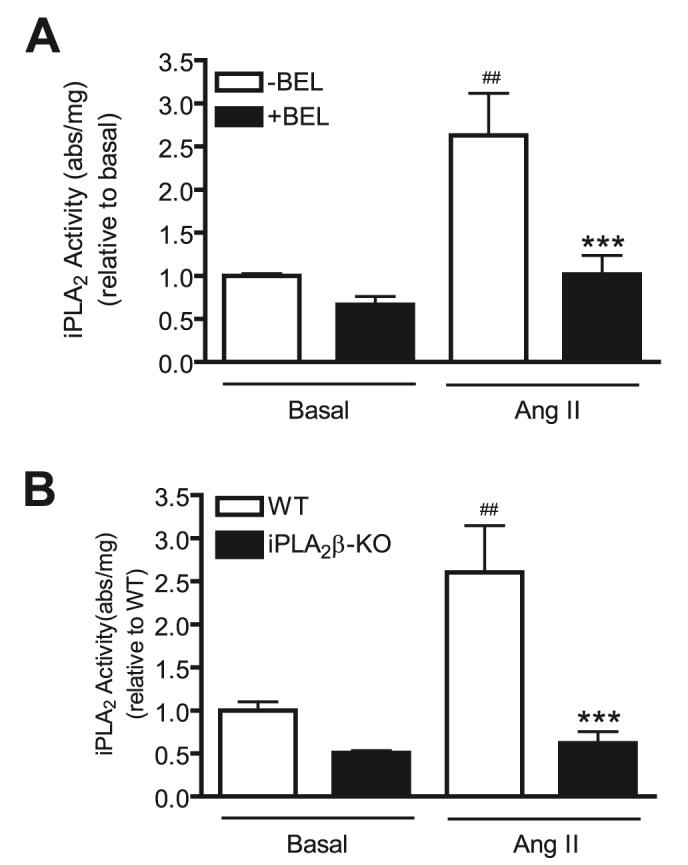
Prior to Ang II stimulation (100 nm, 1 h), wild-type cells (A), but not iPLA2β-null cells (B), were pretreated with BEL (3 μm, 30 min). Equal amounts of protein from basal and stimulated cells were assayed for iPLA2 enzymatic activity (in absorbance/mg of protein units) using a commercially available kit. The summary data are from at least three time independent experiments. ##, p < 0.01 versus basal; ***, p < 0.01 versus Ang II stimulation alone.
The assay used in Fig. 7A measures all Ca2+-independent phospholipase A2 activity, and BEL inhibits many iPLA2 family members. This leaves ambiguity with respect to whether iPLA2β or another iPLA2 enzyme accounts for the Ang II-induced increase in PLA2 activity observed in Fig. 7A. To address this issue, we performed similar studies with VSMC from wild-type and iPLA2β-null mice. Fig. 7B illustrates that Ang II stimulates iPLA2 activity in mouse VSMC, as it does in rat VSMC (Fig. 7A), but Ang II failed to stimulate iPLA2 activity in iPLA2β-null mouse VSMC. This suggests that the Ang II-induced increase in VSMC iPLA2 activity is attributable to iPLA2β. In principle, the increase in activity might be attributable to increased levels of iPLA2β protein or to an increase in the specific activity of existing iPLA2β protein. We therefore determined the effects of Ang II (100 nm, 1 h) on the level of iPLA2β immunoreactive protein in VSMC and observed no change in expression (data not shown).
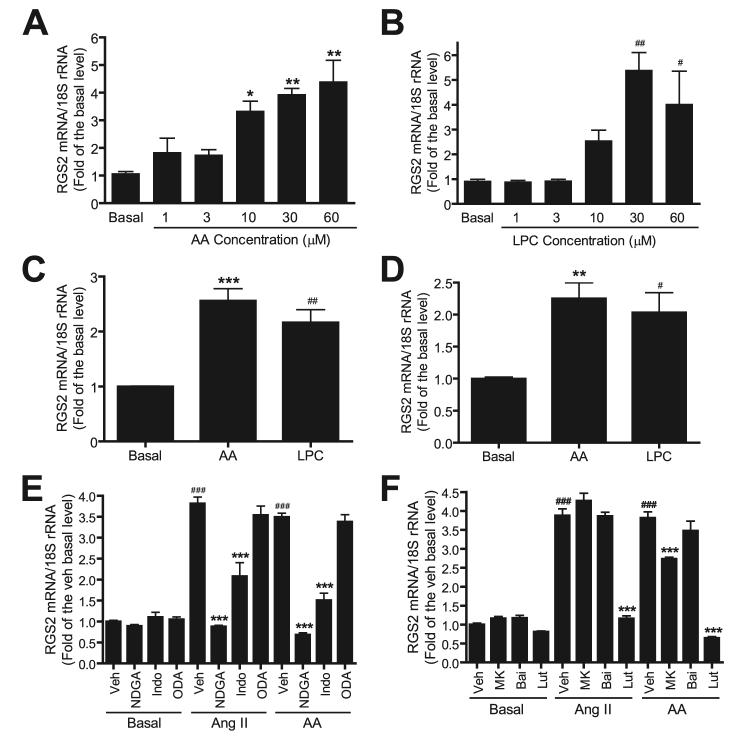
The products of iPLA2β action on phospholipid substrates are a free fatty acid, e.g. AA, and a 2-lysophospholipid, e.g. LPC. It is possible that one or both of these products or their metabolites mediate the participation of iPLA2β in Ang II signaling. We therefore determined the effects of incubating VSMC with exogenous AA (Fig. 8A) or LPC (Fig. 8B) on RGS2 mRNA expression levels, and we observed that both AA and LPC induced a concentration-dependent increase in RGS2 mRNA expression. This is compatible with the possibility that the participation of iPLA2β in Ang II signaling in VSMC involves enzymatic activation and accumulation of its reaction products.
