Abstract
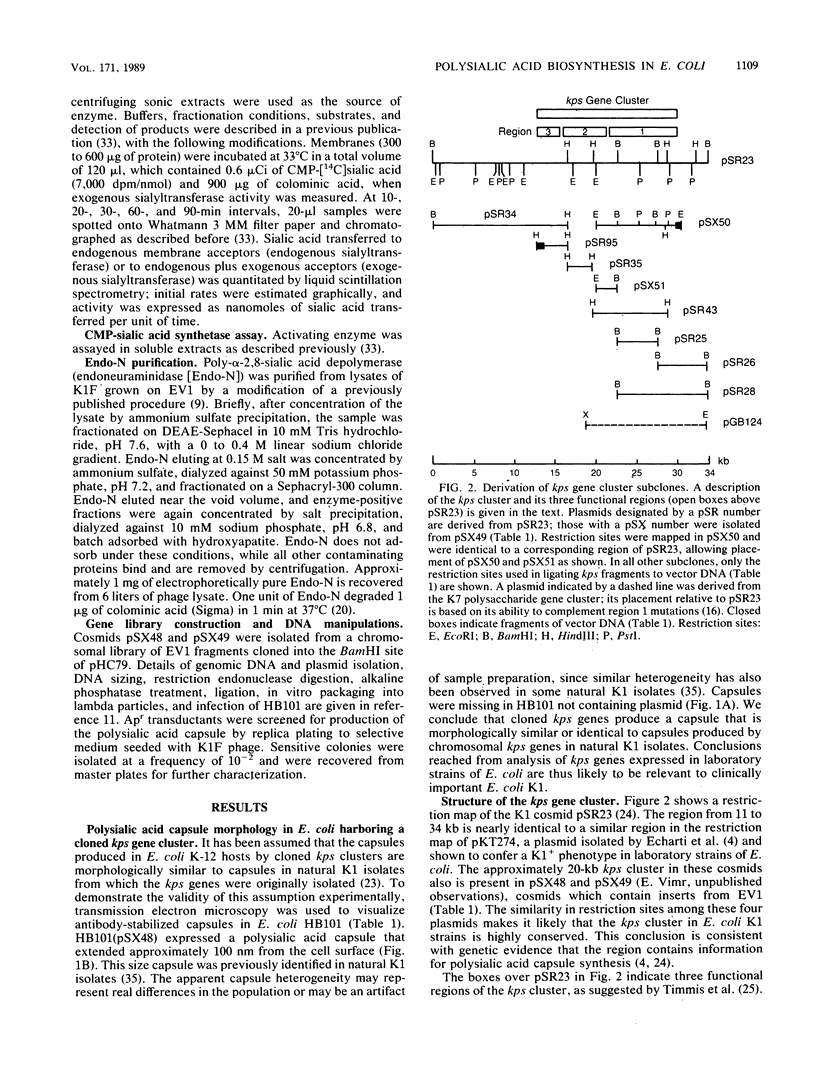
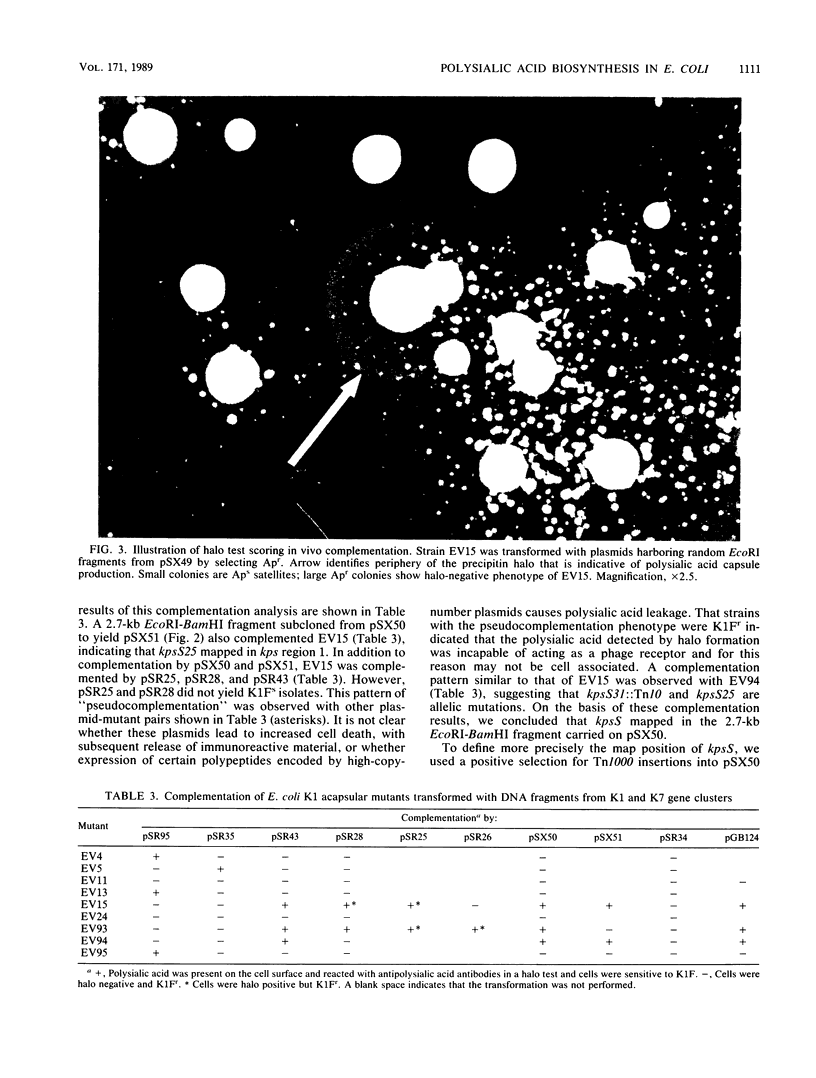
The kps gene cluster of Escherichia coli K1 encodes functions for sialic acid synthesis, activation, polymerization, and possibly translocation of polymer to the cell surface. The size and complexity of this membrane polysaccharide biosynthetic cluster have hindered genetic mapping and functional descriptions of the kps genes. To begin a detailed investigation of the polysialic acid synthetic mechanism, acapsular mutants were characterized to determine their probable defects in polymer synthesis. The mutants were tested for complementation with kps fragments subcloned from two separately isolated, functionally intact kps gene clusters. Complementation was assayed by immunological and biochemical methods and by sensitivity to the K1-specific bacteriophage K1F. The kps cluster consisted of a central 5.8-kilobase region that contained at least two genes coding for sialic acid synthetic enzymes, a gene encoding the sialic acid-activating enzyme, and a gene encoding the sialic acid polymerase. This biosynthetic region is flanked on one side by an approximately 2.8-kilobase region that contains a potential regulatory locus and at least one structural gene for a polypeptide that appears to function in polysialic acid assembly. Flanking the biosynthetic region on the opposite side is a 6- to 8.4-kilobase region that codes for at least three proteins which may also function in polymer assembly and possibly in translocating polymer to the outer cell surface. Results of transduction crosses supported these conclusions and indicated that some of the kps genes flanking the central biosynthetic region may not function directly in transporting polymer to the cell surface. The results also demonstrate that the map position and probable function of most of the kps cluster genes have been identified.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Echarti C., Hirschel B., Boulnois G. J., Varley J. M., Waldvogel F., Timmis K. N. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect Immun. 1983 Jul;41(1):54–60. doi: 10.1128/iai.41.1.54-60.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Leinonen M., Mäkelä P. H. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983 Aug 13;2(8346):355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- GHOSH S., ROSEMAN S. THE SIALIC ACIDS. IV. N-ACYL--D-GLUCOSAMINE 6-PHOSPHATE 2-EPIMERASE. J Biol Chem. 1965 Apr;240:1525–1530. [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Guyer M. S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- Hallenbeck P. C., Vimr E. R., Yu F., Bassler B., Troy F. A. Purification and properties of a bacteriophage-induced endo-N-acetylneuraminidase specific for poly-alpha-2,8-sialosyl carbohydrate units. J Biol Chem. 1987 Mar 15;262(8):3553–3561. [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson L., Holbein B. E. