Abstract
Bone marrow-derived mesenchymal stem cells (MSCs) have been reported to prevent the development of liver fibrosis in a number of pre-clinical studies. Marked changes in liver histopathology and serological markers of liver function have been observed without a clear understanding of the therapeutic mechanism by which stem cells act. We sought to determine if MSCs could modulate the activity of resident liver cells, specifically hepatic stellate cells (SCs) by paracrine mechanisms using indirect cocultures. Indirect coculture of MSCs and activated SCs led to a significant decrease in collagen deposition and proliferation, while inducing apoptosis of activated SCs. The molecular mechanisms underlying the modulation of SC activity by MSCs were examined. IL-6 secretion from activated SCs induced IL-10 secretion from MSCs, suggesting a dynamic response of MSCs to the SCs in the microenvironment. Blockade of MSC-derived IL-10 and TNF-α abolished the inhibitory effects of MSCs on SC proliferation and collagen synthesis. In addition, release of HGF by MSCs was responsible for the marked induction of apoptosis in SCs as determined by antibody-neutralization studies. These findings demonstrate that MSCs can modulate the function of activated SCs via paracrine mechanisms provide a plausible explanation for the protective role of MSCs in liver inflammation and fibrosis, which may also be relevant to other models of tissue fibrosis.
Keywords: stem cell, fibrosis, immunomodulation, liver injury
INTRODUCTION
Liver fibrosis, the precursor to cirrhosis, is the result of an imbalance in extracellular matrix (ECM) synthesis and degradation mediated primarily by activated hepatic stellate cells (SCs). Following liver injury, SCs undergo a phenotypic switch from a quiescent, vitamin-A storing cell into a proliferative, α-smooth muscle actin (SMA) positive, myofibroblast-like cell, which shows an upregulation in collagen synthesis [1]. In vivo activation of SCs is divided into a fibrogenic and hyperplastic response [2] that is mediated by many autocrine and paracrine signals. Spontaneous resolution of liver fibrosis has been reported in different rat models of chronic liver injury [3, 4]. This resolution has been correlated with decreased synthesis of type I collagen and tissue inhibitor of matrix metalloproteinases (TIMP) 1 and 2 transcripts, with a concomitant decrease in the number of α-SMA positive SCs [4]. Yet, it remains unclear whether the decrease in the number of activated SCs is due to selective apoptosis [5, 6] or reversion to a quiescent state by microenvironmental cues [7-9].
A new technique in the treatment of inflammatory conditions involves the infusion of bone marrow-derived mesenchymal stem cells (MSCs). Recent studies have demonstrated that MSCs can be of therapeutic benefit in the prevention of fibrotic lesions, such as pulmonary fibrosis after bleomycin challenge [10], and in the protection of cardiac function after a myocardial infarction [11]. In particular, studies using MSCs for cellular cardiomyoplasty showed that paracrine factors produced by MSCs may contribute to their therapeutic benefit [11]. Systemic delivery of MSCs prior to, and during the induction of experimental liver fibrosis significantly inhibits changes in liver histology and clinical serum parameters [12-14], but the preventative mechanisms have yet to be elucidated.
Here, we demonstrate that MSCs indirectly modulate the activity of activated SCs in vitro via paracrine stimulation with specific cytokines and growth factors. Suppression of proliferation and collagen synthesis was mediated by MSC-derived interleukin (IL)-10 and tumor necrosis factor (TNF)-α. IL-10 secretion, in particular, was found to be a dynamic response to IL-6 secreted by activated SCs. In addition, secretion of HGF by MSCs led to the apoptotic death of activated SCs.
MATERIALS AND METHODS
Materials were purchased from Sigma-Aldrich, St. Louis, MO unless otherwise stated.
MSC isolation, ex vivo expansion and characterization
Human MSCs were isolated and cultured as previously reported [15]. The surface antigen profile as analyzed by flow cytometry (FACS Calibur, Becton Dickinson) was consistently CD14-, CD34-, CD45-, CD105+, CD106+ and CD44+. Cells were shown to have adipogenic and osteogenic differentiation potential (suppl. fig. 1) and were used during passages 4-7.
