Abstract
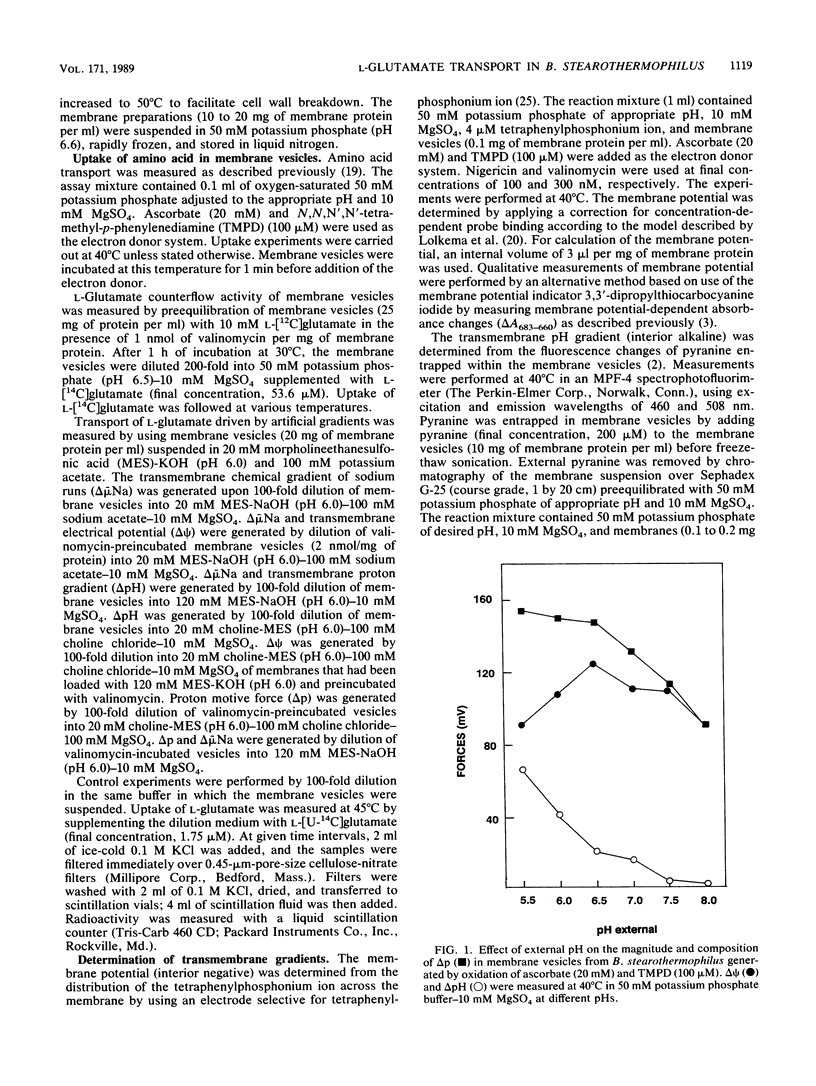
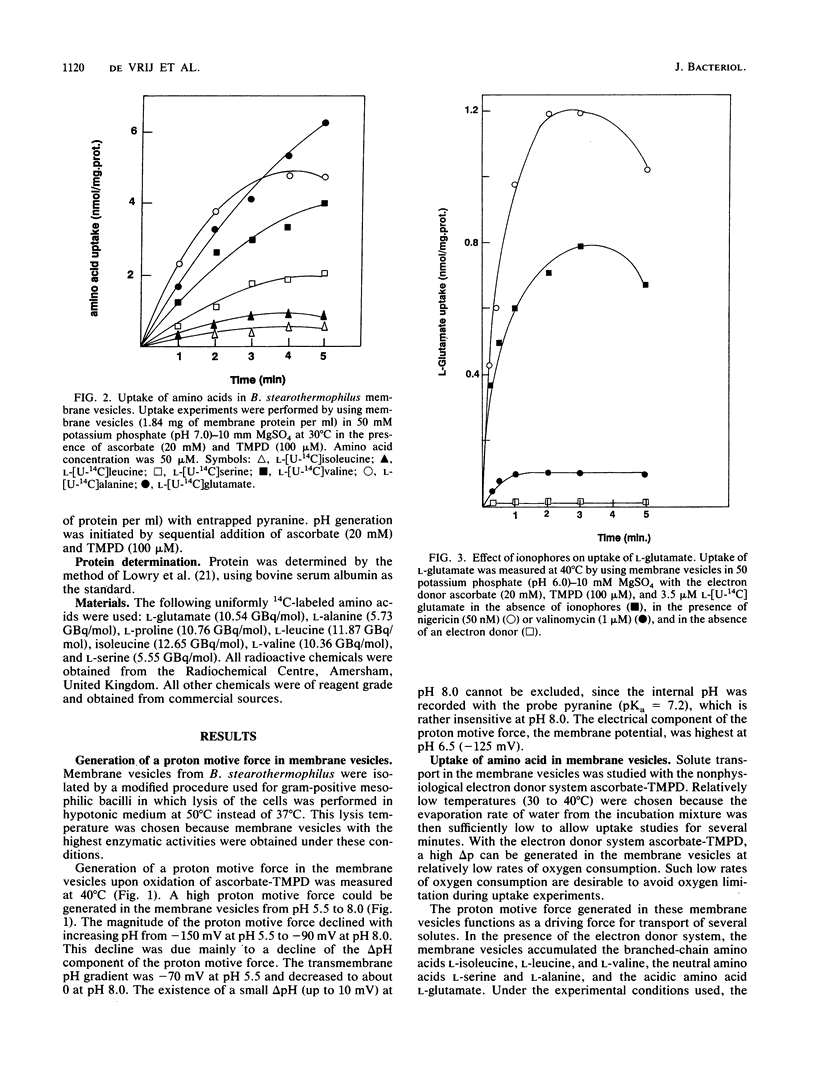
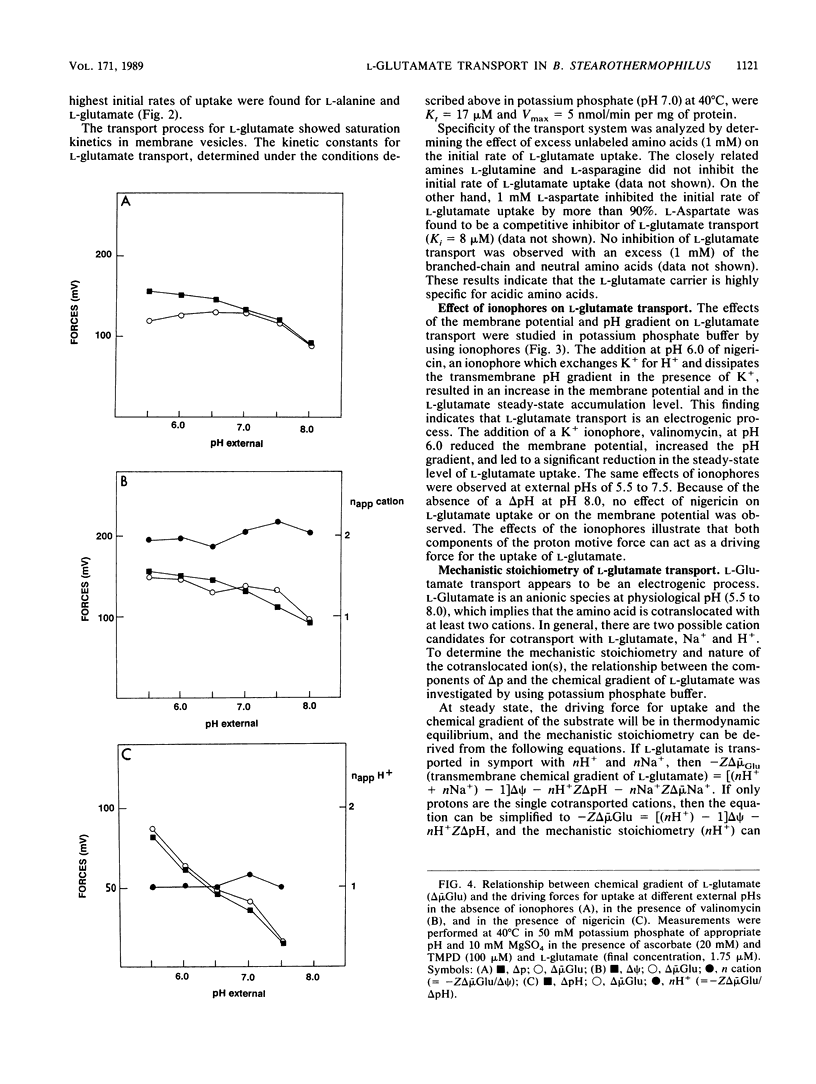
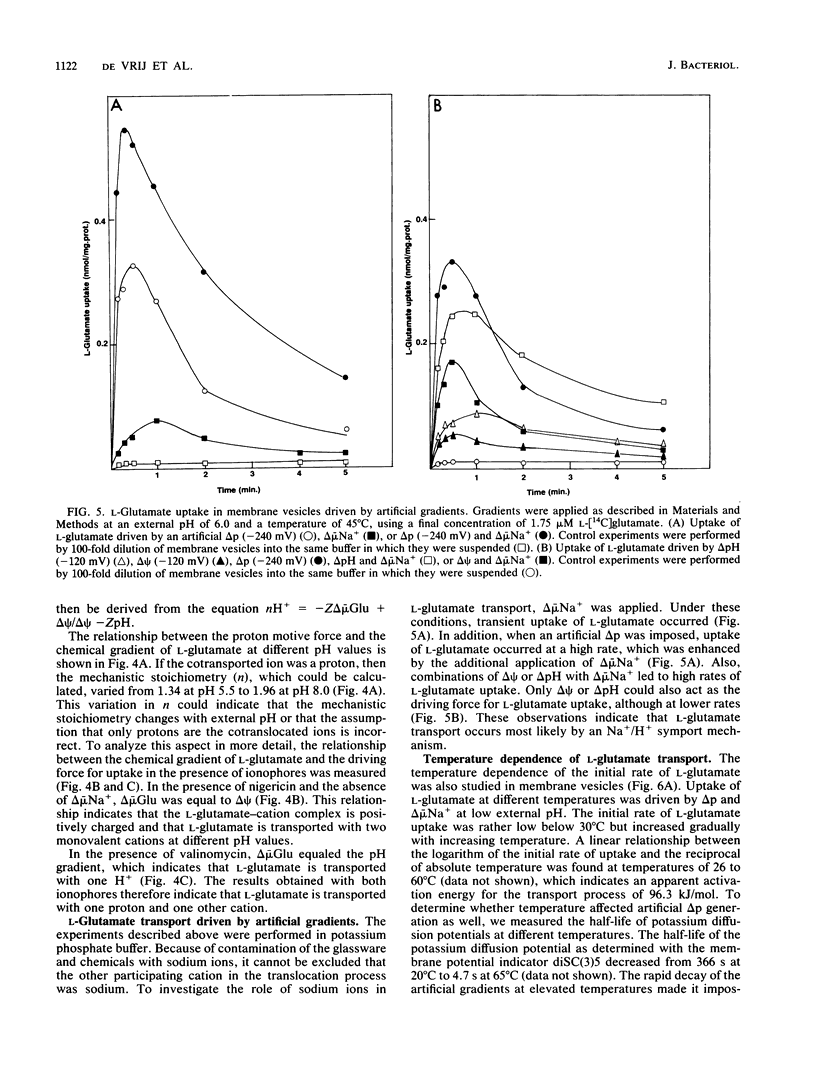
In the presence of electrochemical energy, several branched-chain neutral and acidic amino acids were found to accumulate in membrane vesicles of Bacillus stearothermophilus. The membrane vesicles contained a stereo-specific transport system for the acidic amino acids L-glutamate and L-aspartate, which could not translocate their respective amines, L-glutamine and L-asparagine. The transport system was thermostable (Ti = 70 degrees C) and showed highest activities at elevated temperatures (60 to 65 degrees C). The membrane potential or pH gradient could act as the driving force for L-glutamate uptake, which indicated that the transport process of L-glutamate is electrogenic and that protons are involved in the translocation process. The electrogenic character implies that the anionic L-glutamate is cotransported with at least two monovalent cations. To determine the mechanistic stoichiometry of L-glutamate transport and the nature of the cotranslocated cations, the relationship between the components of the proton motive force and the chemical gradient of L-glutamate was investigated at different external pH values in the absence and presence of ionophores. In the presence of either a membrane potential or a pH gradient, the chemical gradient of L-glutamate was equivalent to that specific gradient at different pH values. These results cannot be explained by cotransport of L-glutamate with two protons, assuming thermodynamic equilibrium between