Abstract
During a critical period of postnatal development, between postnatal days 6 and 14, a high-frequency stimulation train (100 Hz for 1 s) to the mossy fibers induces a long-term depression (LTD) of synaptic efficacy of 29 ± 5.2%. This form of LTD is homosynaptic. It is independent of the activation of N-methyl-d-aspartate or metabotropic glutamate receptors but needs an increase in calcium into the postsynaptic cell for its induction. At the same synapse LTD also could be induced by low-frequency stimulation of the mossy fibers (1 Hz for 15 min). In this case the magnitude of the depression is 37 ± 4.2%. This form of LTD is N-methyl-d-aspartate independent but requires the activation of metabotropic glutamate receptors because it is prevented by (S)-α-methyl-4-carboxyphenylglycine (1 mM). Moreover its induction appears to be presynaptic, because, in contrast with the high-frequency one, it is not blocked by loading the postsynaptic cell with the calcium chelator EGTA or bis-(-o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA). Saturation of one form of LTD does not occlude the other, suggesting that high and low frequency-induced LTD depend on distinct mechanisms of induction and expression. Quantal (noise deconvolution) analysis of minimal excitatory postsynaptic potentials shows, similarly to high-frequency LTD, a decrease in quantal content without any change in quantal size after low-frequency LTD, suggesting that in both forms of LTD the site where maintenance mechanisms are located is presynaptic.
Activity-dependent changes in synaptic strength such as those occurring during long-term potentiation (LTP) or long-term depression (LTD) are considered to be crucial for information storage in the brain. In particular, these processes play an essential role during the period of synapse formation where they are thought to contribute to the development of the mature neuronal circuitry (1). It commonly is accepted that LTD prevails in neonatal and juvenile animals where it precedes the developmental onset of LTP (2, 3). In the CA1 region, prolonged low-frequency stimulation (LFS) of the Schaffer collateral pathway induces a LTD, which is homosynaptic and N-methyl-d-aspartate (NMDA) dependent (4, 5). In contrast, during the first postnatal week a sustained stimulation of the same pathway produces another form of LTD, which depends on the combined activation of voltage-dependent calcium channels and metabotropic glutamate receptors (mGluRs; ref. 6). This suggests that a developmentally regulated switch in the mechanisms underlying LTD occurs in the immediate postnatal period. In spite of the increasing number of reports on LTD in the CA1 hippocampal region, in the CA3 area LTD is not fully characterized. This is partially because of the fact that within this area, mossy fibers start to develop immediately after birth and reach complete maturation toward the end of the third postnatal week (7). Previous work from this laboratory has shown that during the second postnatal week, a high-frequency stimulation (HFS) of the mossy fibers induces a homosynaptic, NMDA-independent form of LTD (8). Although the induction of this form of LTD appears to be postsynaptic (9), its expression seems to be presynaptic as indicated by the reduction in transmitter release probability (10). We now report that in the same region a sustained LFS of the mossy fibers induces another form of LTD that is NMDA independent and requires activation of presynaptic mGluR for its induction. Both of these forms of LTD might coexist in the same neuron, because saturation of one does not impair the other. Despite a different site of induction, the maintenance of both HFS or LFS LTD is presynaptic as suggested by quantal analysis of minimal excitatory postsynaptic potentials (EPSPs).
METHODS
Experiments were performed on hippocampal slices obtained from postnatal day (P) 9–14 Wistar rats (P0 is the day of birth) according to the methods already described (8). Briefly, animals were decapitated after being anesthesized with i.p. injection of uretane (2 g Kg−1). The brain was quickly removed from the skull, and the hippocampi were dissected free. Transverse 500- to 600-μm thick slices were cut with a tissue chopper or with a vibroslicer and maintained at room temperature (22–24°C) in oxygenated artificial cerebrospinal fluid (ACSF) containing: 126 mM NaCl, 3.5 mM KCl, 1.2 mM NaH2PO4, 1.3 mM MgCl2, 2 mM CaCl2, 25 mM NaHCO3, 11 mM glucose (pH 7.3), saturated with 95% O2 and 5% CO2. After incubation in ACSF for at least 1 hr, an individual slice was transferred to a submerged recording chamber, continuously superfused at 33–34°C with oxygenated ACSF at a rate of 3 ml/min.
