Abstract
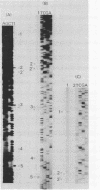
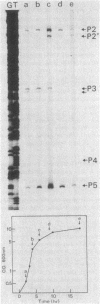
The genes encoding the major cell wall proteins, middle wall protein and outer wall protein, of Bacillus brevis 47 constitute a cotranscriptional unit (cwp [cell wall protein gene] operon). Primer extension assay of cwp operon transcripts showed the existence of six different 5' ends. This confirmed the results of the previous S1 nuclease protection assay and suggested the existence of several tandemly arranged promoters in the 5' region of the cwp operon. Promoter probe vectors carrying the Bacillus licheniformis alpha-amylase gene were constructed and used for deletion analysis of the 5' region. Three (P1, P2, and P3) of the six suggested promoters were shown to be located within three distinct fragments derived from the 5' region. The -35 and -10 regions of the P1 and P3 promoters resemble the consensus sequence recognized by the sigma-43-type RNA polymerase of Bacillus subtilis. The P2 promoter resembles only the consensus sequence in the -10 region. The P1 and P3 promoters were used to the same extents in Bacillus subtilis as in B. brevis, whereas the P2 promoter was used much less frequently in B. subtilis than in B. brevis. The P2 promoter is used constitutively in B. brevis 47 at all stages of growth, whereas P3 is used only at the exponential phase of growth. P2 could be a promoter of an unknown type that is preferentially used in B. brevis and might be responsible for the constitutive synthesis and secretion of the cell wall proteins into the medium at the stationary phase of growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Raibaud O. Expression of the Escherichia coli malPQ operon remains unaffected after drastic alteration of its promoter. J Bacteriol. 1983 Mar;153(3):1221–1227. doi: 10.1128/jb.153.3.1221-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7000–7004. doi: 10.1073/pnas.77.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin T. M., Sonenshein A. L. Mutations of the Escherichia coli lacUV5 promoter resulting in increased expression in Bacillus subtilis. Mol Gen Genet. 1987 Oct;209(3):467–474. doi: 10.1007/BF00331151. [DOI] [PubMed] [Google Scholar]
- Igo M., Lampe M., Ray C., Schafer W., Moran C. P., Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Kop J., Kopylov A. M., Magrum L., Siegel R., Gupta R., Woese C. R., Noller H. F. Probing the structure of 16 S ribosomal RNA from Bacillus brevis. J Biol Chem. 1984 Dec 25;259(24):15287–15293. [PubMed] [Google Scholar]
- Le Grice S. F., Sonenshein A. L. Interaction of Bacillus subtilis RNA polymerase with a chromosomal promoter. J Mol Biol. 1982 Dec 15;162(3):551–564. doi: 10.1016/0022-2836(82)90388-6. [DOI] [PubMed] [Google Scholar]
- Lewandoski M., Dubnau E., Smith I. Transcriptional regulation of the spo0F gene of Bacillus subtilis. J Bacteriol. 1986 Nov;168(2):870–877. doi: 10.1128/jb.168.2.870-877.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marahiel M. A., Zuber P., Czekay G., Losick R. Identification of the promoter for a peptide antibiotic biosynthesis gene from Bacillus brevis and its regulation in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2215–2222. doi: 10.1128/jb.169.5.2215-2222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., Banner C. D., Haldenwang W. G., Losick R. Promoter for a developmentally regulated gene in Bacillus subtilis. Cell. 1981 Sep;25(3):783–791. doi: 10.1016/0092-8674(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Saito N. A thermophilic extracellular -amylase from Bacillus licheniformis. Arch Biochem Biophys. 1973 Apr;155(2):290–298. doi: 10.1016/0003-9861(73)90117-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi W., Yamagata H., Yamaguchi K., Tsukagoshi N., Udaka S. Genetic transformation of Bacillus brevis 47, a protein-secreting bacterium, by plasmid DNA. J Bacteriol. 1983 Dec;156(3):1130–1134. doi: 10.1128/jb.156.3.1130-1134.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Kawano N. Cloning vehicles for the homologous Bacillus subtilis host-vector system. Gene. 1980 Jul;10(2):131–136. doi: 10.1016/0378-1119(80)90130-4. [DOI] [PubMed] [Google Scholar]
- Tatti K. M., Moran C. P., Jr Utilization of one promoter by two forms of RNA polymerase from Bacillus subtilis. Nature. 1985 Mar 14;314(6007):190–192. doi: 10.1038/314190a0. [DOI] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Adachi T., Sasaki T., Hayakawa S., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988 Feb;170(2):935–945. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Tabata R., Takahashi Y., Hashiba H., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: complete nucleotide sequence of the outer wall protein gene. J Bacteriol. 1986 Oct;168(1):365–373. doi: 10.1128/jb.168.1.365-373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Tabata R., Takemura T., Yamagata H., Udaka S. Molecular cloning of a major cell wall protein gene from protein-producing Bacillus brevis 47 and its expression in Escherichia coli and Bacillus subtilis. J Bacteriol. 1984 Jun;158(3):1054–1060. doi: 10.1128/jb.158.3.1054-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Z., Doi R. H. Overlapping promoters transcribed by bacillus subtilis sigma 55 and sigma 37 RNA polymerase holoenzymes during growth and stationary phases. J Biol Chem. 1984 Jul 10;259(13):8619–8625. [PubMed] [Google Scholar]
- Wong H. C., Schnepf H. E., Whiteley H. R. Transcriptional and translational start sites for the Bacillus thuringiensis crystal protein gene. J Biol Chem. 1983 Feb 10;258(3):1960–1967. [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Adachi T., Tsuboi A., Takao M., Sasaki T., Tsukagoshi N., Udaka S. Cloning and characterization of the 5' region of the cell wall protein gene operon in Bacillus brevis 47. J Bacteriol. 1987 Mar;169(3):1239–1245. doi: 10.1128/jb.169.3.1239-1245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Nakagawa K., Tsukagoshi N., Udaka S. A stable plasmid vector and control of its copy number in Bacillus brevis 47, a protein-producing bacterium. Appl Environ Microbiol. 1985 May;49(5):1076–1079. doi: 10.1128/aem.49.5.1076-1079.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Uemura J., Seki T., Oshima Y. Construction of a promoter-probe vector for a Bacillus subtilis host by using the trpD+ gene of Bacillus amyloliquefaciens. J Bacteriol. 1984 Sep;159(3):905–912. doi: 10.1128/jb.159.3.905-912.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuuki T., Nomura T., Tezuka H., Tsuboi A., Yamagata H., Tsukagoshi N., Udaka S. Complete nucleotide sequence of a gene coding for heat- and pH-stable alpha-amylase of Bacillus licheniformis: comparison of the amino acid sequences of three bacterial liquefying alpha-amylases deduced from the DNA sequences. J Biochem. 1985 Nov;98(5):1147–1156. doi: 10.1093/oxfordjournals.jbchem.a135381. [DOI] [PubMed] [Google Scholar]