SC isolation and culture
Immortalized human SCs were derived as previously reported [16]. Primary rat SCs were isolated from 150-200 g female Lewis rats using a two-step step collagenase perfusion [17] followed by a Percoll density gradient separation as previously described [18]. SCs were activated by culturing them for 10-14 days on tissue culture plastic in DMEM supplemented with 10% FBS before use in experiments. Characterization by immunofluorescence for desmin and α-smooth muscle cell actin (SMA) revealed a purity of >96%.
Coculture Systems
For direct coculture of MSCs and SCs, cells were seeded at a 1:1 ratio in each well of a six-well plate (Corning Costar, Acton, MA). An indirect coculture system between SCs and MSCs was assembled using Transwell membranes (24 mm diameter, 0.4 μm pore size; Corning Costar, Acton, MA). Approximately 1.0 × 105 SCs were placed in the lower chamber with either 0-1.0 × 105 MSCs placed on the membrane insert. Cocultures were maintained in SC medium for 4 days.
Cytokine treatment, neutralization and protein quantification
Human MSCs were treated with IL-6 (2.5 ng/ml; R&D Systems, Minneapolis, MN), IL-1 (5 ng/ml; R&D Systems, Minneapolis, MN), or tumor necrosis factor-α (TNF-α; 25 ng/ml; R&D Systems, Minneapolis, MN) supplemented MSC expansion medium for 24 hours. MSCs cultured in expansion medium served as a negative control. After treatment, cells were harvested and analyzed for changes in gene expression.
Quantification of human TNF-α, IL-10 and rat IL-6 and HGF was determined using an ELISA as per vendor instructions (Endogen, Rockford, IL). Supernatants were sampled after 48 hours of coculture and stored at -20 °C until analysis.
Neutralization of specific cytokines was performed during indirect cocultures. For all neutralization experiments, the ratio of MSCs to SCs was 1:1. Anti-human IL-10 (BioLegend, San Diego, CA), TNF-α, (BioLegend, San Diego, CA), or HGF and anti-rat IL-6 (Cell Sciences, Canton, MA) were diluted in SC medium based on the half maximal inhibition concentrations given by the manufacturer. Fresh medium with neutralizing antibodies was added after 48 hours of coculture.
Total RNA isolation and RT-PCR
RNA was extracted from 0.1-1.0 × 106 MSCs using the Nucleospin RNA purification kit (BD Biosciences, Palo Alto, CA) per the manufacturer’s instructions. Approximately 1 μg of total mRNA was reverse transcribed to cDNA using the OneStep RT-PCR Kit (Qiagen, Valencia, CA) per manufacturer’s instructions and amplified in a Perkin Etus Thermal Cycler 480. Cycling conditions were: 1) 50 °C for 30 minutes; 2) 95 °C for 15 minutes ; 3) 30 cycles at 94 °C for 30 seconds, 55 °C for 30 seconds, and 72 °C for 1 minute; and 4) a final extension step at 72 °C for 10 minutes. Primers used for amplification were: IL-10 (364 bp) AAGCCTGACCACGCTTTCTA, GTAGAGCGGGGTTTCACCA; and GAPDH (238 bp) GAGTCAACGGATTTGGTCGT, TTGATTTTGGAGGGATCTCG.
Collagen synthesis
Collagen synthesis was quantified using an ELISA for procollagen type-I C-peptide (Takara-Bio Inc., Shiga, Japan). After the coculture period, the Transwell insert containing MSCs was removed and the medium on the SCs was replaced with fresh medium. Twenty-four hours later, medium was collected and procollagen type-I C-peptide concentration was measured by ELISA.
Proliferation assay
The proliferative capacity of SCs in culture was evaluated by measuring incorporation of 5-bromo-2′-deoxyuridine (BrdU) after incubation with 10 μM BrdU for 24 h. Cells were collected by trypsinization and fixed in 70% ethanol for 45 min at room temperature. Cells were subsequently incubated in 4 M HCl and a solution of 10% FBS in PBS for 15 min. After incubation with Alexa-Fluor 488 conjugated anti-BrdU antibody, cells were analyzed by flow cytometry.