Field EPSP (fEPSP) Recordings.
fEPSPs were recorded with a glass microelectrode filled with NaCl (2 M, pipette resistance 2–5 MΩ) placed in the stratum lucidum of the CA3 area. fEPSPs were evoked at the frequency of 0.05 Hz with bipolar twisted NiCr-insulated electrodes (50 μm o.d.), positioned on the mossy fiber tract close to the recording electrode. The duration of the stimulus was 100 μs; the stimulation intensity corresponded to that necessary to obtain a response equal to 50% of the maximal fEPSP. Because of the complexity of the dentate gyrus CA3 circuitry (11) and the small number of mossy fibers present in young animals, a site that produced a synaptic response with minimal stimulation current was searched. To induce LTD, a HFS or LFS paradigm was used (referred to as HFS or LFS LTD). HFS LTD was obtained by stimulating the mossy fibers with two trains at 100 Hz for 1 s (10 s apart). LFS LTD was obtained by stimulating the same afferent pathway at 1 Hz for 15 min. For occlusion experiments, to ensure a maximal recruitment of presynaptic fibers, the intensity of stimulation during LFS and HFS was increased to obtain the maximal field response. Responses were recorded with a Dam 80 Differential Amplifier (WPI Instruments, Waltham, MA), acquired, and analyzed with the LTP software package (courtesy of W. W. Anderson, Bristol University, United Kingdom).
Patch Clamp Whole-Cell Recordings.
Whole-cell recordings (current clamp configuration) were obtained with patch electrodes (3–5 MΩ) containing: 135 mM K+-gluconate, 2 mM Mg ATP, 10 mM Hepes, 10 mM EGTA or 10 mM bis-(-o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), 1 mM CaCl2; the pH was adjusted to 7.3 with KOH. Minimal EPSPs with occasional failures were recorded (at resting membrane potential, ranging between −50 and −62 mV) with a standard patch clamp amplifier (Axoclamp 2A, Axon Instruments, Foster City, CA), after stimulation (at 0.06 Hz) of the mossy fiber tract. To see whether EPSPs really reflected activation of mossy and not associative-commissural fibers, in five experiments two stimulating electrodes were used: one positioned on the mossy fiber tract to activate the mossy fibers and one on the stratum radiatum to activate the associative-commissural fibers. Then, the effect of 2-(2,3-dicarboxycyclopropyl) glycine (DCG-IV), a selective agonist of mGluR2/3, was tested (in the presence of d-AP5, 50 μM). mGluR2/3 are localized only on the mossy and not on the associative-commissural fibers, and it has been shown that activation of these receptors reduces only the mossy fiber EPSP (12). As expected, DCG-IV (1 μM), reversibly and completely abolished mossy fiber EPSPs although it did not modify associative-commissural EPSPs, indicating that indeed mossy fibers were stimulated by the electrode positioned on the mossy fiber tract. Paired pulses (50-ms interval) were routinely applied. A response having an amplitude smaller than the background noise (0.18 ± 0.02 mV), recorded during the 50-ms interval preceding the stimulus artefact, was considered a failure. This event never occurred in response to a second stimulus elicited 50 ms after the first one, strongly suggesting that a real failure of transmitter release and not a failure of activation of presynaptic fibers occurred.
Drugs were dissolved in artificial cerebrospinal fluid and applied through a three-way tap system by changing the superfusion solution to one that differed only in its content of drug(s). The ratio of flow rate to bath volume ensured complete exchange within 1 min. Drugs used were: (+)-3-(2-carboxy-piperazin-4-yl)-propyl-1-phosphonic acid (CPP), or d(-)-2-amino-5-phosphonopentanoic acid (D-AP5), (S)-α-methyl-4-carboxyphenylglycine (MCPG), 2-(2,3-dicarboxycyclopropyl) glycine (DCG-IV), purchased from Tocris Cookson (Bristol, U.K.), and picrotoxin from Research Biochemicals. In both extracellular and patch clamp whole-cell recordings, to avoid contamination with the NMDA receptor-dependent form of LTP present in the CA3 region after activation of the associative-commissural fibers (13), experiments were routinely performed in the presence of the NMDA receptor antagonist CPP (20 μM) or d-AP5 (50 μM). In patch clamp experiments, picrotoxin (50 μM) was present to prevent activation of γ-aminobutyric acid type A receptors. These compounds were applied in the bath at least 15 min before delivering HFS or LFS and usually maintained for the entire duration of the experiment (>1 hr). Data are expressed as mean ± SEM.