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J Bacteriol. 1985 Mar;161(3):861–867. doi: 10.1128/jb.161.3.861-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Sharma V., Orskov F. Genetic mapping of the K1 and K4 antigens (L) of Escherichia coli. Non-allelism of K(L) antigens with K antigens of O8:K27(A), O8:K8(L) and O9:K57(B). Acta Pathol Microbiol Scand B. 1976 Jun;84(3):125–131. [PubMed] [Google Scholar]
- Paakkanen J., Gotschlich E. C., Mäkelä P. H. Protein K: a new major outer membrane protein found in encapsulated Escherichia coli. J Bacteriol. 1979 Sep;139(3):835–841. doi: 10.1128/jb.139.3.835-841.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Bray D., Dankert B. M., Wright A. Direction of chain growth in polysaccharide synthesis. Science. 1967 Dec 22;158(3808):1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- Roberts I. S., Mountford R., Hodge R., Jann K. B., Boulnois G. J. Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1305–1310. doi: 10.1128/jb.170.3.1305-1310.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I., Mountford R., High N., Bitter-Suermann D., Jann K., Timmis K., Boulnois G. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol. 1986 Dec;168(3):1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Aparicio L. B., Reglero A., Ortiz A. I., Luengo J. M. A protein-sialyl polymer complex involved in colominic acid biosynthesis. Effect of tunicamycin. Biochem J. 1988 Apr 15;251(2):589–596. doi: 10.1042/bj2510589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr T. E., Troy F. A. Structure and biosynthesis of surface polymers containing polysialic acid in Escherichia coli. J Biol Chem. 1980 Mar 25;255(6):2332–2342. [PubMed] [Google Scholar]
- Rutishauser U., Watanabe M., Silver J., Troy F. A., Vimr E. R. Specific alteration of NCAM-mediated cell adhesion by an endoneuraminidase. J Cell Biol. 1985 Nov;101(5 Pt 1):1842–1849. doi: 10.1083/jcb.101.5.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Vann W. F. The K1 capsular polysaccharide of Escherichia coli. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S282–S286. doi: 10.1093/cid/10.supplement_2.s282. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Aaronson W., Vann W. F. Translocation of capsular polysaccharides in pathogenic strains of Escherichia coli requires a 60-kilodalton periplasmic protein. J Bacteriol. 