FIGURE 8. Free AA, LPC, and AA metabolite are involved in Ang II-induced RGS2 mRNA expression in VSMC.
Quiescent rat VSMC were incubated for 1 h with different concentrations of AA (A) or LPC (B). Quiescent mouse wild-type (C) or iPLA2β-null VSMC (D) were stimulated with AA (30 μm) or LPC (30 μm) for 1 h. E and F, quiescent rat VSMC were pretreated with vehicle (Veh, Me2SO), nordihydroguaiaretic acid (NDGA, 30 μm) (60, 61), indomethacin (Indo, 50 μm) (61, 62), or 17-octadecynoic acid (ODA, 10 μm) (63), MK (MK886, 1 μm) (61), baicalein (Bai,10 μm) (62), or luteolin (Lut,50 μm) (64), respectively, prior to stimulation by Ang II (100 nm)or AA (30 μm) for 1 h. The RGS2 mRNA expression was determined by real-time PCR. Shown in the figure is a summary of three independent experiments. * and #, p < 0.05; and ** and ##, p < 0.01 versus basal. ***, p < 0.001 versus basal/Veh, and ###, p < 0.001 versus Ang II/Veh or AA/Veh.
To further test this possibility, we determined whether AA or LPC stimulates RGS2 mRNA up-regulation in the iPLA2β-null cells, and we observed that both AA and LPC were capable of inducing RGS2 mRNA up-regulation in iPLA2β-null cells (Fig. 8D), just as they stimulated RGS2 mRNA up-regulation in wild-type cells (Fig. 8C). Importantly, although the basal and Ang II-stimulated RGS2 mRNA expression levels are significantly lower in the iPLA2β-null cells than in wild-type cells (Figs. 3 and 6), the AA- or LPC-stimulated fold-increase in RGS2 mRNA expression between wild-type and the iPLA2β-null cells are quite similar, suggesting that the signaling pathway that links the iPLA2β and RGS2 mRNA up transcriptional regulation in the iPLA2β-null cells is unimpaired.
15-Lipoxygenases Is Downstream of iPLA2β Activation in Ang II-induced RGS2 mRNA Up-regulation
AA is metabolized to a variety of biologically active eicosanoids via lipoxygenases, cyclooxygenases, and cytochrome P450-dependent epoxygenases (59). To determine whether AA metabolites are involved in Ang II-induced RGS2 mRNA up-regulation in VSMC, cells were incubated with the lipoxygenase inhibitor nordihydroguaiaretic acid, the cyclooxygenase inhibitor indomethacin, or the cytochrome P450-dependent epoxygenases inhibitor 17-octadecynoic acid, respectively, prior to Ang II or AA stimulation. Interestingly, we found that nordihydroguaiaretic acid (30 μm) (60, 61) completely and indomethacin (50 μm) (61, 62) partially, but significantly, inhibited Ang II or AA-stimulated RGS2 mRNA up-regulation, whereas 17-octadecynoic acid (10 μm) (63) had no inhibitory effect (Fig. 8E). These results suggest that AA metabolites, in particular lipoxygenase-mediated products, are primarily responsible for Ang II- and AA-induced RGS2 mRNA up-regulation in VSMC.
The three major types of lipoxygenase enzymes in VSMC are 5-, 12-, and 15-lipoxygenases. To determine which of these lipoxygenase enzymes is involved in Ang II-induced RGS2 mRNA up-regulation, VSMC were pretreated with MK886 (a selective 5-lipoxygenase inhibitor), Bai (baicalein, a selective 12-lipoxygenase inhibitor), or Lut (luteolin, a selective 15-lipoxygenase inhibitor). We observed that luteolin (50 μm, (64)) abolished Ang II or AA-stimulated RGS2 mRNA up-regulation. In contrast, baicalein (10 μm) (62) had no effect, and MK886 (1 μm) (61) exerted only slight inhibition (Fig. 8F). These results indicate that 15-lipoxygenase plays a major role in Ang II-induced RGS2 mRNA up-regulation in VSMC.
Up-regulation of RGS2 by Ang II/iPLA2β Plays an Important Role in Ang II-induced VASP and Akt Phosphorylation
To address the functional significance of up-regulation of RGS2 mRNA by the Ang II/iPLA2β pathway, we first investigated whether the RGS2 protein, similar to RGS2 mRNA, was also up-regulated by Ang II. Using siRNA that specifically knocks down RGS2, we demonstrated that the anti-RGS2 antibody is specific (Fig. 9A). Using this RGS2 antibody, consistent with the recent report (3), we show that Ang II was able to stimulate RGS2 protein expression. Importantly, when cells were pre-treated with BEL, Ang II-stimulated RGS2 protein up-regulation was inhibited in a dose-dependent manner, but basal RGS2 protein expression was not affected (Fig. 9, B and C).
FIGURE 9. BEL dose-dependently inhibits Ang II-induced RGS2 protein up-regulation in VSMC.
