Abstract
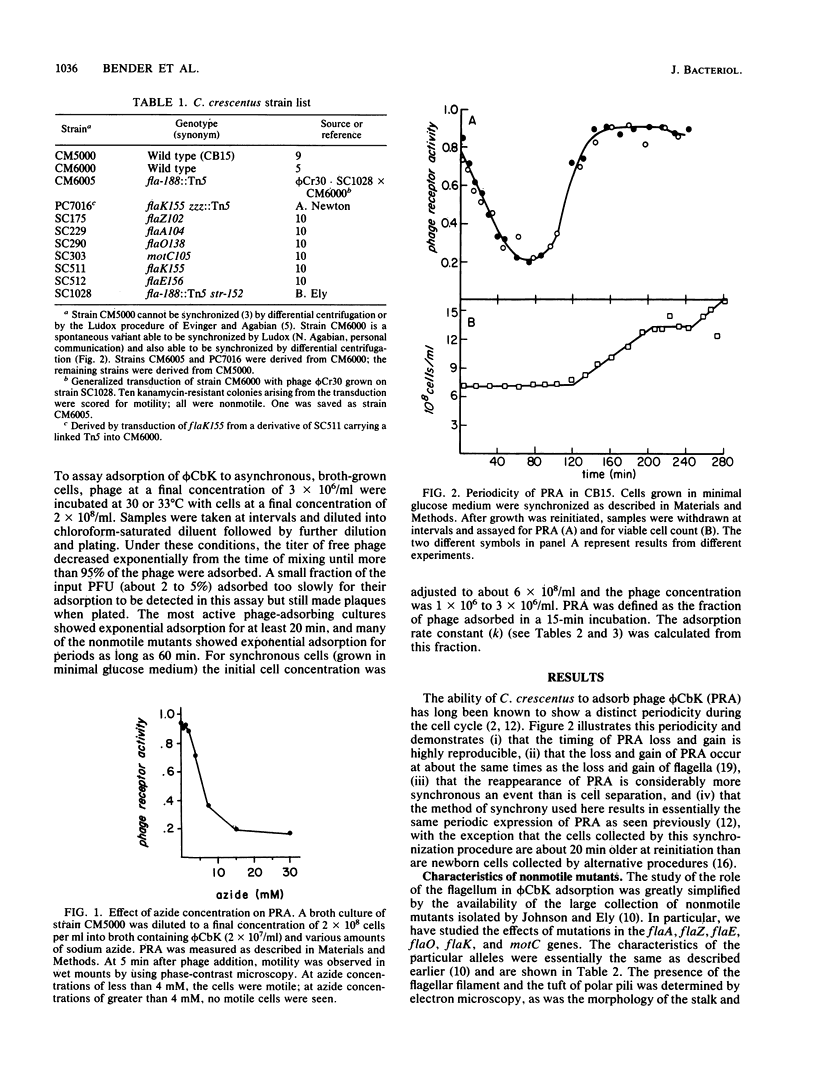
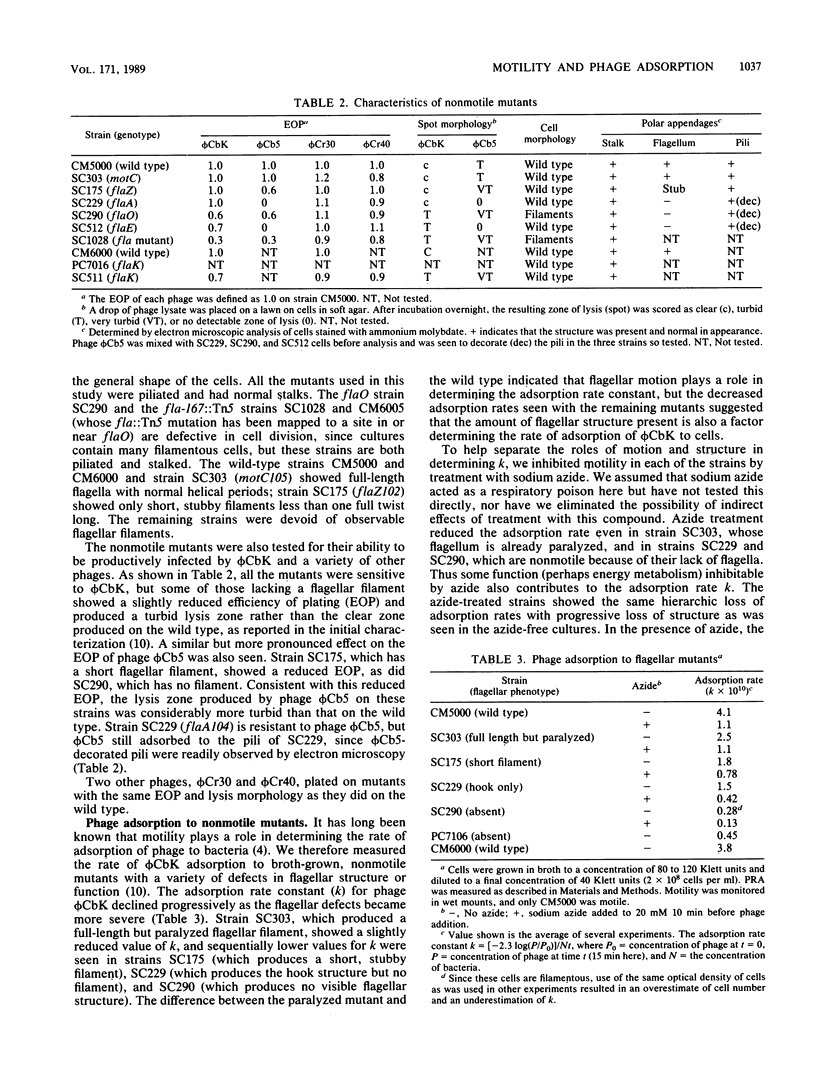
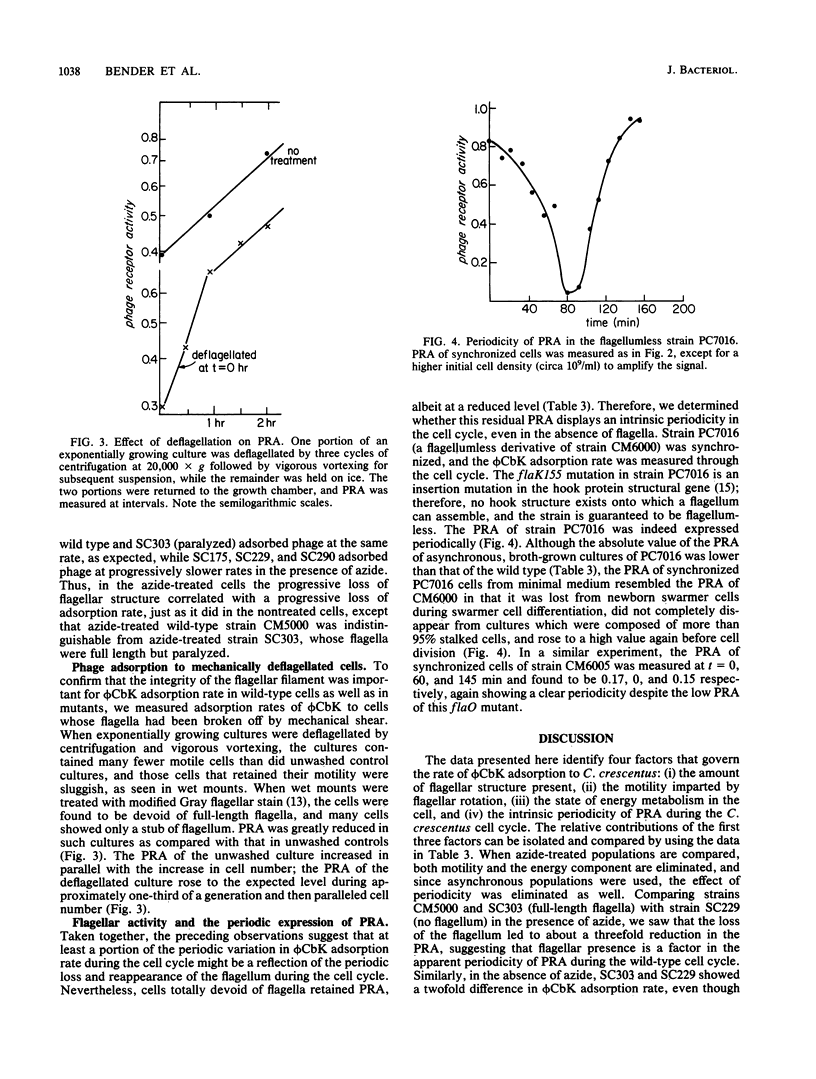
The rate of adsorption of Caulobacter bacteriophage phi CbK to Caulobacter crescentus is dependent on the structural integrity of the flagellum. Cells lacking part or all of the flagellum because of either mutation or mechanical shear were defective in adsorption, and the extent of the defect in adsorption reflected the amount of flagellar structure missing. Maximal adsorption rates were also dependent on cellular motility and energy metabolism, since adsorption to cells with paralyzed flagella was slower than adsorption to motile cells and inhibition of cellular energy metabolism with azide also reduced adsorption rates, even for nonmotile cells. Nevertheless, the flagellum is not the receptor for phage phi CbK, since flagellumless mutants adsorbed phi CbK at detectable rates. While some portion of the fluctuation in the phi CbK receptor activity during the C. crescentus cell cycle can be ascribed to the periodicity of flagellar loss and reappearance, the phage receptor activity remaining in flagellumless mutants was periodic in the cell cycle. Therefore, the periodic expression of phage receptor activity is an intrinsic property of the C. crescentus cell cycle, although the amplitude of the oscillation may be altered by the periodic expression of flagellar motility.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian-Keshishian N., Shapiro L. Bacterial differentiation and phage infection. Virology. 1971 Apr;44(1):46–53. doi: 10.1016/0042-6822(71)90151-6. [DOI] [PubMed] [Google Scholar]