Quantal (Noise Deconvolution) Analysis.
To determine the mean quantal content m, the “unconstrained” noise deconvolution technique has been used. EPSPs were measured by using the first component score obtained with application of the principal component analysis (14). The scores were determined from a window from the onset to the peak of the EPSP. The integral covariance measure is strongly correlated with the peak amplitude of the EPSP but gives a better signal-to-noise ratio. The covariance amplitudes are termed EPSP amplitudes for simplicity in Figs. 4 and 5. The algorithm searched for discrete distributions (bars) with coordinates xi (distance from 0) and Pi (heights). The weighted mean interval between the bars was used to define the quantal size v
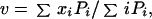 |
and m = E/v, where E is the mean EPSP amplitude; 0.15 v was taken as intrinsic quantal variance (14). Because stimuli had to be applied at a low rate to avoid amplitude depression, the sample size n was usually small (≈100). Computer simulations (14) show that small samples can give reliable estimates provided v/Sn >2.5, where Sn is the SD of the noise.
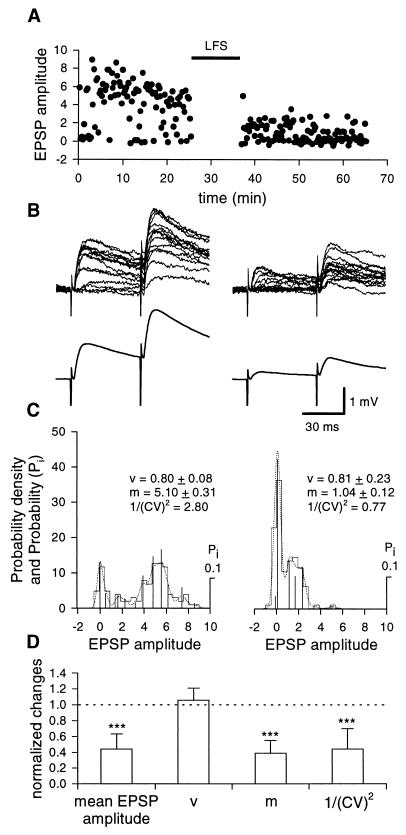
Figure 4.
Presynaptic expression of LFS LTD. (A) Plot of EPSPs amplitude (dots) against time, before and after LFS (filled bar) evoked at 0.06 Hz by minimal stimulation of the mossy fibers (representative experiment performed at postnatal day 12 with a patch pipette containing EGTA). (B) Upper traces are superimposed single EPSPs evoked by two stimuli (50 ms apart), before (Left) and 30 min after (Right) LFS. Note the increased number of failures after LFS. Lower traces are the average of all responses evoked before (Left) or after (Right) LFS. (C) Probability density and probability (Pi) calculated by deconvolution analysis of minimal evoked EPSPs, obtained from the representative cell shown in A and B, before (Left) and after (Right) LFS LTD. Deconvolved components are shown by vertical bars; experimental and predictive distributions are represented by the histograms and dotted lines, respectively. (Insets) Estimated quantal size (v), mean quantal content, (m), and the inverse square of the coefficient of variation (1/(CV)2). Numbers of samples were 98 and 102 before and after LFS, respectively. (D) Each column represents the normalized change in mean EPSP amplitude, quantal size, quantal content, and inverse square of the coefficient of variation after LFS LTD in comparison to prestimulus controls obtained in seven cells loaded with EGTA or BAPTA. Bars are SD. ∗∗∗, P < 0.001.