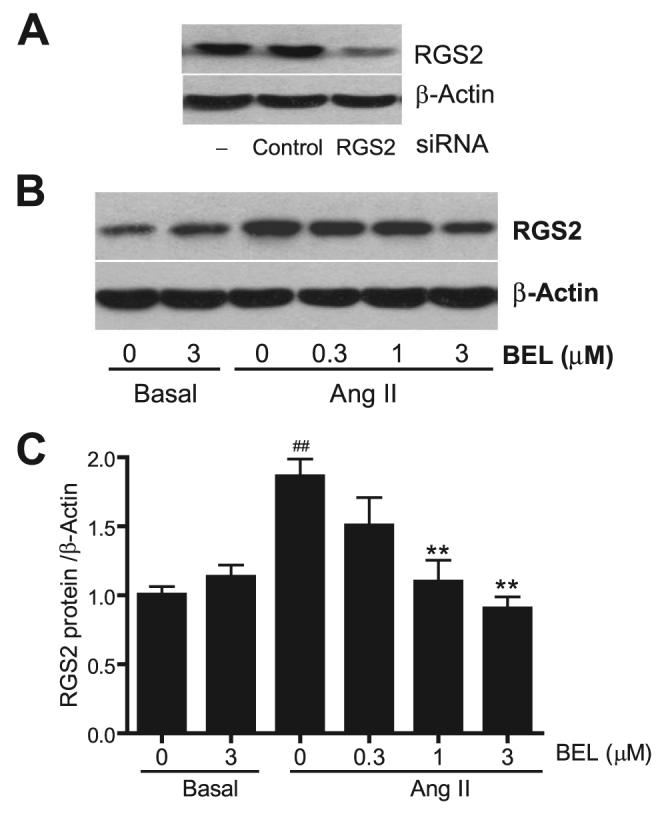
A, a representative Western blot. Cells were transfected with 20 nm RGS2 siRNA, control siRNA, or Lipofectamine 2000 only. RGS2 and β-actin protein expressions were blotted by anti-RGS2 and β-actin antibodies, respectively. B and C, quiescent VSMC were incubated with different concentrations of BEL or vehicle (Me2SO) for 30 min, respectively, before Ang II stimulation (100 nm Ang II, 2 h). RGS2 protein expression was determined by Western blot using an anti-RGS2 antibody and normalized to β-actin and then to the basal level. B, A representative RGS2 Western blot. The summary data are from at least four time independent experiments (C). ##, p < 0.01 versus basal; **, p < 0.01 versus Ang II stimulation alone.
To directly address whether disrupting the Ang II/iPLA2β/RGS2 pathway has a functional impact on Ang II signaling, we determined whether Ang II-induced VASP phosphorylation was affected by genetic iPLA2β ablation. We determined the time course of Ang II-induced VASP phosphorylation in wild-type and iPLA2β-null cells (Fig. 10, A and B) and found that Ang II (100 nm, 5 min) potently induced VASP phosphorylation in both wild-type and iPLA2β-null cells to a similar extent, as illustrated in Fig. 5. Interestingly, Ang II-induced VASP phosphorylation is transient, presumably because Ang II initiates a negative feedback pathway (e.g. RGS2 up-regulation) that causes a decrease in VASP phosphorylation over time. Indeed, we found that, in iPLA2β-null cells, Ang II-induced VASP phosphorylation was partially, but significantly, restored over time and was more sustained than in wild-type cells. Because the Ang II/iPLA2β/RGS2 feedback pathway was disrupted in iPLA2β-null cells, these data strongly argue that the Ang II/iPLA2β/RGS2 feedback pathway contributes to regulation of Ang II-induced VASP phosphorylation in VSMC.
FIGURE 10. Genetic deletion of iPLA2β partially, but significantly, potentiates Ang II-induced VASP and Akt phosphorylation in a time-dependent manner.
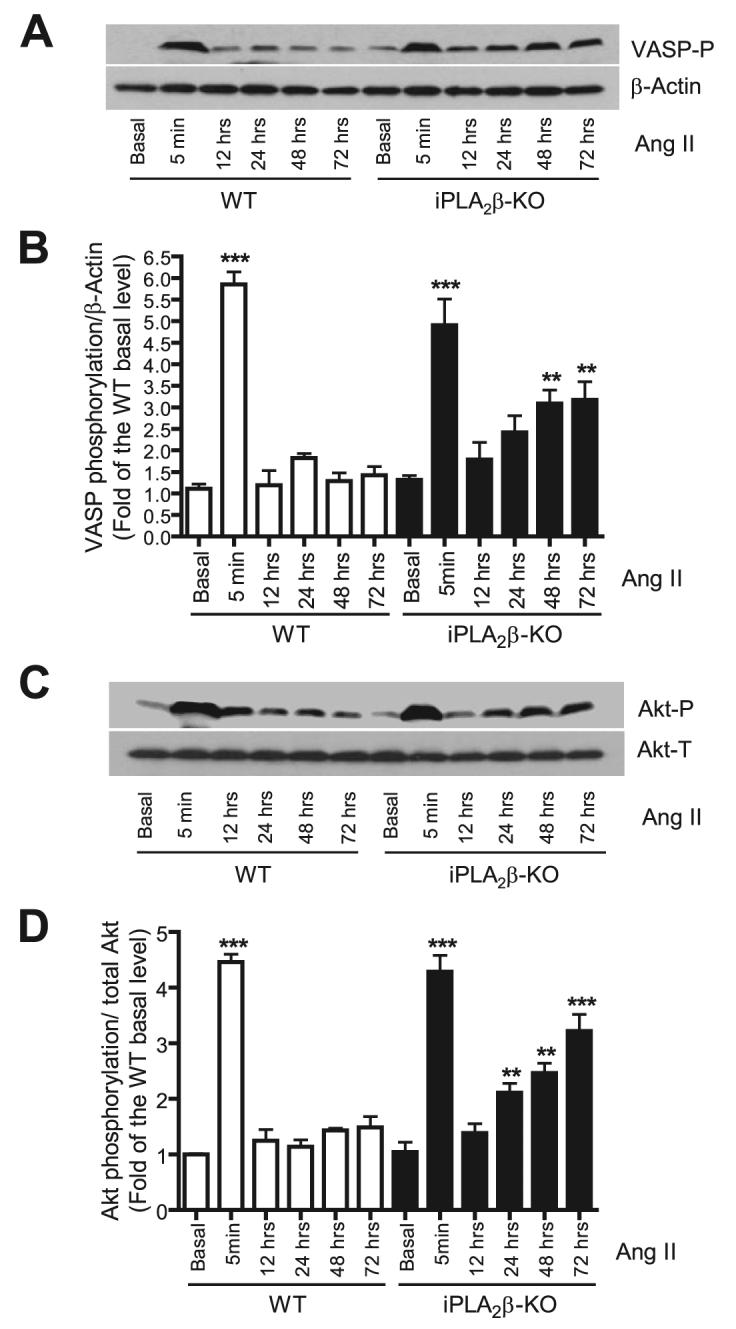
Quiescent mouse WT and iPLA2β-null VSMC were stimulated with Ang II (100 nm) for the indicated intervals. VASP phosphorylation levels (VASP-P, A and B) were determined with anti-VASP Ser-157 phospho-specific antibody and normalized to β-actin and then to the WT basal level. Akt phosphorylation levels (C and D) were determined with an anti-Akt Ser-473 phospho-specific antibody. The phosphorylation level was normalized to the total Akt (Akt-T) level and then to the WT basal level. The representative Western blots (A and C) are from at least three independent experiments and quantitative data are summarized in B and D. **, p < 0.01; ***, p < 0.001 versus WT basal. KO, knock-out.