- Agabian-Keshishian N., Shapiro L. Stalked bacteria: properties of deoxriybonucleic acid bacteriophage phiCbK. J Virol. 1970 Jun;5(6):795–800. doi: 10.1128/jvi.5.6.795-800.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Weissborn A., Amemiya K., Mansour J., Henry S., Shapiro L., Bender R. The effect of termination of membrane phospholipid synthesis on cell-dependent events in Caulobacter. J Mol Biol. 1980 Apr;138(2):401–409. doi: 10.1016/0022-2836(80)90295-8. [DOI] [PubMed] [Google Scholar]
- Evinger M., Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J Bacteriol. 1977 Oct;132(1):294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Iba H., Okada Y. Stalkless mutants of Caulobacter crescentus. J Bacteriol. 1977 Jul;131(1):280–287. doi: 10.1128/jb.131.1.280-287.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Miyakawa K., Iba H., Okada Y. A flagellotropic bacteriophage and flagella formation in Caulobacter. Virology. 1976 Jun;71(2):583–592. doi: 10.1016/0042-6822(76)90383-4. [DOI] [PubMed] [Google Scholar]
- Huguenel E. D., Newton A. Localization of surface structures during procaryotic differentiation: role of cell division in Caulobacter crescentus. Differentiation. 1982;21(2):71–78. doi: 10.1111/j.1432-0436.1982.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Analysis of nonmotile mutants of the dimorphic bacterium Caulobacter crescentus. J Bacteriol. 