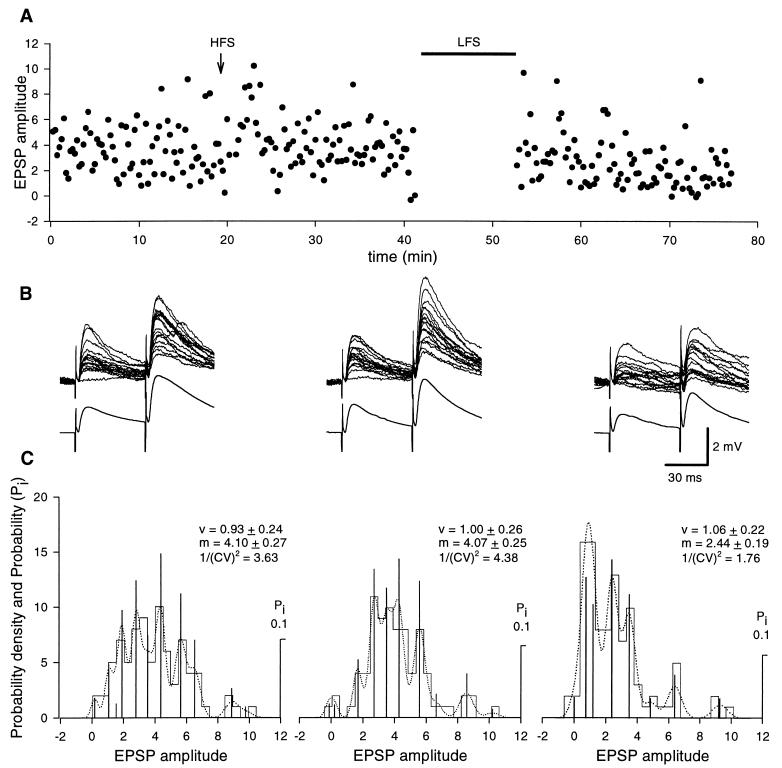
Figure 5.
Chelation of postsynaptic [Ca2+]i with EGTA prevents HFS, but not LFS, LTD. (A) Plot of EPSPs amplitude (dots) against time, before and after HFS (arrow) and LFS (filled bar) protocol, evoked at 0.06 Hz by minimal stimulation of the mossy fibers. (B) Upper traces are superimposed single EPSPs evoked by two stimuli (50 ms apart), before (Left), 20 min after HFS (Center), and LFS (Right). Note the increased number of failures after LFS. Lower traces are the average of all responses evoked before or after HFS and LFS. (C) Probability density and probability (Pi) calculated by deconvolution analysis of minimal evoked EPSPs, obtained from the representative cell shown in A and B (from a postnatal day 11 rat), before (Left) and after HFS (Center), or LFS (Right). Deconvolved components are shown by vertical bars; experimental and predictive distributions are represented by the histograms and dotted lines, respectively. Numbers of samples were 100, 103, 101, in control, after HFS, and after LFS, respectively. (Insets) Estimated quantal size (v), mean quantal content (m), and the inverse square of the coefficient of variation (1/(CV)2). No changes in quantal content or quantal size were observed after HFS, whereas a clear reduction in quantal content was found after LFS.
RESULTS
Extracellular Recordings.
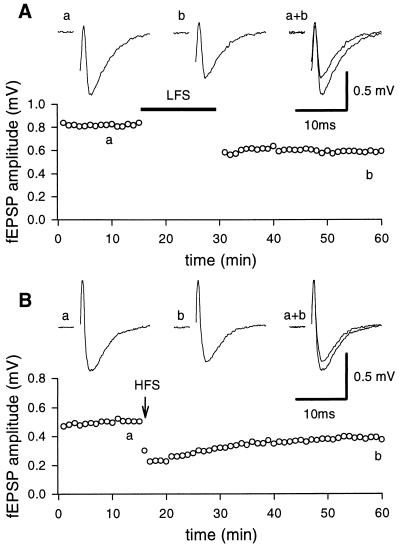
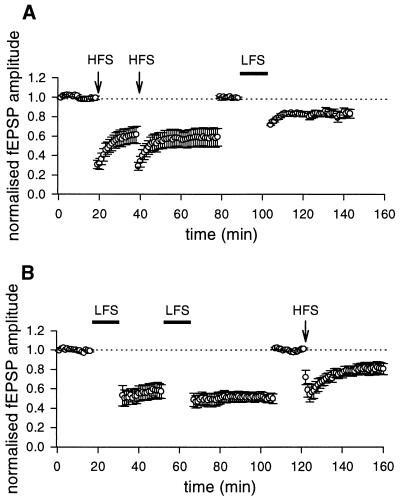
As shown in Fig. 1B, a HFS train delivered to the mossy fibers during a critical period of postnatal development, in the presence of d-AP5 (50 μM), induced a LTD of the fEPSP in the CA3 area. In five slices, 40 min after the HFS train, a depression of 29 ± 5.2% was observed. The depression increased further after a second or third conditioning train and eventually reached saturation (36.3 ± 7.1%).