In addition, we also determined whether Ang II-induced Akt phosphorylation was affected by iPLA2β ablation because Akt phosphorylation is negatively regulated by RGS2 (28). Our data indicate that genetic deletion of iPLA2β potentiates Ang II-induced Akt phosphorylation in a manner reminiscent of our findings on VASP phosphorylation (Fig. 10, C and D). Taken together, these data suggest that the Ang II/iPLA2β/RGS2 feedback pathway plays an important role in modulation of Ang II signaling.
DISCUSSION
These studies demonstrate that iPLA2β participates in the signaling pathway whereby Ang II induces an increase in RGS2 mRNA and protein expression in VSMC. Rgs2-deficient mice exhibit severe hypertension (6 - 8), and genetic variations in the RGS2 gene have been observed in hypertensive human subjects (9, 10). It is well established that Ang II induces up-regulation of RGS2 mRNA in VSMC (13), and this is considered to be an important negative feedback mechanism to constrain stimulatory effects of Ang II on VSMC. Loss of this negative feedback mechanism by Rgs2-deficient mice results in hypertension and other disorders. The results of our study suggest that iPLA2β is an obligatory participant in some pathways regulating RGS2 expression.
There are several iPLA2β isoforms that arise from alternative splicing and proteolytic processing events, and these gene products have been implicated in various biologic processes, including membrane phospholipid remodeling, cell proliferation and differentiation, apoptosis, transcriptional regulation, calcium homeostasis, insulin secretion, cardiac ischemia, eicosanoid synthesis, smooth muscle contraction, preadipocyte spreading, membrane traffic, and male fertility (33-35, 65). In particular, iPLA2β-null mice exhibit defects in male fertility (41), macrophage signaling (42), and insulin secretion by pancreatic islets (43).
Our studies demonstrate another signaling role of iPLA2β in Ang II signaling in VSMC using the independent approaches of pharmacologic inhibition, molecular biologic suppression of expression, and genetic deletion of iPLA2β. The signaling defect induced by reduction or elimination of iPLA2β activity in VSMC reflects a failure of a pathway for transcriptional regulation of RGS2 mRNA expression by Ang II G-protein-coupled receptors. Peritoneal macrophages from iPLA2β-null mice exhibit an analogous defect in a pathway of transcriptional regulation of inducible nitric-oxide synthase expression (42).
There are at least seven iPLA2 family members (α,β, γ, δ, ε, ξ, and η) (32), and both iPLA2β and iPLA2γ are expressed in VSMC (49, 53, 66). Interestingly, studies with BEL enantiomers that distinguish between iPLA2β and iPLA2γ indicate that the former but not the latter enzyme catalyzes vasopressin-induced AA release in A10 vascular smooth cells (53). These BEL enantiomers and cells from iPLA2β-null mice were also used in recent studies that demonstrate that iPLA2β and not iPLA2γ participates in the signaling pathway whereby encephalomyocarditis virus induces an increase in macrophage nitric-oxide synthase expression and nitric oxide production (42).