Apoptosis Assay
Quantification of cell apoptosis/necrosis was determined using the Annexin-V Fluos kit (Roche, Indianapolis, IN) as per vendor instructions and analyzed by flow cytometry. Serum deprived SCs served as a positive control for apoptosis.
Statistical Analysis
Results were analyzed using the unpaired Student’s t-test. A p-value <.05 was considered statistically significant. Results are provided as mean ± s.e.m.
RESULTS & DISCUSSION
MSCs inhibit collagen synthesis in activated SCs
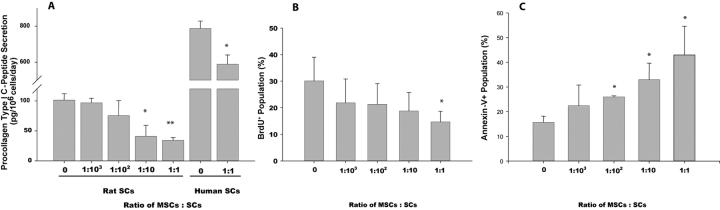
The reversal of experimental liver fibrosis in vivo has been highly correlated with a decrease in tissue collagen content [1]. A similar decrease in tissue collagen has been reported after MSC infusion into animals with fibrotic livers [13, 14] We evaluated whether MSCs can reduce the secretion of procollagen type I C-peptipe (PIP) in SCs. Levels of PIP-secretion by activated SCs (101 ± 11 pg/106 cells/day) were significantly reduced at a MSC:SC coculture ratio of 1:10 (41 ± 18 pg/106 cells/day; p=0.0491), with a 66% reduction at a 1:1 coculture ratio (34 ± 5 pg/106 cells/day; p=0.004; Fig. 1A). A 25% reduction of PIP-secretion was observed in the immortalized human SCs at a 1:1 coculture ratio (787 ± 40 to 588 ± 51 pg/106 cells/day; p=0.02). When cocultured with a control cell type no reduction in secretion of PIP was observed (Supl Fig. 2). These results suggest that soluble factors released by MSCs inhibit the synthesis of collagen by activated SCs.
Figure 1. Inhibited collagen synthesis and proliferation and increased apoptosis in SCs cocultured with MSCs.
Activated SCs were cultured with and without MSCs at increasing ratios, separated by Transwell membranes. Procollagen type-I C-peptide (PIP) secretion from rat and human SCs in Transwell coculture with MSCs was measured by ELISA (A). BrdU incorporation (B) and Annexin-V reactivity (C) were analyzed in rat SCs by flow cytometry. Data represent the mean of two experiments performed in triplicate. Error bars are standard deviation. *p<.05, **p<.01 compared to SCs alone (MSC:SC = 0).
MSCs inhibit proliferation and induce apoptosis in activated SCs
The decrease in hepatic fibrosis observed after transplantation of MSCs is accompanied by a reduction in the number of α-SMA+ (activated stellate) cells [12-14]. It is unclear whether this observation is due to a decrease in the proliferative capacity, increased apoptotic cell death or a reversion of activated SCs to a quiescent phenotype [1]. We examined whether MSC paracrine factors can reduce the number of activated SCs by any of these mechanisms.
At a 1:1 coculture ratio, the number of SCs entering the S-phase of the cell cycle, quantified by flow cytometric analysis of BrdU-incorporation, significantly decreased from 30 ± 9% to 15 ± 4% (Fig. 1B; p=0.043). No significant difference in proliferation could be detected at lower MSC:SC ratios. To determine whether MSCs also have the capacity to reduce activated SC numbers by inducing their apoptosis, we quantified the number of Annexin-V expressing cells. In SCs cultured alone, there was a basal level of apoptosis (16%, Fig. 1C) consistent with previous reports [19]. Coculture with MSCs at a 1:1 ratio increased apoptosis levels approximately 2.5-fold to 43% (Fig. 1C, p=0.0007). Coculture with fibroblasts resulted in a level of apoptosis that was similar to SCs alone (32%, suppl. Fig 3), demonstrating that the pro-apoptotic effect was MSC-specific. Significant SC death also occurred at MSC:SC coculture ratios of 1:100 and 1:10 (26%, p=0.009 and 33%, p=0.004 respectively, Fig. 1C). Flow cytometric analysis did not reveal a decrease in levels of α-SMA expression after coculture with MSCs (suppl. Fig. 4).