Figure 1.
LTD induced by LFS and HFS protocol. The graphs represent the amplitude of the fEPSP plotted against time before and after LFS protocol (filled bar, A) or HFS (arrow, B), respectively. Each point represents the mean of three responses evoked by stimulation of the mossy fiber tract at 0.05 Hz. (Insets) fEPSPs recorded at the time indicated by the letters on the graphs. Each trace is the average of three successive fEPSPs (artefacts of stimulation have been truncated). Postnatal days 12 and 10 rats in A and B, respectively.
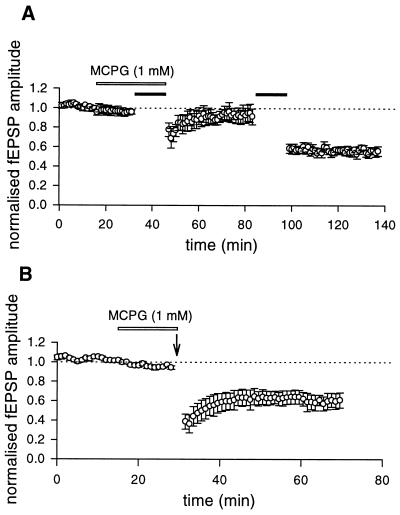
In the CA1 region of neonatal (6) or juvenile rats (15) or in the CA3 area of adult mice (16), a mGluR-dependent form of LTD can be induced by a sustained LFS of afferent pathways. To see whether a similar form of LTD also could be obtained in the CA3 region of neonatal rats, mossy fibers were stimulated (in the presence of NMDA receptor antagonists) at 1 Hz for 15 min. During the LFS paradigm, in the majority of the cases (n = 7) fEPSPs underwent an initial facilitation followed by a partial recovery toward control levels. Only in two cases, the initial facilitation was followed by a depression below the baseline level. After LFS a long-lasting depression of the synaptic response was obtained (40 min after LFS the magnitude of LTD was 36.7 ± 5.5, n = 9, Fig. 1A). A saturation of LFS LTD (43.7 ± 5.1%, n = 9) was achieved after application of two or three conditioning trains. Like HFS LTD, LFS LTD was homosynaptic. When two independent pathways were alternatively stimulated, depression of the synaptic strength occurred only in those synapses receiving the conditioning stimuli, whereas the control synapses were unaffected (not shown). The involvement of mGluR in LFS LTD was tested by using the competitive antagonist MCPG. MCPG (1 mM) prevented the induction of LFS LTD (n = 12). However, a second conditioning LFS train delivered after MCPG was washed out was able to induce a LTD (32.9 ± 9.7%, n = 4, Fig. 2A). As already described (9) MCPG did not block the HFS LTD (Fig. 2B).
Figure 2.
LFS but not HFS LTD depends on the activation of mGluR. (A) Pooled data (mean ± SEM, n = 4) of normalized fEPSPs plotted as a function of time. A LFS protocol (filled bar) delivered in the presence of MCPG (1 mM, open bar) failed to induce LTD. The same stimulus delivered to the same pathway after MCPG was washed out produced LTD. (B) Pooled data (mean ± SEM, n = 4) of normalized fEPSP plotted as a function of time. A HFS protocol (arrow) given in the presence of MCPG (1 mM, open bar) induced LTD.
To test whether the expression of LFS LTD is presynaptic in origin, in six experiments, paired-pulse facilitation, which is a widely accepted index of presynaptic function (17), was measured before and after LFS LTD. A significant (P < 0.01) increase in paired-pulse facilitation ratio was observed after LTD (the magnitude of paired-pulse facilitation ratio was 1.21 ± 0.06 and 1.45 ± 0.06, before and after LTD, respectively). This suggests that LFS LTD occurs at the same site involved in paired-pulse facilitation.