We have obtained analogous findings in our studies that demonstrate that suppression of iPLA2β expression with an antisense oligonucleotide significantly attenuated the Ang II-induced increase in VSMC RGS2 mRNA expression (Fig. 2). We also found that iPLA2β-null VSMC fail to exhibit the increases in iPLA2β activity and in RGS2 mRNA expression in response to Ang II that are observed with wild-type VSMC (Fig. 3). It is not yet known whether compensatory changes in expression of iPLA2γ or other phospholipases occur in iPLA2β-null cells, but our findings establish that any such changes cannot functionally compensate for the loss of iPLA2β in the signaling pathway through which Ang II causes increased RGS2 mRNA expression in VSMC, although this response is restored by adenovirus-mediated restoration of iPLA2β expression.
One unanticipated finding of the current studies is that the basal RGS2 mRNA level is significantly lower in the iPLA2β-null VSMC than in wild-type cells (Figs. 3C and 6B). This suggests that iPLA2β is required for maintaining basal RGS2 transcription. Interestingly, inhibition of iPLA2 with BEL (Fig. 1) or down-regulation of iPLA2β with antisense oligonucleotide (Fig. 2) had no effect on basal RGS2 mRNA levels. The different effects on basal RGS2 mRNA levels by genetic iPLA2β deletion versus BEL/oligonucleotide inhibition may reflect the possibility that only a low iPLA2β level is required and sufficient for maintaining basal RGS2 mRNA levels. Some iPLA2β activity persists, albeit at a reduced level, in VSMS treated with anti-sense oligonucleotides or BEL, and perhaps this residual iPLA2β activity is sufficient to support basal RGS2 mRNA expression. In contrast, no iPLA2β expression occurs in VSMC derived from iPLA2β-null mice, and some iPLA2β activity might be required for basal RGS2 mRNA expression to occur.
Alternatively, the different effects on basal RGS2 mRNA levels produced by different approaches of reducing iPLA2β expression or activity might reflect an effect of endogenous Ang II levels of maintaining basal RGS2 mRNA expression, and ongoing studies are designed to compare Ang II levels in wild-type and iPLA2β-null mice in various (patho)physiologic settings.
It is well established that Ang II induces AA release from VSMC (67, 68). Although it has previously been suggested that iPLA2 might catalyze Ang II-induced AA release from VSMC (67), our study is the first of which we are aware to demonstrate an increase in iPLA2 activity measured under standard assay conditions (47-49) in VSMC stimulated with Ang II (Fig. 7). This increase in activity was prevented by pretreating wild-type VSMC with the iPLA2 suicide substrate BEL, and the increase did not occur in iPLA2β-null VSMC. These observations indicate that the Ang II-induced increase in iPLA2 activity in wild-type VSMC is attributable to activation of iPLA2β rather than to another iPLA2 family member.
Free fatty acids, e.g. AA, and 2-lysophospholipids, e.g. LPC, are produced by iPLA2β action on phospholipid substrates, and these molecules and their metabolites have established messenger functions in various settings. We found that incubating VSMC with either AA or LPC results in a concentration-dependent increase in RGS2 mRNA expression (Fig. 8, A and B). Moreover, we found that incubating VSMC with either AA or LPC could stimulate RGS2 mRNA independent of iPLA2β (Fig. 8, C and D). It is of interest that LPC has been demonstrated to stimulate protein kinase A activity and CREB (cAMP response element-binding protein) activation by phosphorylation in myocardium (69, 70) and macrophages (42, 71). These observations suggest the possibilities that the participation of iPLA2β in Ang II signaling might involve the sequence of enzymatic activation, production of LPC, activation of PKA, CREB phosphorylation, transcriptional activation, and increased expression of RGS2 mRNA. Ongoing studies are designed to investigate these possibilities.
The effects of AA on RGS2 mRNA expression might be direct or mediated by metabolites. Our findings indicate that inhibition of lipoxygenase or cyclooxygenase, but not cyto-chrome P450-dependent epoxygenases, attenuated Ang II- or AA-induced RGS2 mRNA expression (Fig. 8E). Moreover, our findings indicate that, among lipoxygenase enzymes, 15-lipoxygenase plays a major role in Ang II- or AA-induced RGS2 mRNA expression (Fig. 8F). 15-Lipoxygenase has also been implicated in the action of Ang II to activate Syk, Ras, p38 MAPK, and CREB (12, 38, 62, 72). Activation of such pathways by Ang II could account for Ang II-induced RGS2 mRNA expression because these pathways participate in transcriptional regulation. Alternatively, it is possible that activation of iPLA2β/AA/cyclooxygenase/PGF2α is responsible for Ang II-induced RGS2 mRNA up-regulation because PGF2α potently stimulates RGS2 mRNA expression. It should nonetheless be emphasized that potential off-target effects of pharmacological inhibitors necessitate future investigation of these possibilities by molecular and genetic approaches.
The fact that a large number of biologic events have been reported to be influenced by AA, LPC, or their metabolites also raises the possibility that activation of iPLA2β by Ang II could affect multiple downstream signaling pathways that might include both positive and negative regulatory arms. This could complicate interpretation of experiments aimed at determining the functional significance of the Ang II/iPLA2β/RGS2 signaling pathway in VSMC. It is, however, well established that the hypertension that develops in Rgs2-null mice is dependent on Ang II signaling (8), and this indicates that the Ang II/RGS2 signaling pathway is an important determinant of blood pressure homeostasis in vivo. This in turn suggests that the participation of iPLA2β in this pathway demonstrated here has important (patho)physiologic implications that merit further examination.
