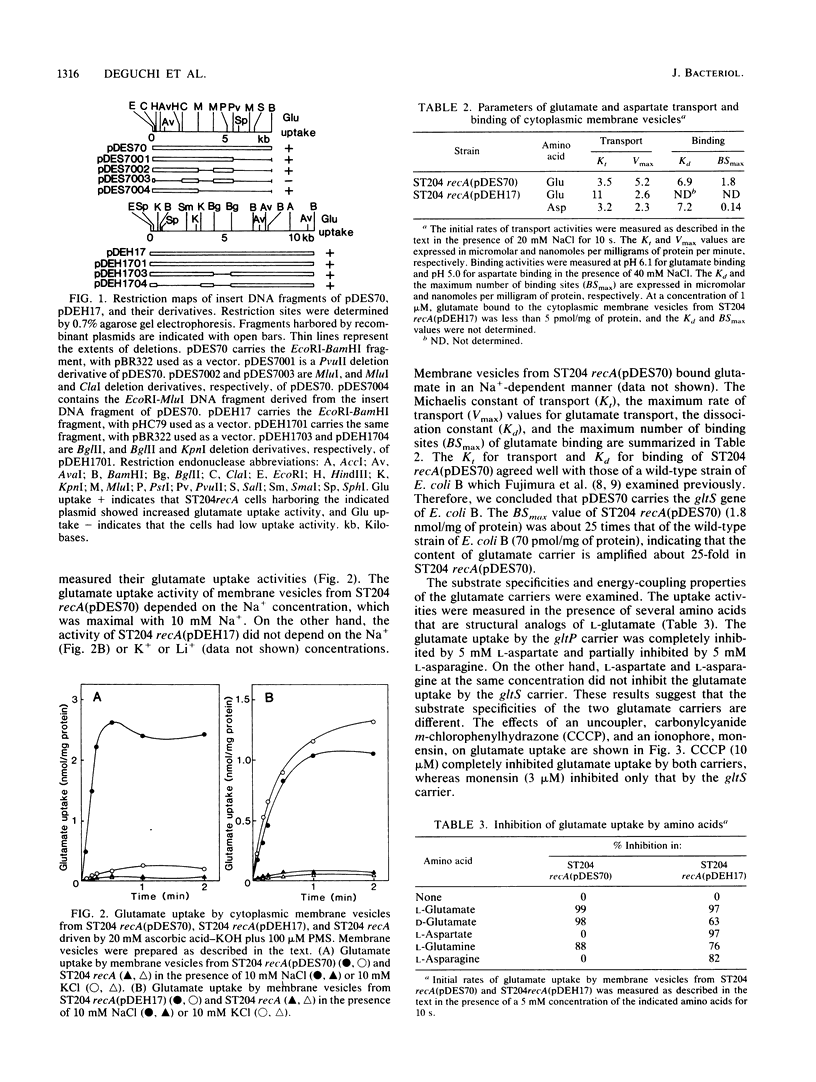
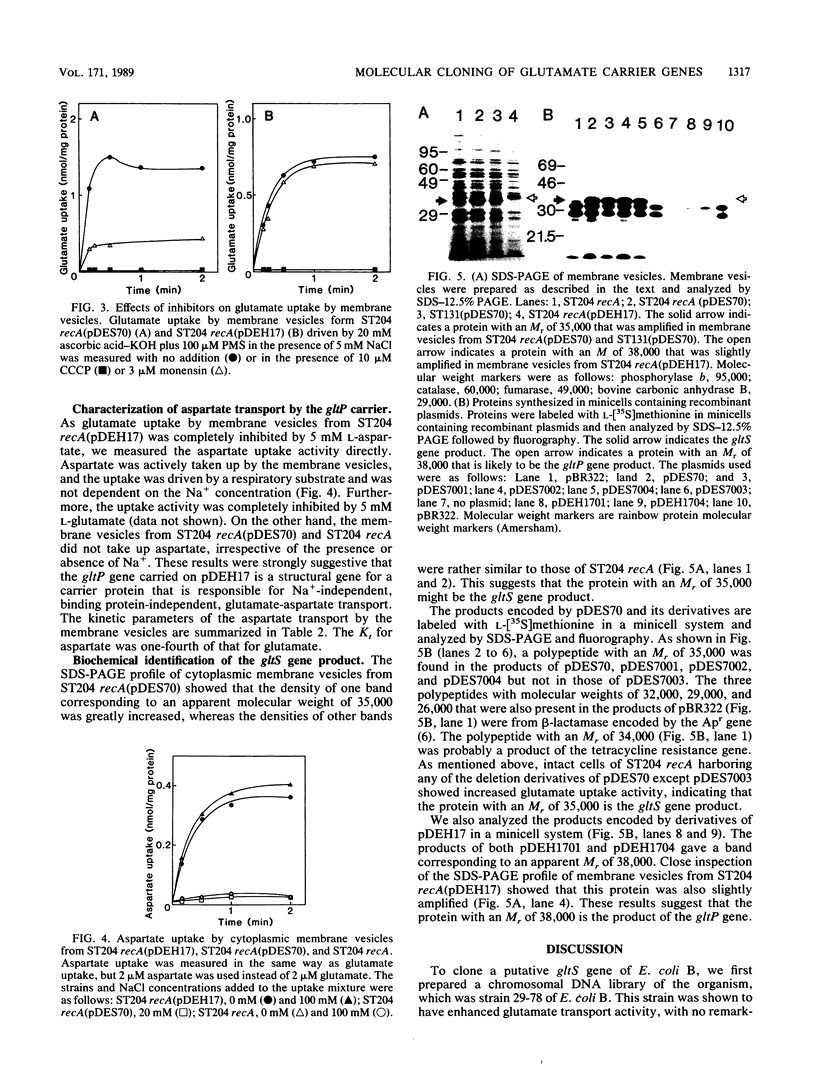
Abstract
Two genes encoding distinct glutamate carrier proteins of Escherichia coli B were cloned into an E. coli K-12 strain by using a cosmid vector, pHC79. One of them was the gltS gene coding for a glutamate carrier of an Na+-dependent, binding protein-independent, and glutamate-specific transport system. The content of the glutamate carrier was amplified about 25-fold in the cytoplasmic membranes from a gltS-amplified strain. The gltS gene was located in a 3.2-kilobase EcoRI-MluI fragment, and the gene product was identified as a membrane protein with an apparent Mr of 35,000 in a minicell system. A gene designated gltP was also cloned. The transport activity of the gltP system in cytoplasmic membrane vesicles from a gltP-amplified strain was driven by respiratory substrates and was independent of the concentrations of Na+, K+, and Li+. An uncoupler, carbonylcyanide m-chlorophenylhydrazone, completely inhibited the transport activities of both systems, whereas an ionophore, monensin, inhibited only that of the gltS system. The Kt value for glutamate was 11 microM in the gltP system and 3.5 microM in the gltS system. L-Aspartate inhibited the glutamate transport of the gltP system but not that of the gltS system. Aspartate was taken up actively by membrane vesicles from the gltP-amplified strain, although no aspartate uptake activity was detected in membrane vesicles from a wild-type E. coli strain. These results suggest that gltP is a structural gene for a carrier protein of an Na+-independent, binding protein-independent glutamate-aspartate transport system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Manning P. A., Edelbluth C., Herrlich P. Export without proteolytic processing of inner and outer membrane proteins encoded by F sex factor tra cistrons in Escherichia coli minicells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4837–4841. doi: 10.1073/pnas.76.10.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Wilson T. H. Solubilization and functional reconstitution of the proline transport system of Escherichia coli. J Biol Chem. 1986 Feb 25;261(6):2599–2604. [PubMed] [Google Scholar]