To see whether the two forms of LTD may coexist at the same synapse, occlusion experiments were performed. In a first set of experiments (n = 5), HFS LTD was saturated by using a stimulation intensity (during the induction protocol only), which induced the maximal response. After saturation, the stimulus intensity was increased to evoke a fEPSP similar in amplitude to the preconditioning one. Then a subsequent LFS train (at higher intensity of stimulation) to the same pathway produced a further LTD of 15.5 ± 3.7% (40 min after LFS protocol, Fig. 3A). In a second set of experiments (n = 7), LFS LTD was similarly saturated by using a high-stimulation intensity during the induction protocol and then a HFS train was delivered to the afferent fibers. Also in this case a persistent depression of 16.8 ± 3.9% of the fEPSP was produced (Fig. 3B). The present data suggest that two developmentally regulated forms of LTD, both NMDA independent, may coexist at the same synapse.
Figure 3.
HFS and LFS LTD do not occlude each other. (A) Pooled data (mean ± SEM, n = 5) of normalized fEPSPs plotted as a function of time. Two HFS (arrows) delivered to the mossy fibers (20 min apart), using a stimulation intensity that induced the maximal response, induced LTD that reached saturation. Forty minutes later, the stimulation intensity was increased to evoke a fEPSP similar in amplitude to the preconditioning one. A subsequent LFS (filled bar) produced a further LTD. (B) Pooled data (mean ± SEM, n = 5) of normalized fEPSPs plotted as a function of time. Two LFS (filled bars) delivered to the mossy fibers, using a stimulation intensity that induced the maximal response (20 min apart), induced LTD that reached saturation. As in A, the stimulation intensity was increased to evoke a fEPSP similar in amplitude to the preconditioning one. A HFS train delivered to the same pathway produced a further depression of the fEPSP.
Whole-Cell Patch Clamp Recordings.
In previous reports, it was shown that HFS LTD is induced postsynaptically and expressed presynaptically (9, 10). To identify the induction and expression mechanisms underlying LFS LTD, quantal analysis on minimal EPSPs evoked in CA3 pyramidal neurons by activation of the mossy fibers was performed in the whole-cell configuration of the patch clamp technique (current clamp mode), with the patch pipette containing EGTA (10 mM, n = 4) or BAPTA (10 mM, n = 5). Because no differences in LTD were found between these two experimental conditions, data from these experiments have been pooled together. As shown in the representative example of Fig. 4, in control conditions, EPSPs fluctuated in amplitude from trial to trial and were associated with occasional failures. Usually two pulses, 50 ms apart, were delivered to the afferent fibers, to study paired-pulse facilitation (17). A clear facilitation of the response to the second stimulus was observed with no failures. On average the paired-pulse facilitation ratio was 2.04 ± 0.3 (n = 6). In the absence of a conditioning stimulation protocol, no clear rundown of synaptic potentials was observed for at least 60 min (not shown). A LFS protocol induced a clear long-lasting depression of synaptic efficacy (44 ± 7%, n = 7/9) and an increase in the number of failures (Fig. 4). The probability to have failures in response to the first pulse increased significantly (P < 0.01) from 0.25 ± 0.06 in control to 0.56 ± 0.07 after LTD (n = 7). In spite of the reduction in amplitude of both conditioning and test responses, the second EPSP was still facilitated: the paired-pulse facilitation ratio was 2.46 ± 0.3, a value significantly (P < 0.05) different from the control one. The increased number of failures as well as the increase in paired-pulse facilitation ratio suggests that maintenance of LFS LTD is a presynaptic phenomenon. Consistent with a presynaptic site of expression are the deconvolution analysis data. As shown in the representative example of Fig. 4C, amplitude distribution histograms were peaky and contained similar equidistant components before and after LTD. Accordingly, the quantal size v did not change after LTD, but the quantal content m was significantly reduced (P < 0.001) in parallel with a reduction in the larger amplitude events. The data from seven cells are summarized in Fig. 4D. It is clear from the figure that a reduction in quantal content was associated to a significant (P < 0.001) decrease in the inverse square of the coefficient of variation. In contrast to LFS LTD and in keeping with previous results (9), the HFS LTD was blocked by intracellular EGTA (Fig. 5), indicating that induction of this form of synaptic plasticity occurs at a postsynaptic site.