Taken together, these results suggest that MSCs have the capacity to inhibit proliferation and induce apoptosis of SCs through secreted factors, while SCs do not revert to a quiescent state. In the context of relatively low levels of MSC engraftment in vivo, a 1:1 ratio of MSCs to SCs may not be a realistic model for the differences seen in α-SMA+ cells in vivo. Induction of apoptosis, which was observed at low MSC:SC ratios, may therefore be a more important mechanism.
MSCs secrete IL-10 when stimulated by IL-6 and constitutively secrete TNF-α
In an effort to understand the molecular basis of the observed effects of MSCs on SCs, we evaluated whether MSCs secrete immunomodulatory molecules when exposed to cytokines known to be involved in liver fibrosis. Using species-specific reagents, MSCs were found to express and secrete increased levels of the anti-inflammatory cytokine IL-10 in response to IL-6 secreted by the SCs, suggesting a dynamic response of MSCs to a pro-inflammatory environment. In addition, MSCs were found to constitutively secrete TNF-α (suppl. Fig. 5A-E).
Paracrine stimulation with IL-10 and TNF-α from MSCs synergistically inhibits collagen synthesis and proliferation in SCs
Autocrine IL-10 stimulation in SCs has been shown in vitro to modulate the expression of collagen type I and favor a more quiescent state [8, 9]. However, expression of IL-10 is suppressed upon activation in vivo. TNF-α has also been observed to be an anti-proliferative/fibrogenic stimulus for activated SCs [20]. Thus, we determined the role of these expressed cytokines on proliferation and collagen synthesis of SCs.
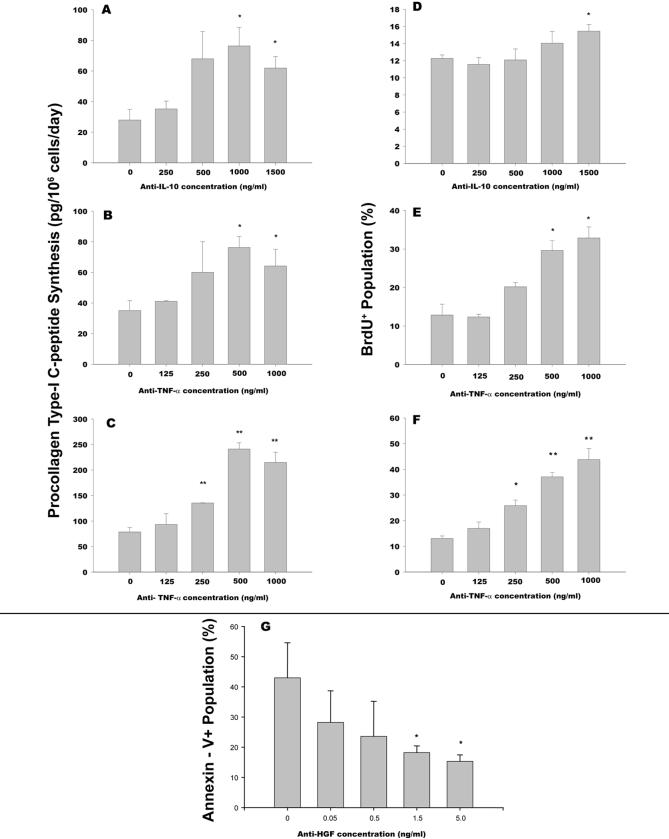
Partial reversal of high levels of PIP-secretion was observed after neutralization of IL-10 or TNF-α by antibody concentrations of 1000 ng/ml (Fig. 2A) and 500 ng/ml (Fig. 2B) or greater, respectively. Neutralization of both IL-10 and TNF-α in coculture led to a synergistic rise in PIP levels from 79 ± 9 pg/106 cells/day to 215 ± 20 pg/106 cells/day (p=0.002) at maximal antibody concentrations (Fig. 2C).
