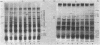Abstract
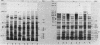
Salinity altered the protein synthesis patterns in two cyanobacterial strains: Anabaena torulosa, a salt-tolerant brackish water strain, and Anabaena sp. strain L-31, a salt-sensitive freshwater strain. The cyanobacterial response to salinity was very rapid, varied with time, and was found to be correlated with the external salt (NaCl) concentration during stress. Salinity induced three prominent types of modification. First, the synthesis of several proteins was inhibited, especially in the salt-sensitive strain; second, the synthesis of certain proteins was significantly enhanced; and third, synthesis of a specific set of proteins was induced de novo by salinity stress. Proteins which were selectively synthesized or induced de novo during salt stress, tentatively called the salt-stress proteins, were confined to an isoelectric pI range of 5.8 to 7.5 and were distributed in a molecular mass range of 12 to 155 kilodaltons. These salt-stress proteins were unique to each Anabaena strain, and their expression was apparently regulated coordinately during exposure to salt stress. In Anabaena sp. strain L-31, most of the salt-stress-induced proteins were transient in nature and were located mainly in the cytoplasm. In A. torulosa, salt-stress-induced proteins were evenly distributed in the membrane and cytoplasmic fractions and were persistent, being synthesized at high rates throughout the period of salinity stress. These initial studies reveal that salinity-induced modification of protein synthesis, as has been demonstrated in higher plant species, also occurs in cyanobacteria and that at least some of the proteins preferentially synthesized during salt stress may be important to cyanobacterial osmotic adaptation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte S. K., Reddy B. R., Thomas J. Relationship between Sodium Influx and Salt Tolerance of Nitrogen-Fixing Cyanobacteria. Appl Environ Microbiol. 1987 Aug;53(8):1934–1939. doi: 10.1128/aem.53.8.1934-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte S. K., Thomas J. Membrane electrogenesis and sodium transport in filamentous nitrogen-fixing cyanobacteria. Eur J Biochem. 1986 Jan 15;154(2):395–401. doi: 10.1111/j.1432-1033.1986.tb09411.x. [DOI] [PubMed] [Google Scholar]
- Blumwald E., Mehlhorn R. J., Packer L. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc Natl Acad Sci U S A. 1983 May;80(9):2599–2602. doi: 10.1073/pnas.80.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Tel-Or E. Salt adaptation of the cyanobacterium synechococcus 6311 growing in a continuous culture (turbidostat). Plant Physiol. 1984 Jan;74(1):183–185. doi: 10.1104/pp.74.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély G., Surányi G., Korcz A., Pálfi Z. Effect of heat shock on protein synthesis in the cyanobacterium Synechococcus sp. strain PCC 6301. J Bacteriol. 1985 Mar;161(3):1125–1130. doi: 10.1128/jb.161.3.1125-1130.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Demmerle S., Mackay M. A., Norton R. S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980 Nov 7;210(4470):650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Miller D. M., Jones J. H., Yopp J. H., Tindall D. R., Schmid W. E. Ion metabolism in a halophilic blue-green alga, Aphanothece halophytica. Arch Microbiol. 1976 Dec 1;111(1-2):145–149. doi: 10.1007/BF00446561. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ostrem J. A., Olson S. W., Schmitt J. M., Bohnert H. J. Salt Stress Increases the Level of Translatable mRNA for Phosphoenolpyruvate Carboxylase in Mesembryanthemum crystallinum. Plant Physiol. 1987 Aug;84(4):1270–1275. doi: 10.1104/pp.84.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S. Salinity stress induced tissue-specific proteins in barley seedlings. Plant Physiol. 1987 Jun;84(2):324–331. doi: 10.1104/pp.84.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. Absence of the pigments of photosystem II of photosynthesis in heterocysts of a blue-green alga. Nature. 1970 Oct 10;228(5267):181–183. doi: 10.1038/228181b0. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]