1979 Jan;137(1):627–634. doi: 10.1128/jb.137.1.627-634.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurn N., Ammer S., Shapiro L. A pleiotropic mutation affecting expression of polar development events in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3157–3161. doi: 10.1073/pnas.71.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Farmer S., Agabian N. Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology. 1977 Mar;77(1):401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
- Mayfield C. I., Inniss W. E. A rapid, simple method for staining bacterial flagella. Can J Microbiol. 1977 Sep;23(9):1311–1313. doi: 10.1139/m77-198. [DOI] [PubMed] [Google Scholar]
- Newton A. Role of transcription in the temporal control of development in Caulobacter crescentus (stalk-rifampin-RNA synthesis-DNA synthesis-motility). Proc Natl Acad Sci U S A. 1972 Feb;69(2):447–451. doi: 10.1073/pnas.69.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill E. A., Bender R. A. Periodic synthesis of phospholipids during the Caulobacter crescentus cell cycle. J Bacteriol. 1987 Jun;169(6):2618–2623. doi: 10.1128/jb.169.6.2618-2623.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta N., Swanson E., Ely B., Newton A. Physical mapping and complementation analysis of transposon Tn5 mutations in Caulobacter crescentus: organization of transcriptional units in the hook gene cluster. J Bacteriol. 1984 Jun;158(3):897–904. doi: 10.1128/jb.158.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]