Figure 2. Reversal of inhibited collagen synthesis and proliferation in SCs cocultured with MSCs by neutralizing IL-10 and TNF-α and reversal of induced apoptosis by neutralizing HGF.
Activated stellate cells were cultured with MSCs separated by Transwell membranes at a fixed ratio of 1:1. PIP-secretion with increasing concentrations of neutralizing antibody for IL-10 (A), TNF-α (B) or TNF-α with a fixed concentration of anti-IL-10 at 1500 ng/ml (C) was measured by ELISA. BrdU staining was measured by flow cytometry with increasing concentrations of neutralizing antibody for IL-10 (D), TNF-α (E) or TNF-α with a fixed concentration of anti-IL-10 at 1500 ng/ml (F). Annexin-V reactivity was analyzed in rat SCs by flow cytometry at increasing concentrations of neutralizing anti-HGF (G). Data represent the mean of two experiments performed in triplicate. Error bars are standard deviation. *p<.05, **p<.01 compared to SCs alone (MSC:SC = 0).
TNF-α and IL-10 from MSCs were also found to be involved in the inhibition of proliferation of activated SCs. Maximal neutralization of IL-10 led to an increase in the number of BrdU-positive cells from 12% to 15% (p=0.0197, Fig. 2D), while anti-TNF-α induced an increase to 33% (p=0.035, Fig. 2E). Neutralization of both cytokines synergistically increased proliferation to 44% (p=0.009, Fig. 2F). These results indicated that IL-10 and TNF-α secreted by MSCs are both involved in inhibition of collagen synthesis and proliferation in activated SCs.
MSC-derived HGF induces apoptosis in activated SCs
Given the observation that a relatively small number of MSCs could cause SC apoptosis, we analyzed the Transwell coculture supernatants for potent pro-apoptotic signals. ELISAs for nerve growth factor and MMP-9, proteins previously reported to induce apoptosis [5] in SCs were negative (data not shown). In contrast, we detected a considerable amount of hepatocyte growth factor (HGF) that was produced at approximately equivalent levels by SCs and MSCs (suppl. Fig. 5F). HGF has been shown to have a pro-apoptotic on liver myofibroblasts [16], which we have reproduced in vitro (suppl. Fig. 6). Neutralization of HGF resulted in a normalization of apoptosis levels to non-coculture levels from 43 to 15 % (p=0.04, Fig. 2G). No reduction was observed with an isotype control antibody at the same concentration (data not shown). These data support a role for MSC-derived HGF in accelerating the rate of SC apoptosis.
DISCUSSION
Prior studies have shown that transplantation of a CD45-population of bone marrow cells prevented histopathological changes during chronic exposure to hepatotoxins [12-14]. These observations were correlated with a co-localization of transplanted cells and SCs, a reduction in the number of α-SMA+ cells, decreased tissue collagen content, and increased gelatinase gene expression. These in vivo findings provided a rationale for the therapeutic benefit of MSCs, although evidence supporting this mechanism in vitro does not exist. Thus, we investigated the effects of paracrine factors secreted by MSCs on activated SCs, the primary extracellular matrix-producing cell type in the liver.
We observed that MSCs inhibit the proliferative and fibrogenic function of activated SCs in a paracrine manner and as a function of MSC number. This inhibition was caused by MSC-derived IL-10 and TNF-α, which acted synergistically. The secretion of IL-10 by MSCs was found to be a dynamic response to IL-6 secretion by activated SCs. Secretion of IL-10 by MSCs in response to TNF-α was observed after exogenous stimulation, but not during mono- or coculture. This result is presumably due to the high levels of stimulation used in vitro (25 ng/ml) compared to the low levels measured in coculture (∼2.5 ng/ml) indicating a potential threshold concentration of TNF-α necessary for IL-10 expression. Furthermore, MSCs induced apoptosis in activated SCs that is, in part, mediated by HGF. These results support the hypothesis that the therapeutic effect of MSCs may be due to paracrine factors that modulate the proliferation, viability, and function of resident SCs.