DISCUSSION
The present results clearly show that during the second postnatal week, two NMDA-independent forms of LTD coexist at the same mossy fiber-CA3 synapse. These forms of LTD differ in their induction and expression mechanisms. Although HFS LTD is associative and requires concomitant presynaptic and postsynaptic activation, LFS LTD is nonassociative and appears to depend on only the sustained activation of afferent pathways.
Induction Mechanisms.
It has been reported (8, 9) that the HFS LTD is homosynaptic as well as NMDA and mGluR independent, but needs a rise in [Ca2+]i in the postsynaptic cell for its induction, possibly through the activation of voltage-dependent calcium channels. Thus, depression of synaptic strength does not occur when the HFS train is delivered in voltage-clamp conditions (at −70 mV) or when the postsynaptic cell is loaded with the calcium chelator BAPTA (9). A homosynaptic and NMDA-independent form of LTD could be induced in the CA3 region of the hippocampus of adult rats in vivo, after a HFS train to the mossy fibers (18). However, in contrast to the juvenile form of LTD (8), this form of plasticity requires the activation of opioid receptors by endogenous opioid peptides released by mossy fiber synapses, because it could be prevented by selective μ opioid receptor antagonists. The present results show that, like HFS LTD, LFS LTD is homosynaptic and NMDA independent. However, in contrast to HFS LTD, chelation of [Ca2+]i in the postsynaptic cell with EGTA or BAPTA failed to prevent LTD induction. Moreover, this form of LTD requires the activation of mGluRs, probably localized on presynaptic terminals. In this respect, LFS LTD closely resembles that recently described in adult mice by Kobayashi et al. (16) where the role of presynaptic mGluR in LTD was assessed by targeted disruption of the gene encoding mGluR2 (19). Hence, the possibility that this form of synaptic plasticity may persist in adulthood cannot be excluded.
Expression Mechanisms.
In a previous report it was shown that, in spite of a postsynaptic induction mechanism, the maintenance of the HFS LTD is likely to occur at presynaptic sites, as indicated by quantal analysis that has revealed a decrease in mean quantal content after induction of depression without any significant change in quantal size (10). Therefore, it is likely that in common with other forms of synaptic plasticity (20) HFS LTD requires the production of a retrograde messenger whose nature has not been identified yet. The expression of LFS LTD is presynaptic as suggested by the changes in paired-pulse facilitation ratio, the reduction in quantal content m (but not in quantal size, v) and by a significant increase in the number of failures. We cannot exclude that the reduction in m, in both HFS and LFS LTD, may reflect a down-regulation of previous active α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (21). However, this hypothesis is unlikely for the following reasons: (i) a decrease in m not associated to changes in v would require a selective all-or-none modification at some releasing sites without changes in other sites; (ii) after LTD, large amplitude responses still could be occasionally evoked by the second pulse; and (iii) LTD was associated to a significant increase in paired-pulse facilitation ratio, which is a clear index of presynaptic changes in release probability (17). Although expression of both forms of LTD appears to be presynaptic, the underlying mechanisms may be distinct as demonstrated by their coexistence at the same synapse. It should be stressed, however, that the magnitude of LTD obtained with HFS or LFS protocol after saturation of the corresponding pathway with LFS or HFS, respectively, was smaller than that obtained with the same stimulation protocol before saturation, indicating that at least a partial occlusion was present. Occlusion may take place at the level of presynaptic mGluR that would be activated by spillover of glutamate during both stimulation protocols (22) or at a site localized downstream of the transduction pathway that follows the induction of either forms of LTD. Although MCPG experiments allow to exclude the first hypothesis, further studies will be needed to determine the convergence processes involved in these two forms of synaptic depression. Coexistence of two distinct forms of LTD has been described in the CA1 hippocampal region of juvenile rats (15). However, in the CA1 subfield, the induction of the NMDA-dependent versus the mGluR-dependent form of LTD was influenced by the recording conditions, namely the Ca2+/Mg2+ ratio and the level of γ-aminobutyric acid type A (GABAA)-mediated synaptic inhibition. In our case HFS or LFS LTD were obtained in the same recording conditions and were both insensitive to picrotoxin, indicating that GABAA-mediated inhibition was not involved. Moreover, in the CA1 region the two forms of LTD have the same site of induction (postsynaptic), whereas the expression appears to be presynaptic for mGluR-LTD and postsynaptic for NMDA-LTD. This suggests that coexistence of various forms of LTD may occur through different mechanisms of induction and expression in different synapses.
