Abstract
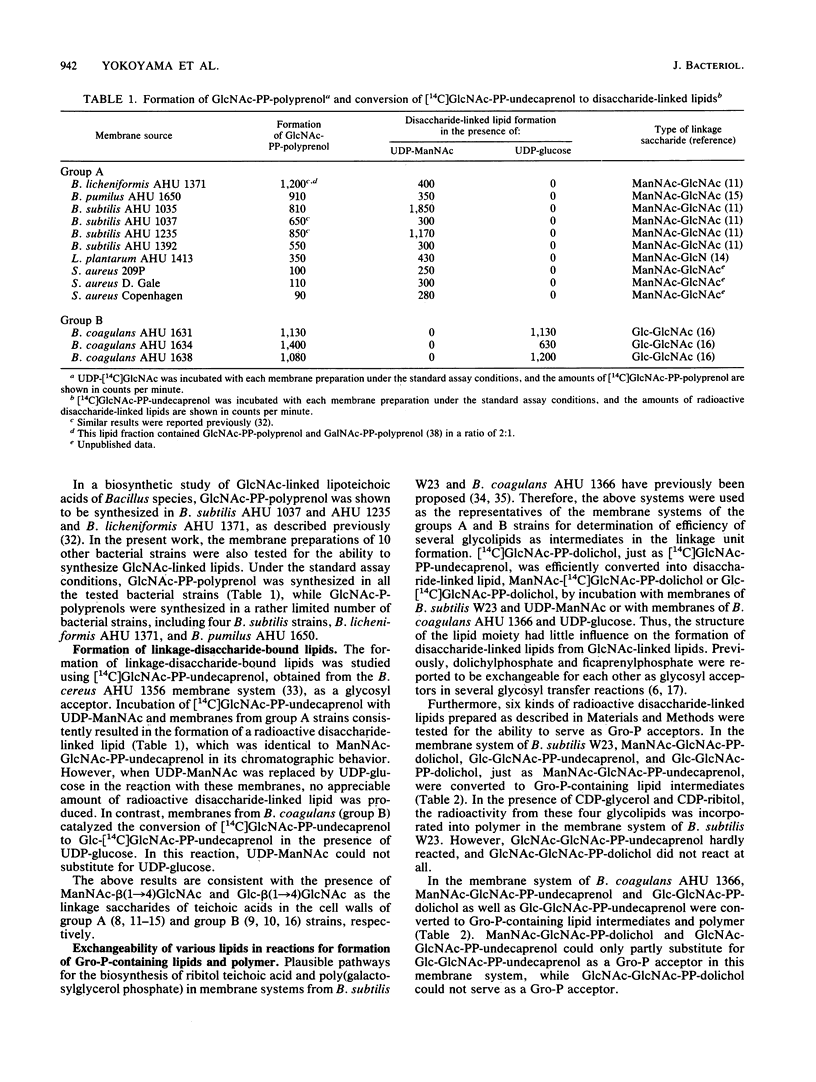
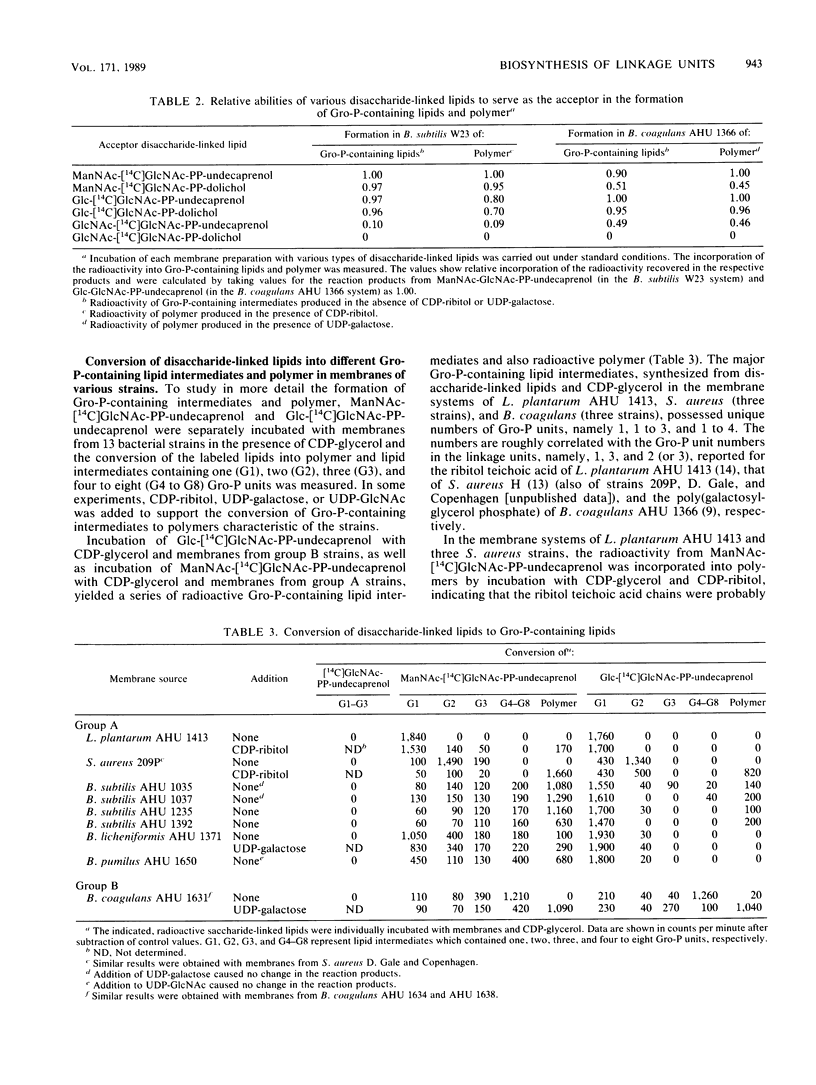
The distribution and substrate specificities of enzymes involved in the formation of linkage units which contain N-acetylglucosamine (GlcNAc) and N-acetylmannosamine (ManNAc) or glucose and join teichoic acid chains to peptidoglycan were studied among membrane systems obtained from the following two groups of gram-positive bacteria: group A, including Bacillus subtilis, Bacillus licheniformis, Bacillus pumilus, Staphylococcus aureus, and Lactobacillus plantarum; group B, Bacillus coagulans. All the membrane preparations tested catalyzed the synthesis of N-acetylglucosaminyl pyrophosphorylpolyprenol (GlcNAc-PP-polyprenol). The enzymes transferring glycosyl residues to GlcNAc-PP-polyprenol were specific to either UDP-ManNAc (group A strains) or UDP-glucose (group B strains). In the synthesis of the disaccharide-bound lipids, GlcNAc-PP-dolichol could substitute for GlcNAc-PP-undecaprenol. ManNAc-GlcNAc-PP-undecaprenol, ManNAc-GlcNAc-PP-dolichol, Glc-GlcNAc-PP-undecaprenol, Glc-GlcNAc-PP-dolichol, and GlcNAc-GlcNAc-PP-undecaprenol were more or less efficiently converted to glycerol phosphate-containing lipid intermediates and polymers in the membrane systems of B. subtilis W23 and B. coagulans AHU 1366. However, GlcNAc-GlcNAc-PP-dolichol could not serve as an intermediate in either of these membrane systems. Further studies on the exchangeability of ManNAc-GlcNAc-PP-undecaprenol and Glc-GlcNAc-PP-undecaprenol revealed that in the membrane systems of S. aureus strains and other B. coagulans strains both disaccharide-inked lipids served almost equally as intermediates in the synthesis of polymers. In the membrane systems of other B. subtilis strains as well as B. licheniformis and B. pumilus strains, however, the replacement of ManNAc-GlcNAc-PP-undecaprenol by Glc-GlcNAc-PP-undecaprenol led to a great accumulation of (glycerol phosphate)-Glc-GlcNAc-PP-undecaprenol accompanied by a decrease in the formation of polymers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coley J., Tarelli E., Archibald A. R., Baddiley J. The linkage between teichoic acid and peptidoglycan in bacterial cell walls. FEBS Lett. 1978 Apr 1;88(1):1–9. doi: 10.1016/0014-5793(78)80594-8. [DOI] [PubMed] [Google Scholar]
- Hancock I. C., Baddiley J. Biosynthesis of the wall teichoic acid in Bacillus licheniformis. Biochem J. 1972 Mar;127(1):27–37. doi: 10.1042/bj1270027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. R., Baddiley J. Biosynthesis of wall teichoic acids in Staphylococcus aureus H, Micrococcus varians and Bacillus subtilis W23. Involvement of lipid intermediates containing the disaccharide N-acetylmannosaminyl N-acetylglucosamine. Eur J Biochem. 1985 Dec 16;153(3):639–645. doi: 10.1111/j.1432-1033.1985.tb09348.x. [DOI] [PubMed] [Google Scholar]