the driving force for uptake and the chemical gradient of the substrate. To determine the character of the cotranslocated cations, L-glutamate uptake was monitored with artificial gradients. It was established that either the membrane potential, pH gradient, or chemical gradient of sodium ions could act as the driving force for L-glutamate uptake, which indicated that L-glutamate most likely is cotranslocated in symport with one proton and on sodium ion.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clement N. R., Gould J. M. Pyranine (8-hydroxy-1,3,6-pyrenetrisulfonate) as a probe of internal aqueous hydrogen ion concentration in phospholipid vesicles. Biochemistry. 1981 Mar 17;20(6):1534–1538. doi: 10.1021/bi00509a019. [DOI] [PubMed] [Google Scholar]
- De Vrij W., Bulthuis R. A., Konings W. N. Comparative study of energy-transducing properties of cytoplasmic membranes from mesophilic and thermophilic Bacillus species. J Bacteriol. 1988 May;170(5):2359–2366. doi: 10.1128/jb.170.5.2359-2366.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. Sodium ion transport decarboxylases and other aspects of sodium ion cycling in bacteria. Microbiol Rev. 1987 Sep;51(3):320–340. doi: 10.1128/mr.51.3.320-340.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 1. Proton-dependent and sodium ion dependent binding of glutamate to a glutamate carrier in the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1954–1959. doi: 10.1021/bi00277a033. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 2. Kinetics of glutamate transport driven by artificially imposed proton and sodium ion gradients across the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1959–1965. doi: 10.1021/bi00277a034. [DOI] [PubMed] [Google Scholar]
