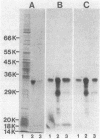
Abstract
Surface antigens of Rhizobium leguminosarum biovar viciae strain 248 were characterized by using polyclonal and monoclonal antibodies. With Western immunoblotting as the criterion, an antiserum raised against living whole cells recognized mainly flagellar antigens and the O-antigen-containing part of the lipopolysaccharide (LPS). Immunization of mice with a peptidoglycan-outer membrane complex yielded eight monoclonal antibodies, of which three reacted with LPS and five reacted with various sets of outer membrane protein antigens. The observation that individual monoclonal antibodies react with sets of related proteins is discussed. Studies of the influence of calcium deficiency and LPS alterations on surface antigenicity showed that in normally grown wild-type cells, the O-antigenic side chain of LPS blocks binding of an antibody to a deeper-lying antigen. This antigen is accessible to antibodies in cells grown under calcium limitation as well as in O-antigen-lacking mutant cells. Two of the antigen groups which can be distinguished in cell envelopes of free-living bacteria were depleted in cell envelopes of isolated bacteroids, indicating that the monoclonal antibodies could be useful tools for studying the differentiation process from free-living bacteria to bacteroids.
Full text
PDF

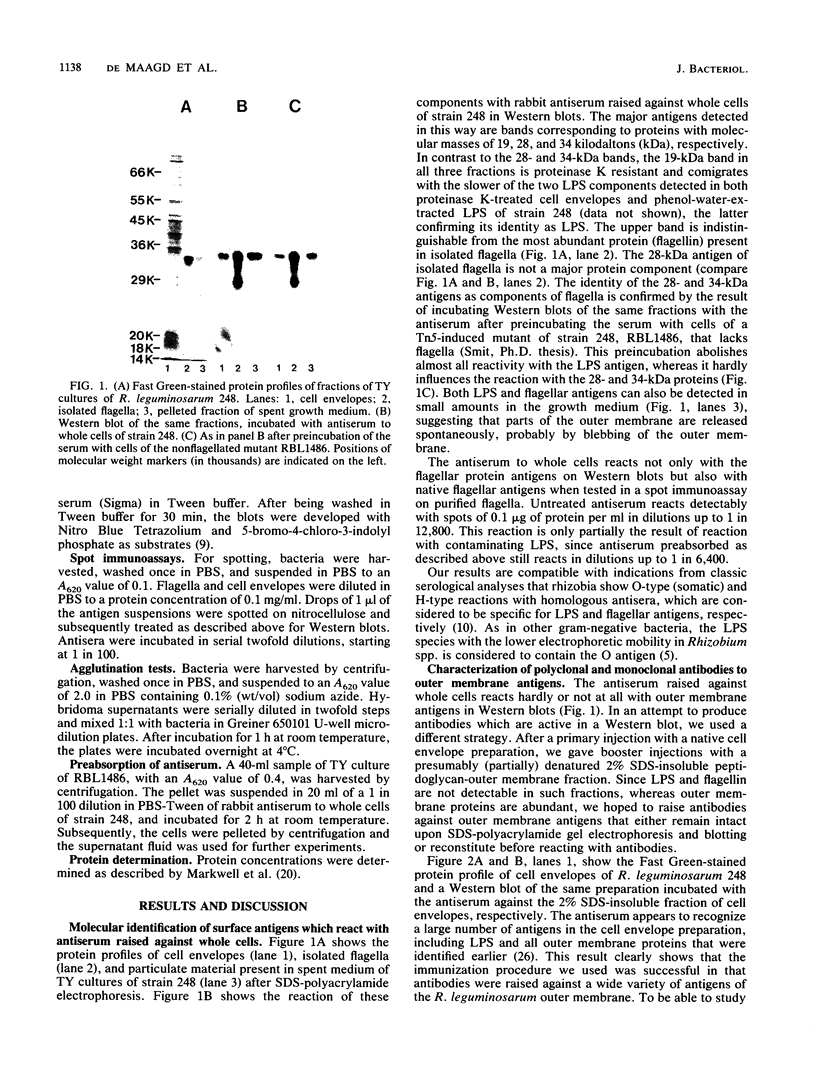



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Brewin N. J., Robertson J. G., Wood E. A., Wells B., Larkins A. P., Galfre G., Butcher G. W. Monoclonal antibodies to antigens in the peribacteroid membrane from Rhizobium-induced root nodules of pea cross-react with plasma membranes and Golgi bodies. EMBO J. 1985 Mar;4(3):605–611. doi: 10.1002/j.1460-2075.1985.tb03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W. Heterogeneity of Rhizobium lipopolysaccharides. J Bacteriol. 1984 Jun;158(3):1012–1017. doi: 10.1128/jb.158.3.1012-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Ashman L. K. The use of alkaline phosphatase-conjugated anti-immunoglobulin with immunoblots for determining the specificity of monoclonal antibodies to protein mixtures. Methods Enzymol. 1986;121:497–509. doi: 10.1016/0076-6879(86)21050-2. [DOI] [PubMed] [Google Scholar]
- HUMPHREY B., VINCENT J. M. Calcium in cell walls of Rhizobium trifolii. J Gen Microbiol. 1962 Nov;29:557–561. doi: 10.1099/00221287-29-3-557. [DOI] [PubMed] [Google Scholar]
- Humphrey B. A., Vincent J. M. The effect of calcium nutrition on the production of diffusible antigens by Rhizobium trifolii. J Gen Microbiol. 1965 Oct;41(1):109–118. doi: 10.1099/00221287-41-1-109. [DOI] [PubMed] [Google Scholar]
- John M., Schmidt J., Wieneke U., Kondorosi E., Kondorosi A., Schell J. Expression of the nodulation gene nod C of Rhizobium meliloti in Escherichia coli: role of the nod C gene product in nodulation. EMBO J. 1985 Oct;4(10):2425–2430. doi: 10.1002/j.1460-2075.1985.tb03951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M., Schmidt J., Wieneke U., Krüssmann H. D., Schell J. Transmembrane orientation and receptor-like structure of the Rhizobium meliloti common nodulation protein NodC. EMBO J. 1988 Mar;7(3):583–588. doi: 10.1002/j.1460-2075.1988.tb02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Noel K. D., Vandenbosch K. A., Kulpaca B. Mutations in Rhizobium phaseoli that lead to arrested development of infection threads. J Bacteriol. 