- Heckels J. E., Archibald A. R., Baddiley J. Studies on the linkage between teichoic acid and peptidoglycan in a bacteriophage-resistant mutant of Staphylococcus aureus H. Biochem J. 1975 Sep;149(3):637–647. doi: 10.1042/bj1490637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey H., Baddiley J. Lipid intermediates in the biosynthesis of the wall teichoic acid in Staphylococcus lactis 13. Biochem J. 1972 Mar;127(1):39–50. doi: 10.1042/bj1270039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski W., Chojnacki T. Formation of lipid-linked sugars in rat liver and brain microsomes. Biochim Biophys Acta. 1972 Jan 27;260(1):93–97. doi: 10.1016/0005-2760(72)90078-1. [DOI] [PubMed] [Google Scholar]
- Kawamura T., Kimura M., Yamamori S., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosamine. J Biol Chem. 1978 May 25;253(10):3595–3601. [PubMed] [Google Scholar]
- Kaya S., Araki Y., Ito E. Characterization of a novel linkage unit between ribitol teichoic acid and peptidoglycan in Listeria monocytogenes cell walls. Eur J Biochem. 1985 Feb 1;146(3):517–522. doi: 10.1111/j.1432-1033.1985.tb08682.x. [DOI] [PubMed] [Google Scholar]
- Kaya S., Yokoyama K., Araki Y., Ito E. N-acetylmannosaminyl(1----4)N-acetylglucosamine, a linkage unit between glycerol teichoic acid and peptidoglycan in cell walls of several Bacillus strains. J Bacteriol. 1984 Jun;158(3):990–996. doi: 10.1128/jb.158.3.990-996.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya S., Yokoyama K., Araki Y., Ito E. Structural and biosynthetic studies on linkage region between poly(galactosylglycerol phosphate) and peptidoglycan in Bacillus coagulans. Biochem Biophys Res Commun. 1983 Feb 28;111(1):312–318. doi: 10.1016/s0006-291x(83)80153-3. [DOI] [PubMed] [Google Scholar]
- Kojima N., Araki Y., Ito E. Structural studies on the linkage unit of ribitol teichoic acid of Lactobacillus plantarum. Eur J Biochem. 1985 Apr 1;148(1):29–34. doi: 10.1111/j.1432-1033.1985.tb08802.x. [DOI] [PubMed] [Google Scholar]
- Kojima N., Araki Y., Ito E. Structure of linkage region between ribitol teichoic acid and peptidoglycan in cell walls of Staphylococcus aureus H. J Biol Chem. 1983 Aug 10;258(15):9043–9045. [PubMed] [Google Scholar]