1987 Dec;169(12):5489–5495. doi: 10.1128/jb.169.12.5489-5495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Finn C. W., Vann W. F., Aaronson W., Schneerson R., Kretschmer P. J., Garon C. F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981 Feb 19;289(5799):696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Vann W. F., Aaronson W. Genetic and molecular analyses of Escherichia coli K1 antigen genes. J Bacteriol. 1984 Feb;157(2):568–575. doi: 10.1128/jb.157.2.568-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Troy F. A., 2nd The chemistry and biosynthesis of selected bacterial capsular polymers. Annu Rev Microbiol. 1979;33:519–560. doi: 10.1146/annurev.mi.33.100179.002511. [DOI] [PubMed] [Google Scholar]
- Tsui F. P., Boykins R. A., Egan W. Structural and immunological studies of the Escherichia coli K7 (K56) capsular polysaccharide. Carbohydr Res. 1982 Apr 16;102:263–271. doi: 10.1016/s0008-6215(00)88068-4. [DOI] [PubMed] [Google Scholar]
- Vann W. F., Silver R. P., Abeijon C., Chang K., Aaronson W., Sutton A., Finn C. W., Lindner W., Kotsatos M. Purification, properties, and genetic location of Escherichia coli cytidine 5'-monophosphate N-acetylneuraminic acid synthetase. J Biol Chem. 1987 Dec 25;262(36):17556–17562. [PubMed] [Google Scholar]
- Vijay I. K., Troy F. A. Properties of membrane-associated sialyltransferase of Escherichia coli. J Biol Chem. 1975 Jan 10;250(1):164–170. [PubMed] [Google Scholar]
- Vimr E. R., Green L., Miller C. G. Oligopeptidase-deficient mutants of Salmonella typhimurium. J Bacteriol. 1983 Mar;153(3):1259–1265. doi: 10.1128/jb.153.3.1259-1265.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
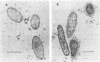
- Vimr E. R., McCoy R. D., Vollger H. F., Wilkison N. C., Troy F. A. Use of prokaryotic-derived probes to identify poly(sialic acid) in neonatal neuronal membranes. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1971–1975. doi: 10.1073/pnas.81.7.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J Bacteriol. 1985 Nov;164(2):845–853. doi: 10.1128/jb.164.2.845-853.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimr E. R., Troy F. A. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J Bacteriol. 1985 Nov;164(2):854–860. doi: 10.1128/jb.164.2.854-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Whitfield C., Vimr E. R., Costerton J. W., Troy F. A. Protein synthesis is required for in vivo activation of polysialic acid capsule synthesis in Escherichia coli K1. J Bacteriol. 1984 Jul;159(1):321–328. doi: 10.1128/jb.159.1.321-328.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T. A model for three-point analysis of random general transduction. Genetics. 1966 Aug;54(2):405–410. doi: 10.1093/genetics/54.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]