1986 Dec;168(3):1392–1401. doi: 10.1128/jb.168.3.1392-1401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puvanesarajah V., Schell F. M., Gerhold D., Stacey G. Cell surface polysaccharides from Bradyrhizobium japonicum and a nonnodulating mutant. J Bacteriol. 1987 Jan;169(1):137–141. doi: 10.1128/jb.169.1.137-141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., John M., Wieneke U., Krüssmann H. D., Schell J. Expression of the nodulation gene nodA in Rhizobium meliloti and localization of the gene product in the cytosol. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9581–9585. doi: 10.1073/pnas.83.24.9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman C. A., Rossen L., Johnston A. W., Downie J. A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl-carrier protein and is regulated by nodD plus a factor in pea root exudate. EMBO J. 1986 Apr;5(4):647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Ugalde R. A., Coira J. A., Brill W. J. Biosynthesis of a galactose-and galacturonic acid-containing polysaccharide in Rhizobium meliloti. J Bacteriol. 1986 Oct;168(1):270–275. doi: 10.1128/jb.168.1.270-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M., HUMPHREY B. A. PARTITION OF DIVALENT CATIONS BETWEEN BACTERIAL WALL AND CELL CONTENTS. Nature. 1963 Jul 13;199:149–151. doi: 10.1038/199149a0. [DOI] [PubMed] [Google Scholar]
- VINCENT J. M. Influence of calcium and magnesium on the growth of rhizobium. J Gen Microbiol. 1962 Sep;28:653–663. doi: 10.1099/00221287-28-4-653. [DOI] [PubMed] [Google Scholar]
- Vincent J. M., Humphrey B. A. Modification of the antigenic surface of Rhizobium trifolii by a deficiency of calcium. J Gen Microbiol. 1968 Dec;54(3):397–405. doi: 10.1099/00221287-54-3-397. [DOI] [PubMed] [Google Scholar]
- Westgeest A. A., Bons J. C., Van den Brink H. G., Aarden L. A., Smeenk R. J. An improved incubation apparatus for Western blots used for the detection of antinuclear antibodies. J Immunol Methods. 1986 Dec 24;95(2):283–288. doi: 10.1016/0022-1759(86)90417-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson T. The problems and the values of objective nursing observations in psychiatric nursing care. J Adv Nurs. 1979 Mar;4(2):151–159. doi: 10.1111/j.1365-2648.1979.tb02996.x. [DOI] [PubMed] [Google Scholar]
- de Maagd R. A., Lugtenberg B. Fractionation of Rhizobium leguminosarum cells into outer membrane, cytoplasmic membrane, periplasmic, and cytoplasmic components. J Bacteriol. 1986 Sep;167(3):1083–1085. doi: 10.1128/jb.167.3.1083-1085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R. A., Rao A. S., Mulders I. H., Goosen-de Roo L., van Loosdrecht M. C., Wijffelman C. A., Lugtenberg B. J. Isolation and characterization of mutants of Rhizobium leguminosarum bv. viciae 248 with altered lipopolysaccharides: possible role of surface charge or hydrophobicity in bacterial release from the infection thread. J Bacteriol. 1989 Feb;171(2):1143–1150. doi: 10.1128/jb.171.2.1143-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Maagd R., van Rossum C., Lugtenberg B. J. Recognition of individual strains of fast-growing rhizobia by using profiles of membrane proteins and lipopolysaccharides. J Bacteriol. 1988 Aug;170(8):3782–3785. doi: 10.1128/jb.170.8.3782-3785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ley P., Kuipers O., Tommassen J., Lugtenberg B. O-antigenic chains of lipopolysaccharide prevent binding of antibody molecules to an outer membrane pore protein in Enterobacteriaceae. Microb Pathog. 1986 Feb;1(1):43–49. doi: 10.1016/0882-4010(86)90030-6. [DOI] [PubMed] [Google Scholar]