- Kojima N., Araki Y., Ito E. Structure of the linkage units between ribitol teichoic acids and peptidoglycan. J Bacteriol. 1985 Jan;161(1):299–306. doi: 10.1128/jb.161.1.299-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Iida J., Araki Y., Ito E. Structural studies on the linkage unit between poly(N-acetylglucosamine 1-phosphate) and peptidoglycan in cell walls of Bacillus pumilus AHU 1650. Eur J Biochem. 1985 Jun 3;149(2):331–336. doi: 10.1111/j.1432-1033.1985.tb08930.x. [DOI] [PubMed] [Google Scholar]
- Kojima N., Uchikawa K., Araki Y., Ito E. A common linkage saccharide unit between teichoic acids and peptidoglycan in cell walls of Bacillus coagulans. J Biochem. 1985 Apr;97(4):1085–1092. doi: 10.1093/oxfordjournals.jbchem.a135152. [DOI] [PubMed] [Google Scholar]
- Lehle L., Fartaczek F., Tanner W., Kauss H. Formation of polyprenol-linked mono- and oligosaccharides in Phaseolus aureus. Arch Biochem Biophys. 1976 Aug;175(2):419–426. doi: 10.1016/0003-9861(76)90529-4. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J. Lipid linked sugars in glycoprotein synthesis. Science. 1975 Jun 6;188(4192):986–991. doi: 10.1126/science.167438. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Scher M. G. Metabolism and function of polyisoprenol sugar intermediates in membrane-associated reactions. Biochim Biophys Acta. 1972 Aug 4;265(3):417–441. doi: 10.1016/0304-4157(72)90015-9. [DOI] [PubMed] [Google Scholar]
- McArthur H. A., Hancock I. C., Baddiley J. Attachment of the main chain to the linkage unit in biosynthesis of teichoic acids. J Bacteriol. 1981 Mar;145(3):1222–1231. doi: 10.1128/jb.145.3.1222-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur H. A., Hancock I. C., Roberts F. M., Baddiley J. Biosynthesis of teichoic acid in Micrococcus varians ATCC 29750. Characterization of a further lipid intermediate. FEBS Lett. 1980 Mar 10;111(2):317–323. doi: 10.1016/0014-5793(80)80818-0. [DOI] [PubMed] [Google Scholar]
- McArthur H. A., Roberts F. M., Hancock I. C., Baddiley J. Lipid intermediates in the biosynthesis of the linkage unit between teichoic acids and peptidoglycan. FEBS Lett. 1978 Feb 15;86(2):193–200. doi: 10.1016/0014-5793(78)80561-4. [DOI] [PubMed] [Google Scholar]
- Mizuno M., Shimojima Y., Sugawara T., Takeda I. An antibiotic 24010. J Antibiot (Tokyo) 1971 Dec;24(12):896–899. doi: 10.7164/antibiotics.24.896. [DOI] [PubMed] [Google Scholar]
- Morton R. A. Polyprenols, their nature, occurrence and biological roles. Biochem Soc Symp. 1972;(35):203–217. [PubMed] [Google Scholar]
- Murazumi N., Sasaki Y., Okada J., Araki Y., Ito E. Biosynthesis of glycerol teichoic acid in Bacillus cereus: formation of linkage unit disaccharide on a lipid intermediate. Biochem Biophys Res Commun. 1981 Mar 31;99(2):504–510. doi: 10.1016/0006-291x(81)91773-3. [DOI] [PubMed] [Google Scholar]