- Dougan G., Saul M., Twigg A., Gill R., Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979 Apr;138(1):48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 1. Proton-dependent and sodium ion dependent binding of glutamate to a glutamate carrier in the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1954–1959. doi: 10.1021/bi00277a033. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Yamato I., Anraku Y. Mechanism of glutamate transport in Escherichia coli B. 2. Kinetics of glutamate transport driven by artificially imposed proton and sodium ion gradients across the cytoplasmic membrane. Biochemistry. 1983 Apr 12;22(8):1959–1965. doi: 10.1021/bi00277a034. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Lupo M. Glutamate transport in wild-type and mutant strains of Escherichia coli. J Bacteriol. 1965 Nov;90(5):1288–1295. doi: 10.1128/jb.90.5.1288-1295.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K., Yamato I., Anraku Y. Construction and properties of bifunctionally active membrane-bound fusion proteins. Escherichia coli proline carrier linked with beta-galactosidase. J Biol Chem. 1987 Oct 15;262(29):14100–14104. [PubMed] [Google Scholar]
- Hanada K., Yamato I., Anraku Y. Purification and reconstitution of Escherichia coli proline carrier using a site specifically cleavable fusion protein. J Biol Chem. 1988 May 25;263(15):7181–7185. [PubMed] [Google Scholar]
- Hanada K., Yamato I., Anraku Y. Solubilization and reconstitution of proline carrier in Escherichia coli; quantitative analysis and optimal conditions. Biochim Biophys Acta. 1988 Apr 7;939(2):282–288. doi: 10.1016/0005-2736(88)90072-7. [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Tsuchiya T. Glutamate transport driven by an electrochemical gradient of sodium ion in membrane vesicles of Escherichia coli B. Biochem Biophys Res Commun. 1977 Sep 9;78(1):122–128. doi: 10.1016/0006-291x(77)91229-3. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Kahane S., Marcus M., Barash H., Halpern Y. S. Sodium-dependent glutamate transport in membrane vesicles of Escherichia coli K-12. FEBS Lett. 1975 Aug 15;56(2):235–239. doi: 10.1016/0014-5793(75)81099-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Lanyi J. K., Greene R. V. Sodium-stimulated glutamate uptake in membrane vesicles of Escherichia coli: the role of ion gradients. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3167–3170. doi: 10.1073/pnas.74.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Genetic analysis of the glutamate permease in Escherichia coli K-12. J Bacteriol. 1969 Mar;97(3):1118–1128. doi: 10.1128/jb.97.3.1118-1128.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner K. M., Frank L. Sodium-stimulated glutamate transport in osmotically shocked cells and membrane vesicles of Escherichia coli. J Bacteriol. 