Based on these findings, we propose a model describing the cross-talk between MSCs and SCs, and the resulting SC phenotype (Fig. 3). Our studies demonstrate that MSCs act through multiple mechanisms to coordinate a dynamic, integrated response to fibrosis. It is also likely that similar immunomodulatory mechanisms may influence the phenotype of resident hepatocytes, Kupffer cells, sinusoidal endothelial cells, and immune cells that infiltrate the liver during inflammation, warranting future studies to examine these multi-cellular effects.
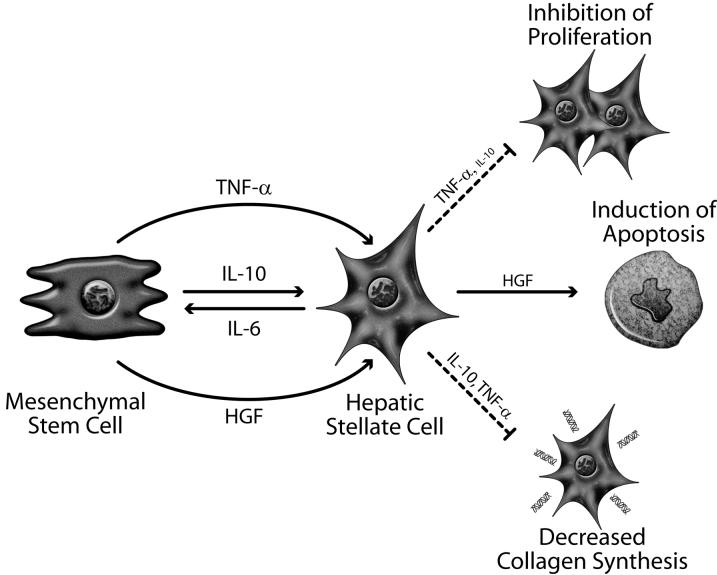
Figure 3.
A schematic model of the paracrine effects of MSC-derived factors on activated SCs. Autocrine factors synthesized by SCs are not represented. Release of IL-6 by activated SCs leads to the secretion of IL-10 by MSCs. Induced IL-10, along with constitutively secreted TNF-α, inhibit SC proliferation and collagen synthesis. The marginal effect of IL-10 on SC proliferation is denoted by the smaller font size. SCs undergo apoptosis after coculture with MSCs due to increased levels of HGF.
Several prior studies have documented the presence of host bone marrow-derived cells in the liver and mobilization of stem cell progenitors during liver fibrosis. Endogenous bone marrow-derived, CD45- cells have been observed in human livers during fibrosis [22], although their role in disease progression was not determined. A recent study by Russo et al. demonstrated that bone marrow-derived MSCs actively contributed to the SC population of the liver during the progression of fibrosis, although the total SC number during fibrosis did not change [23]. In another report, the bone marrow was found to renew the myofibroblast population, not SCs, by CD45+ fibrocytes which localized around fibrotic nodules [24]. We hypothesize that MSCs may be mobilized in response to injury and displace the scar-forming SC mass, but their therapeutic gains may be subdued after chronic insults. Our investigation documents an acute, one-way response of MSCs to activated SCs, however studies of long-term coculture of MSCs and SCs focused on the reciprocal reaction in the phenotype of MSCs may add to a more global assessment of a potential in vivo outcome.
In conclusion, immunomodulation of SCs by MSC soluble factors provides the first mechanistic evidence that MSCs can exert a protective role through paracrine signaling to liver SCs. These findings may be relevant to the beneficial effects of transplanted MSCs in various models of acute and chronic liver injury.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Avrum Leeder, Pohun Chris Chen and Luke Selby for the isolation of cells from rat livers. We also acknowledge Robert Crowther’s assistance in cytological and histological preparations and Donald Poulsen’s contributions for medical illustrations. Imaging studies were made possible by the Special Shared Facility in Morphology at the Shriners Hospitals for Children. B.P. is supported by a National Science Foundation predoctoral fellowship. D.V.P. is supported by a fellowship from the Fulbright Foundation. Z.M. is supported by the National Institutes of Health (F32 DK070496).