- Murazumi N., Yokoyama K., Araki Y., Ito E. An enzyme catalyzing the liberation of N-acetylglucosamine from N-acetylglucosaminyl pyrophosphorylpolyprenol in Bacillus polymyxa membranes. FEBS Lett. 1987 Jun 22;218(1):131–134. doi: 10.1016/0014-5793(87)81032-3. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Araki Y., Ito E. The formation of a mannose-containing trisaccharide on a lipid and its transfer to proteins in yeast. FEBS Lett. 1976 Dec 31;72(2):287–290. doi: 10.1016/0014-5793(76)80988-x. [DOI] [PubMed] [Google Scholar]
- RUPLEY J. A. THE HYDROLYSIS OF CHITIN BY CONCENTRATED HYDROCHLORIC ACID, AND THE PREPARATION OF LOW-MOLECULAR-WEIGHT SUBSTRATES FOR LYSOZYME. Biochim Biophys Acta. 1964 Nov 1;83:245–255. doi: 10.1016/0926-6526(64)90001-1. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Araki Y., Ito E. Structure of teichoic-acid--glycopeptide complexes from cell walls of Bacillus cereus AHU 1030. Eur J Biochem. 1983 Apr 15;132(1):207–213. doi: 10.1111/j.1432-1033.1983.tb07349.x. [DOI] [PubMed] [Google Scholar]
- Shimada A., Ohta M., Iwasaki H., Ito E. The function of beta-N-acetyl-D-glucosaminyl monophosphorylundecaprenol in biosynthesis of lipoteichoic acids in a group of Bacillus strains. Eur J Biochem. 1988 Oct 1;176(3):559–565. doi: 10.1111/j.1432-1033.1988.tb14314.x. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Curtis C. A. The biosynthesis and linkage of teichuronic acid to peptidoglycan in Bacillus licheniformis. Eur J Biochem. 1982 Feb;122(1):125–132. doi: 10.1111/j.1432-1033.1982.tb05857.x. [DOI] [PubMed] [Google Scholar]
- Yamamori S., Murazumi N., Araki Y., Ito E. Formation and function of N-acetyloglucosamine-linked phosphoryl- and pyrophosphorylundecaprenols in membranes from Bacillus cereus. J Biol Chem. 1978 Sep 25;253(18):6516–6522. [PubMed] [Google Scholar]
- Yokoyama K., Araki Y., Ito E. Biosynthesis of poly(galactosylglycerol phosphate) in Bacillus coagulans. Eur J Biochem. 1987 May 15;165(1):47–53. doi: 10.1111/j.1432-1033.1987.tb11192.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Miyashita T., Araki Y., Ito E. Structure and functions of linkage unit intermediates in the biosynthesis of ribitol teichoic acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur J Biochem. 1986 Dec 1;161(2):479–489. doi: 10.1111/j.1432-1033.1986.tb10469.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama T., Koike Y., Arakawa H., Yokoyama K., Sasaki Y., Kawamura T., Araki Y., Ito E., Takao S. Distribution of mannosamine and mannosaminuronic acid among cell walls of Bacillus species. J Bacteriol. 1982 Jan;149(1):15–21. doi: 10.1128/jb.149.1.15-21.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