Consistent with its in vivo significance, our findings indicate that, in cultured VSMC, RGS2 protein is also significantly up-regulated by Ang II, to an extent similar to that of its transcript, and these effects are attenuated by inhibiting iPLA2β activity with BEL (Fig. 9). Moreover, genetic deletion of iPLA2β potentiates Ang II-induced VASP and Akt phosphorylation in a time-dependent manner (Fig. 10), and loss of the Ang II/iPLA2β/RGS2 negative feedback pathway in iPLA2β-null cells probably contributes to or accounts for this phenomenon. Because the transience of Ang II-induced phosphorylation of VASP and Akt might reflect a decline in kinase activity or to an increase in phosphatase activity, the Ang II/iPLA2β/RGS2 negative feedback pathway might affect either kinases or phosphatases that regulate the phosphorylation state of VASP and Akt. Further studies are required to investigate these possibilities.
In summary, our studies provide multiple lines of evidence that demonstrate that iPLA2β is an obligatory participant in the signaling pathway whereby Ang II causes transcriptional activation of the Rgs2 gene and increased expression of RGS2 mRNA and protein in VSMC. These findings provide new detail to the understanding of signal transduction cascades initiated by agonist binding of G-protein-coupled receptors and suggest that iPLA2β might be a fruitful target for development of strategies for prevention and treatment of cardiovascular diseases.
Acknowledgment
We thank the Cardiovascular Research Group of the University of Kentucky for invaluable advice and assistance.
Footnotes
This work was supported by a Scientist Development Grant from the American Heart Association (to Z. G.), National Institutes of Health Grants HL67284 and HL082791, and Career Development Award 1-04-CD-04 from American Diabetes Association (to M. G.).
The abbreviations used are: RGS2, regulator of G-protein signaling-2; Ang II, angiotensin II; VSMC, vascular smooth muscle cells; PLA2, phospholipase A2; iPLA2β, calcium-independent phospholipase A2β; AA, arachidonic acid; LPC, lysophosphatidylcholine; BEL, bromoenol lactone; CREB, cAMP response element-binding protein; sPLA2, secretory phospholipase A2; cPLA2, cytosolic phospholipase A2; PGF2α, prostaglandin F2α; VASP, vasodilator-stimulated phosphoprotein; WT, wild type; siRNA, small interfering RNA; DOX, doxycycline.
REFERENCES
- 1.Wieland T, Chen CK. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:14–26. doi: 10.1007/s002109900031. [DOI] [PubMed] [Google Scholar]
- 2.Heximer SP, Srinivasa SP, Bernstein LS, Bernard JL, Linder ME, Hepler JR, Blumer KJ. J. Biol. Chem. 1999;274:34253–34259. doi: 10.1074/jbc.274.48.34253. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Hashim S, Anand-Srivastava MB. Cardiovasc. Res. 2005;66:503–511. doi: 10.1016/j.cardiores.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Sinnarajah S, Dessauer CW, Srikumar D, Chen J, Yuen J, Yilma S, Dennis JC, Morrison EE, Vodyanoy V, Kehrl JH. Nature. 2001;409:1051–1055. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- 5.Kehrl JH, Sinnarajah S. Int. J. Biochem. Cell Biol. 2002;34:432–438. doi: 10.1016/s1357-2725(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 6.Tang M, Wang G, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, Mendelsohn ME. Nat. Med. 2003;9:1506–1512. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. Mol. Pharmacol. 2005;67:631–639. doi: 10.1124/mol.104.007724. [DOI] [PubMed] [Google Scholar]
- 8.Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, Oliveira-dos-Santos A, Penninger JM, Muslin AJ, Steinberg TH, Wyss JM, Mecham RP, Blumer KJ. J. Clin. Investig. 2003;111:445–452. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Kamide K, Kokubo Y, Takiuchi S, Tanaka C, Banno M, Miwa Y, Yoshii M, Horio T, Okayama A, Tomoike H, Kawano Y, Miyata T. J. Hypertens. 2005;23:1497–1505. doi: 10.1097/01.hjh.0000174606.41651.ae. [DOI] [PubMed] [Google Scholar]
- 10.Riddle EL, Rana BK, Murthy KK, Rao F, Eskin E, O'Connor DT, Insel PA. Hypertension. 2006;47:415–420. doi: 10.1161/01.HYP.0000200714.81990.61. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham ML, Waldo GL, Hollinger S, Hepler JR, Harden TK. J. Biol. Chem. 2001;276:5438–5444. doi: 10.1074/jbc.M007699200. [DOI] [PubMed] [Google Scholar]