FINANCIAL SUPPORT: This work was partially supported by grants from the National Institutes of Health (F32 DK070496, K08 DK66040 and R01 DK43371) and the Shriners Hospitals for Children.
ABBREVIATIONS
- MSC
mesenchymal stem cell
- SC
hepatic stellate cell
- ECM
extracellular matrix
- RT-PCR
reverse-transcriptase polymerase chain reaction
- mRNA
messenger ribonucleic acid
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of matrix metalloproteinase
- DMEM
Dulbecco’s modified Eagle’s medium
- IL
interleukin
- TNF
tumor necrosis factor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ELISA
enzyme-linked immunosorbent assay
- PBS
phosphate buffered saline
- BrdU
bromo-deoxyuridine
- α-SMA
α-smooth muscle actin
- FITC
fluorescein isothiocynate
- DAPI
4′,6-diamidino-2-phenylindole
- HGF
hepatocyte growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275(4):2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 2.Grotendorst GR, Rahmanie H, Duncan MR. Combinatorial signaling pathways determine fibroblast proliferation and myofibroblast differentiation. Faseb J. 2004;18(3):469–79. doi: 10.1096/fj.03-0699com. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Aziz G, et al. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137(6):1333–42. [PMC free article] [PubMed] [Google Scholar]
- 4.Iredale JP, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102(3):538–49. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10(5):927–39. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 6.Fischer R, et al. Caspase 9-dependent killing of hepatic stellate cells by activated Kupffer cells. Gastroenterology. 2002;123(3):845–61. doi: 10.1053/gast.2002.35384. [DOI] [PubMed] [Google Scholar]
- 7.Senoo H, et al. Molecular mechanisms in the reversible regulation of morphology, proliferation and collagen metabolism in hepatic stellate cells by the three-dimensional structure of the extracellular matrix. J Gastroenterol Hepatol. 1998;13(Suppl):S19–32. [PubMed] [Google Scholar]
- 8.Thompson KC, et al. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation In vitro. Hepatology. 1998;28(6):1518–24. doi: 10.1002/hep.510280611. [DOI] [PubMed] [Google Scholar]
- 9.Thompson K, et al. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28(6):1597–606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100(14):8407–11. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnecchi M, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 12.Sakaida I, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40(6):1304–11. doi: 10.1002/hep.20452. [DOI] [PubMed] [Google Scholar]
- 13.Fang B, et al. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78(1):83–8. doi: 10.1097/01.tp.0000128326.95294.14. [DOI] [PubMed] [Google Scholar]
- 14.Zhao DC, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11(22):3431–40. doi: 10.3748/wjg.v11.i22.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauney JR, Volloch V, Kaplan DL. Matrix-mediated retention of adipogenic differentiation potential by human adult bone marrow-derived mesenchymal stem cells during ex vivo expansion. Biomaterials. 2005;26(31):6167–75. doi: 10.1016/j.biomaterials.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Shibata N, et al. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12(5):499–507. doi: 10.3727/000000003108747064. [DOI] [PubMed] [Google Scholar]
- 17.Dunn JC, et al. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. Faseb J. 1989;3(2):174–7. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 18.Blomhoff R, Berg T. Isolation and cultivation of rat liver stellate cells. Methods Enzymol. 1990;190:58–71. doi: 10.1016/0076-6879(90)90009-p. [DOI] [PubMed] [Google Scholar]
- 19.Saile B, et al. Rat liver myofibroblasts and hepatic stellate cells differ in CD95-mediated apoptosis and response to TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2002;283(2):G435–44. doi: 10.1152/ajpgi.00441.2001. [DOI] [PubMed] [Google Scholar]
- 20.Saile B, et al. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30(1):196–202. doi: 10.1002/hep.510300144. [DOI] [PubMed] [Google Scholar]
- 21.Kim WH, Matsumoto K, Bessho K, Nakamura T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am J Pathol. 2005;166:1017–1028. doi: 10.1016/S0002-9440(10)62323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forbes SJ, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126(4):955–63. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Russo FP, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130(6):1807–21. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Kisseleva T, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–38. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.