CONCLUSIONS
We have presented evidence that, during a critical period of postnatal development, two independent forms of LTD can be induced by a HFS or a LFS protocol at the mossy fiber-CA3 synapse. In this area, LFS LTD also persists with similar characteristics in adulthood (16), indicating that this form of synaptic plasticity may represent a more general, nondevelopmentally regulated phenomenon. At the same synapse, while during the second postnatal week, a HFS train to afferent inputs induces a postsynaptic form of LTD, the same stimulation protocol delivered a few days later, produces a LTP (8), which, as in adult (13) is presynaptic. It should be noticed that in adulthood at the mossy fiber-CA3 synapse a postsynaptically induced form of LTP (NMDA independent) can be observed (23, 24). However, this form of LTP requires a paradigm of induction different from HFS.
Coexistence of distinct associative and nonassociative activity-dependent forms of synaptic plasticity at the mossy fiber-CA3 synapse may allow to store a considerable amount of information, thus greatly expanding the “mnemonic” capacity of the hippocampal network during postnatal development.
Acknowledgments
We are grateful to Dr. A. Treves for critically reading the manuscript. This work was partially supported by a grant from Ministero dell’Universita’ e Ricerca Scientifica e Tecnologica (MURST) (to E.C.). N.B. was supported by a fellowship from Novartis Pharmaceuticals.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: LTD, long-term depression; LTP, long-term potentiation; NMDA, N-methyl-d-aspartate; mGluR, metabotropic glutamate receptor; MCPG, (S)-α-methyl-4-carboxyphenylglycine; HFS, high-frequency stimulation; LFS, low-frequency stimulation; EPSP, excitatory postsynaptic potential; fEPSP, field EPSP; BAPTA, bis-(-o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
References
- 1.Bear M F, Malenka R C. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 2.Dudek S M, Bear M F. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolshakov V Y, Siegelbaum S A. Science. 1995;269:1730–1734. doi: 10.1126/science.7569903. [DOI] [PubMed] [Google Scholar]
- 4.Dudek S M, Bear M F. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulkey M M, Malenka R C. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 6.Bolshakov V Y, Siegelbaum S A. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 7.Amaral D G, Dent J A. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- 8.Battistin T, Cherubini E. Eur J Neurosci. 1994;6:1750–1755. doi: 10.1111/j.1460-9568.1994.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 9.Györi J, Atzori M, Cherubini E. NeuroReport. 1996;7:1660–1664. doi: 10.1097/00001756-199607080-00027. [DOI] [PubMed] [Google Scholar]
- 10.Berretta N, Cherubini E, Rossokhin A V, Voronin L. J Physiol. 1997;504:180P. (abstr.). [Google Scholar]
- 11.Claiborne B J, Xiang Z, Brown T H. Hippocampus. 1993;3:115–122. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- 12.Kamiya H, Shinozaki H, Yamamoto C. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalutsky R A, Nicoll R A. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 14.Astrelin A V, Sokolov M V, Behnisch T, Reymann K G, Voronin L L. J Neurosci Methods. 1998;73:17–27. doi: 10.1016/s0165-0270(96)02206-6. [DOI] [PubMed] [Google Scholar]
- 15.Oliet S H R, Malenka R C, Nicoll R A. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Manabe T, Takahashi T. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 17.Zucker R S. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- 18.Derrick B E, Martinez J L. Nature (London) 1996;381:429–434. doi: 10.1038/381429a0. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, et al. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 20.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 21.Malenka R C, Nicoll R A. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 22.Scanziani M, Salin P A, Vogt K E, Malenka R C, Nicoll R A. Nature (London) 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe D, Johnston D. J Neurophysiol. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- 24.Urban N N, Barrionuevo G. J Neurosci. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]