- 12.Dronadula N, Rizvi F, Blaskova E, Li Q, Rao GN. J. Lipid Res. 2006;47:767–777. doi: 10.1194/jlr.M500369-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Grant SL, Lassegue B, Griendling KK, Ushio-Fukai M, Lyons PR, Alexander RW. Mol. Pharmacol. 2000;57:460–467. doi: 10.1124/mol.57.3.460. [DOI] [PubMed] [Google Scholar]
- 14.Cho H, Harrison K, Schwartz O, Kehrl JH. Biochem. J. 2003;371:973–980. doi: 10.1042/BJ20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miles RR, Sluka JP, Santerre RF, Hale LV, Bloem L, Boguslawski G, Thirunavukkarasu K, Hock JM, Onyia JE. Endocrinology. 2000;141:28–36. doi: 10.1210/endo.141.1.7229. [DOI] [PubMed] [Google Scholar]
- 16.Reif K, Cyster JG. J. Immunol. 2000;164:4720–4729. doi: 10.4049/jimmunol.164.9.4720. [DOI] [PubMed] [Google Scholar]
- 17.Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, Barnes CA, Lanahan AA, Siderovski DP, Ross EM, Gilman AG, Worley PF. J. Neurosci. 1998;18:7178–7188. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burchett SA, Bannon MJ, Granneman JG. J. Neurochem. 1999;72:1529–1533. doi: 10.1046/j.1471-4159.1999.721529.x. [DOI] [PubMed] [Google Scholar]
- 19.Beadling C, Druey KM, Richter G, Kehrl JH, Smith KA. J. Immunol. 1999;162:2677–2682. [PubMed] [Google Scholar]
- 20.Song L, De Sarno P, Jope RS. J. Biol. Chem. 1999;274:29689–29693. doi: 10.1074/jbc.274.42.29689. [DOI] [PubMed] [Google Scholar]
- 21.Zmijewski JW, Song L, Harkins L, Cobbs CS, Jope RS. Biochim. Biophys. Acta. 2001;1541:201–211. doi: 10.1016/s0167-4889(01)00144-6. [DOI] [PubMed] [Google Scholar]
- 22.Wilson LD, Ross SA, Lepore DA, Wada T, Penninger JM, Thomas PQ. Gene Expr. Patterns. 2005;5:305–311. doi: 10.1016/j.modgep.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Calo LA, Pagnin E, Davis PA, Sartori M, Ceolotto G, Pessina AC, Semplicini A. J. Clin. Endocrinol. Metab. 2004;89:4153–4157. doi: 10.1210/jc.2004-0498. [DOI] [PubMed] [Google Scholar]
- 24.Eszlinger M, Holzapfel HP, Voigt C, Arkenau C, Paschke R. Eur. J. Endocrinol. 2004;151:383–390. doi: 10.1530/eje.0.1510383. [DOI] [PubMed] [Google Scholar]
- 25.Taymans JM, Kia HK, Claes R, Cruz C, Leysen J, Langlois X. Eur. J. Neurosci. 2004;19:2249–2260. doi: 10.1111/j.0953-816X.2004.03336.x. [DOI] [PubMed] [Google Scholar]
- 26.Gan XT, Rajapurohitam V, Haist JV, Chidiac P, Cook MA, Karmazyn M. J. Pharmacol. Exp. Ther. 2005;312:27–34. doi: 10.1124/jpet.104.073122. [DOI] [PubMed] [Google Scholar]
- 27.Kveberg L, Ryan JC, Rolstad B, Inngjerdingen M. Immunology. 2005;115:358–365. doi: 10.1111/j.1365-2567.2005.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwable J, Choudhary C, Thiede C, Tickenbrock L, Sargin B, Steur C, Rehage M, Rudat A, Brandts C, Berdel WE, Muller-Tidow C, Serve H. Blood. 2005;105:2107–2114. doi: 10.1182/blood-2004-03-0940. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. J. Biol. Chem. 2002;277:24949–24958. doi: 10.1074/jbc.M203802200. [DOI] [PubMed] [Google Scholar]
- 30.Touyz RM. Curr. Hypertens. Rep. 2003;5:155–164. doi: 10.1007/s11906-003-0073-2. [DOI] [PubMed] [Google Scholar]
- 31.Dennis EA. J. Biol. Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 32.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. J. Biol. Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 33.Balsinde J, Balboa MA. Cell Signal. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, Turk J. Prog. Nucleic Acids Res. Mol. Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- 35.Akiba S, Sato T. Biol. Pharm. Bull. 2004;27:1174–1178. doi: 10.1248/bpb.27.1174. [DOI] [PubMed] [Google Scholar]
- 36.Lehman JJ, Brown KA, Ramanadham S, Turk J, Gross RW. J. Biol. Chem. 1993;268:20713–20716. [PubMed] [Google Scholar]
- 37.Jenkins CM, Wolf MJ, Mancuso DJ, Gross RW. J. Biol. Chem. 2001;276:7129–7135. doi: 10.1074/jbc.M010439200. [DOI] [PubMed] [Google Scholar]
- 38.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. J. Biol. Chem. 2002;277:9920–9928. doi: 10.1074/jbc.M111305200. [DOI] [PubMed] [Google Scholar]
- 39.Rao GN, Lassegue B, Griendling KK, Alexander RW. Oncogene. 1993;8:2759–2764. [PubMed] [Google Scholar]
- 40.Yamakawa T, Eguchi S, Yamakawa Y, Motley ED, Numaguchi K, Utsunomiya H, Inagami T. Hypertension. 1998;31:248–253. doi: 10.1161/01.hyp.31.1.248. [DOI] [PubMed] [Google Scholar]
- 41.Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. J. Biol. Chem. 2004;279:38194–38200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran JM, Buller RM, McHowat J, Turk J, Wohltmann M, Gross RW, Corbett JA. J. Biol. Chem. 2005;280:28162–28168. doi: 10.1074/jbc.M500013200. [DOI] [PubMed] [Google Scholar]