1974 Mar;117(3):1093–1098. doi: 10.1128/jb.117.3.1093-1098.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T., Anraku Y. Mechanism of proline transport in Escherichia coli K12. II. Effect of alkaline cations on binding of proline to a H+/proline symport carrier in cytoplasmic membrane vesicles. J Biol Chem. 1984 Jun 25;259(12):7797–7801. [PubMed] [Google Scholar]
- Motojima K., Yamato I., Anraku Y., Nishimura A., Hirota Y. Amplification and characterization of the proline transport carrier of Escherichia coli K-12 by using proT+ hybrid plasmids. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6255–6259. doi: 10.1073/pnas.76.12.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H., Kita K., Anraku Y. Cloning of cybB, the gene for cytochrome b561 of Escherichia coli K12. Mol Gen Genet. 1984;198(2):1–6. doi: 10.1007/BF00328692. [DOI] [PubMed] [Google Scholar]
- Nakao T., Yamato I., Anraku Y. Nucleotide sequence of putP, the proline carrier gene of Escherichia coli K12. Mol Gen Genet. 1987 Jun;208(1-2):70–75. doi: 10.1007/BF00330424. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Furlong C. E. Resolution of the multiplicity of the glutamate and aspartate transport systems of Escherichia coli. J Biol Chem. 1977 Dec 25;252(24):9055–9064. [PubMed] [Google Scholar]
- Tsuchiya T., Hasan S. M., Raven J. Glutamate transport driven by an electrochemical gradient of sodium ions in Escherichia coli. J Bacteriol. 1977 Sep;131(3):848–853. doi: 10.1128/jb.131.3.848-853.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Raven J., Wilson T. H. Co-transport of Na+ and methul-beta-D-thiogalactopyranoside mediated by the melibiose transport system of Escherichia coli. Biochem Biophys Res Commun. 1977 May 9;76(1):26–31. doi: 10.1016/0006-291x(77)91663-1. [DOI] [PubMed] [Google Scholar]
- Velten J., Fukada K., Abelson J. In vitro construction of bacteriophage lambda and plasmid DNA molecules containing DNA fragments from bacteriophage T4. Gene. 1976;1(1):93–106. doi: 10.1016/0378-1119(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Yamato I., Anraku Y., Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem. 1975 Apr;77(4):705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- Yazyu H., Shiota-Niiya S., Shimamoto T., Kanazawa H., Futai M., Tsuchiya T. Nucleotide sequence of the melB gene and characteristics of deduced amino acid sequence of the melibiose carrier in Escherichia coli. J Biol Chem. 1984 Apr 10;259(7):4320–4326. [PubMed] [Google Scholar]




