Abstract
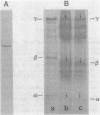
A brown carbon monoxide dehydrogenase from CO-autotrophically grown cells of Acinetobacter sp. strain JC1, which is unstable outside the cells, was purified 80-fold in seven steps to better than 95% homogeneity, with a yield of 44% in the presence of the stabilizing agents iodoacetamide (1 mM) and ammonium sulfate (100 mM). The final specific activity was 474 mumol of acceptor reduced per min per mg of protein as determined by an assay based on the CO-dependent reduction of thionin. Methyl viologen, NAD(P), flavin mononucleotide, flavin adenine dinucleotide, and ferricyanide were not reduced by the enzyme, but methylene blue, thionin, and dichlorophenolindophenol were reduced. The molecular weight of the native enzyme was determined to be 380,000. Sodium dodecyl sulfate-gel electrophoresis revealed at least three nonidentical subunits of molecular weights 16,000 (alpha), 34,000 (beta), and 85,000 (gamma). The purified enzyme contained particulate hydrogenase-like activity. Selenium did not stimulate carbon monoxide dehydrogenase activity. The isoelectic point of the native enzyme was found to be 5.8; the Km of CO was 150 microM. The enzyme was rapidly inactivated by methanol. One mole of native enzyme was found to contain 2 mol of each of flavin adenine dinucleotide and molybdenum and 8 mol each of nonheme iron and labile sulfide, which indicated that the enzyme was a molybdenum-containing iron-sulfur flavoprotein. The ratio of densities of each subunit after electrophoresis (alpha:beta:gamma = 1:2:6) and the number of each cofactor in the native enzyme suggest a alpha 2 beta 2 gamma 2 structure of the enzyme. The carbon monoxide dehydrogenase of Acinetobacter sp. strain JC1 was found to have no immunological relationship with enzymes of Pseudomonas carboxydohydrogena and Pseudomonas carboxydovorans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUMBY P. E., MILLER R. W., MASSEY V. THE CONTENT AND POSSIBLE CATALYTIC SIGNIFICANCE OF LABILE SULFIDE IN SOME METALLOFLAVOPROTEINS. J Biol Chem. 1965 May;240:2222–2228. [PubMed] [Google Scholar]
- Bray R. C., George G. N., Lange R., Meyer O. Studies by e.p.r. spectroscopy of carbon monoxide oxidases from Pseudomonas carboxydovorans and Pseudomonas carboxydohydrogena. Biochem J. 1983 Jun 1;211(3):687–694. doi: 10.1042/bj2110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Seiler W. Role of microorganisms in the consumption and production of atmospheric carbon monoxide by soil. Appl Environ Microbiol. 1980 Sep;40(3):437–445. doi: 10.1128/aem.40.3.437-445.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Khalil M. A., Rasmussen R. A. Carbon Monoxide in the Earth's Atmosphere: Increasing Trend. Science. 1984 Apr 6;224(4644):54–56. doi: 10.1126/science.224.4644.54. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Electron transport system of an aerobic carbon monoxide-oxidizing bacterium. J Bacteriol. 1981 Dec;148(3):991–994. doi: 10.1128/jb.148.3.991-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Oxidation of carbon monoxide by bacteria. Int Rev Cytol. 1983;81:1–32. doi: 10.1016/s0074-7696(08)62333-5. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Hegeman G. D. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J Bacteriol. 1981 Dec;148(3):904–911. doi: 10.1128/jb.148.3.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger B., Meyer O. Structural elements of bactopterin from Pseudomonas carboxydoflava carbon monoxide dehydrogenase. Biochim Biophys Acta. 1987 Apr 30;912(3):357–364. doi: 10.1016/0167-4838(87)90040-9. [DOI] [PubMed] [Google Scholar]
- Krüger B., Meyer O. The pterin (bactopterin) of carbon monoxide dehydrogenase from Pseudomonas carboxydoflava. Eur J Biochem. 1986 May 15;157(1):121–128. doi: 10.1111/j.1432-1033.1986.tb09647.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MASSEY V. Studies on succinic dehydrogenase. VII. Valency state of the iron in beef heart succinic dehydrogenase. J Biol Chem. 1957 Dec;229(2):763–770. [PubMed] [Google Scholar]
- MILLER R. W., MASSEY V. DIHYDROOROTIC DEHYDROGENASE. I. SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1453–1465. [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol. 1984 Feb;157(2):643–648. doi: 10.1128/jb.157.2.643-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Selenite binding to carbon monoxide oxidase from Pseudomonas carboxydovorans. Selenium binds covalently to the protein and activates specifically the CO----methylene blue reaction. J Biol Chem. 1984 May 10;259(9):5612–5617. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979 Feb;137(2):811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner) comb. nov. Arch Microbiol. 1978 Jul;118(1):35–43. doi: 10.1007/BF00406071. [DOI] [PubMed] [Google Scholar]
- Robbins R. C., Borg K. M., Robinson E. Carbon monoxide in the atmosphere. J Air Pollut Control Assoc. 1968 Feb;18(2):106–110. doi: 10.1080/00022470.1968.10469094. [DOI] [PubMed] [Google Scholar]
- Rohde M., Mayer F., Meyer O. Immunocytochemical localization of carbon monoxide oxidase in Pseudomonas carboxydovorans. The enzyme is attached to the inner aspect of the cytoplasmic membrane. J Biol Chem. 1984 Dec 10;259(23):14788–14792. [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- WESTLAKE D. W., ROXBURGH J. M., TALBOT G. Microbial production of carbon monoxide from flavonoids. Nature. 1961 Feb 11;189:510–511. doi: 10.1038/189510a0. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]