- 43.Bao S, Song H, Wohltmann M, Ramanadham S, Jin W, Bohrer A, Turk J. J. Biol. Chem. 2006;281:20958–20973. doi: 10.1074/jbc.M600075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pang H, Guo Z, Su W, Xie Z, Eto M, Gong MC. Am. J. Physiol. 2005;289:C352–C360. doi: 10.1152/ajpcell.00111.2005. [DOI] [PubMed] [Google Scholar]
- 45.Sakata Y, Xiang F, Chen Z, Kiriyama Y, Kamei CN, Simon DI, Chin MT. Arterioscler. Thromb. Vasc. Biol. 2004;24:2069–2074. doi: 10.1161/01.ATV.0000143936.77094.a4. [DOI] [PubMed] [Google Scholar]
- 46.Guo Z, Su W, Ma Z, Smith GM, Gong MC. J. Biol. Chem. 2003;278:1856–1863. doi: 10.1074/jbc.M211075200. [DOI] [PubMed] [Google Scholar]
- 47.Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. Nat. Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 48.Smani T, Zakharov SI, Leno E, Csutora P, Trepakova ES, Bolotina VM. J. Biol. Chem. 2003;278:11909–11915. doi: 10.1074/jbc.M210878200. [DOI] [PubMed] [Google Scholar]
- 49.Yellaturu CR, Rao GN. J. Biol. Chem. 2003;278:43831–43837. doi: 10.1074/jbc.M301472200. [DOI] [PubMed] [Google Scholar]
- 50.Balsinde J, Balboa MA, Dennis EA. J. Biol. Chem. 1997;272:29317–29321. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- 51.Xie Z, Su W, Guo Z, Pang H, Post SR, Gong MC. Cardiovasc. Res. 2006;69:491–501. doi: 10.1016/j.cardiores.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Balsinde J, Balboa MA, Insel PA, Dennis EA. Annu. Rev. Pharmacol. Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins CM, Han X, Mancuso DJ, Gross RW. J. Biol. Chem. 2002;277:32807–32814. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- 54.Balsinde J, Dennis EA. J. Biol. Chem. 1996;271:31937–31941. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- 55.Akiba S, Mizunaga S, Kume K, Hayama M, Sato T. J. Biol. Chem. 1999;274:19906–19912. doi: 10.1074/jbc.274.28.19906. [DOI] [PubMed] [Google Scholar]
- 56.Roshak AK, Capper EA, Stevenson C, Eichman C, Marshall LA. J. Biol. Chem. 2000;275:35692–35698. doi: 10.1074/jbc.M002273200. [DOI] [PubMed] [Google Scholar]
- 57.Fuentes L, Perez R, Nieto ML, Balsinde J, Balboa MA. J. Biol. Chem. 2003;278:44683–44690. doi: 10.1074/jbc.M307209200. [DOI] [PubMed] [Google Scholar]
- 58.Dulin NO, Niu J, Browning DD, Ye RD, Voyno-Yasenetskaya T. J. Biol. Chem. 2001;276:20827–20830. doi: 10.1074/jbc.C100195200. [DOI] [PubMed] [Google Scholar]
- 59.Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Annu. Rev. Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- 60.Turk J, Colca JR, McDaniel ML. Biochim. Biophys. Acta. 1985;834:23–36. doi: 10.1016/0005-2760(85)90172-9. [DOI] [PubMed] [Google Scholar]
- 61.Alzoghaibi MA, Walsh SW, Willey A, Yager DR, Fowler AA, 3rd, Graham MF. Am. J. Physiol. 2004;286:G528–G537. doi: 10.1152/ajpgi.00189.2003. [DOI] [PubMed] [Google Scholar]
- 62.Yaghini FA, Li F, Malik KU. J. Biol. Chem. 2007;282:16878–16890. doi: 10.1074/jbc.M610494200. [DOI] [PubMed] [Google Scholar]
- 63.Fatima S, Khandekar Z, Parmentier JH, Malik KU. J. Pharmacol. Exp. Ther. 2001;298:331–338. [PubMed] [Google Scholar]
- 64.Kim JH, Jin YR, Park BS, Kim TJ, Kim SY, Lim Y, Hong JT, Yoo HS, Yun YP. Biochem. Pharmacol. 2005;69:1715–1721. doi: 10.1016/j.bcp.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Balsinde J, Dennis EA. J. Biol. Chem. 1997;272:16069–16072. doi: 10.1074/jbc.272.26.16069. [DOI] [PubMed] [Google Scholar]
- 66.Larsson Forsell PK, Kennedy BP, Claesson HE. Eur. J. Biochem. 1999;262:575–585. doi: 10.1046/j.1432-1327.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- 67.Freeman EJ, Ruehr ML, Dorman RV. Am. J. Physiol. 1998;274:C282–C288. doi: 10.1152/ajpcell.1998.274.1.C282. [DOI] [PubMed] [Google Scholar]
- 68.Rao GN, Lassegue B, Alexander RW, Griendling KK. Biochem. J. 1994;299:197–201. doi: 10.1042/bj2990197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williams SD, Ford DA. FEBS Lett. 1997;420:33–38. doi: 10.1016/s0014-5793(97)01482-8. [DOI] [PubMed] [Google Scholar]
- 70.Williams SD, Ford DA. Am. J. Physiol. 2001;281:H168–H176. doi: 10.1152/ajpheart.2001.281.1.H168. [DOI] [PubMed] [Google Scholar]
- 71.Maggi LB, Jr., Moran JM, Scarim AL, Ford DA, Yoon JW, McHowat J, Buller RM, Corbett JA. J. Biol. Chem. 2002;277:38449–38455. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 72.Kalyankrishna S, Malik KU. J. Pharmacol. Exp. Ther. 2003;304:761–772. doi: 10.1124/jpet.102.040949. [DOI] [PubMed] [Google Scholar]