- Goto K., Hirata H., Kagawa Y. A stable Na+/H+ antiporter of thermophilic bacterium PS3. J Bioenerg Biomembr. 1980 Aug;12(3-4):297–308. doi: 10.1007/BF00744690. [DOI] [PubMed] [Google Scholar]
- Hellingwerf K. J., Konings W. N. The energy flow in bacteria: the main free energy intermediates and their regulatory role. Adv Microb Physiol. 1985;26:125–154. doi: 10.1016/s0065-2911(08)60396-3. [DOI] [PubMed] [Google Scholar]
- Hirata H., Kambe T., Kagawa Y. A purified alanine carrier composed of a single polypeptide from thermophilic bacterium PS3 driven by either proton or sodium ion gradient. J Biol Chem. 1984 Sep 10;259(17):10653–10656. [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Active transport of alanine by thermostable membrane vesicles isolated from a thermophilic bacterium. J Biochem. 1976 Jun;79(6):1157–1166. doi: 10.1093/oxfordjournals.jbchem.a131171. [DOI] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Isolation of the alanine carrier from the membranes of a thermophilic bacterium and its reconstitution into vesicles capable of transport. J Supramol Struct. 1977;6(1):77–84. doi: 10.1002/jss.400060106. [DOI] [PubMed] [Google Scholar]
- Hirata H., Sone N., Yoshida M., Kagawa Y. Solubilization and partial purification of alanine carrier from membranes of a thermophilic bacterium and its reconstitution into functional vesicles. Biochem Biophys Res Commun. 1976 Apr 5;69(3):665–671. doi: 10.1016/0006-291x(76)90927-x. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Daatselaar M. C.C. Transport of L-glutamate and L-aspartate by membrane vesicles of Bacillus subtilis W 23. FEBS Lett. 1972 Aug 15;24(3):260–264. doi: 10.1016/0014-5793(72)80368-5. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Bisschop A., Veenhuis M., Vermeulen C. A. New procedure for the isolation of membrane vesicles of Bacillus subtilis and an electron microscopy study of their ultrastructure. J Bacteriol. 1973 Dec;116(3):1456–1465. doi: 10.1128/jb.116.3.1456-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings W. N., Freese E. Amino acid transport in membrane vesicles of Bacillus subtilis. J Biol Chem. 1972 Apr 25;247(8):2408–2418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Reizer J., Grossowicz N. Properties of alpha-aminoisobutyric acid transport in a thermophilic microorganism. J Bacteriol. 1974 May;118(2):414–424. doi: 10.1128/jb.118.2.414-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J., Driessen A. J., Konings W. N. Sodium-dependent transport of neutral amino acids by whole cells and membrane vesicles of Streptococcus bovis, a ruminal bacterium. J Bacteriol. 1988 Aug;170(8):3531–3536. doi: 10.1128/jb.170.8.3531-3536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Sone N. Cytochrome oxidase from thermophilic bacterium PS3. Methods Enzymol. 1986;126:145–153. doi: 10.1016/s0076-6879(86)26016-4. [DOI] [PubMed] [Google Scholar]
- Wakayama N. Membrane properties of an extreme thermophile. II. Membrane functions underlying leucine transport and their relation with thermotropic phase transitions. J Biochem. 1978 Jun;83(6):1693–1698. doi: 10.1093/oxfordjournals.jbchem.a132082. [DOI] [PubMed] [Google Scholar]



