Abstract
The pharmacological properties of voltage-dependent calcium channel (VDCC) subtypes appear mainly to be determined by the α1 pore-forming subunit but, whether P-and Q-type VDCCs are encoded by the same α1 gene presently is unresolved. To investigate this, we used IgG antibodies to presynaptic VDCCs at motor nerve terminals that underlie muscle weakness in the autoimmune Lambert–Eaton myasthenic syndrome (LEMS). We first studied their action on changes in intracellular free Ca2+ concentration [Ca2+]i in human embryonic kidney (HEK293) cell lines expressing different combinations of human recombinant VDCC subunits. Incubation for 18 h with LEMS IgG (2 mg/ml) caused a significant dose-dependent reduction in the K+-stimulated [Ca2+]i increase in the α1A cell line but not in the α1B, α1C, α1D, and α1E cell lines, establishing the α1A subunit as the target for these autoantibodies. Exploiting this specificity, we incubated cultured rat cerebellar neurones with LEMS IgG and observed a reduction in P-type current in Purkinje cells and both P- and Q-type currents in granule cells. These data are consistent with the hypothesis that the α1A gene encodes for the pore-forming subunit of both P-type and Q-type VDCCs.
Neuronal voltage-dependent calcium channels (VDCCs) play an important role in the control of neurotransmitter release at the synapse (1). High voltage-activated VDCCs can be classified into P, Q, N, L, and R-types according to their electrophysiological and pharmacological properties (2). Neuronal VDCCs consist of an α1 pore-forming subunit together with an intracellular β subunit and a glycosylated α2δ subunit (3). Human genes encoding many of the human VDCC subunits have been cloned and sequenced, including six α1 genes (α1A, α1B, α1C, α1D, α1E, and α1S), four β genes (β1, β2, β3, and β4), and the α2δ gene. The classification of VDCCs into various subtypes (P-type, Q-type, etc.) largely is thought to depend on the α1 subunit, which contains the pore of the channel and possesses binding sites for drugs and peptide neurotoxins (4). The α1B and α1C/D subunits have been assigned unambiguously to the N-type and L-type VDCCs, respectively (5–7). However, whether P-type and Q-type VDCCs are encoded by the same α1 gene is uncertain. P-type calcium currents first were described in Purkinje cells and show marked sensitivity to low nanomolar concentrations of the neurotoxin ω-agatoxin (Aga) IVA (8). In contrast, Q-type currents, which form a major component of calcium currents in cerebellar granule cells, are relatively insensitive to ω-Aga IVA (9). Antisense experiments suggest that the α1A gene encodes a P-type VDCC in Purkinje cells (10), but whether it also encodes the Q-type VDCC remains uncertain.
Lambert–Eaton myasthenic syndrome (LEMS) is an autoimmune neurological disease in which antibodies are directed against presynaptic VDCCs at the neuromuscular junction leading to muscle weakness (11). Many (≈60%) patients have an associated small cell lung carcinoma (SCLC). SCLC cells are known to express VDCCs that are believed to trigger the autoantibody response in these patients (12). Antibodies that immunoprecipitate P/Q-type [125I-ω-Conotoxin (CTX) MVIIC-labeled] VDCCs are found in 85% of LEMS patients and a smaller percentage (30–40%) have antibodies to N-type (125I-ω-CTX GVIA-labeled) VDCCs (refs. 13 and 14; for review see ref. 11).
In the first part of the study, we have investigated the specificity of LEMS IgGs for cloned human neuronal VDCCs by studying their effect on K+-stimulated changes in intracellular free Ca2+ concentration [Ca2+]i in human embryonic kidney (HEK293) cells transfected with different human VDCC subunits. We then investigated the action of these characterized autoantibodies on whole-cell calcium currents in cultured rat cerebellar Purkinje and granule cells to identify the pore-forming subunit of P- and Q-type VDCCs in these neurons.
MATERIALS AND METHODS
Transfected HEK293 Cell Culture.
HEK293 cell lines were transfected stably with cDNAs encoding human VDCC subunits, and characterization of several of these lines has been published (5, 6, 15, 16). The pharmacological sensitivities of the 10–13 (α1A-2, α2bδ, β4a), G1A1 (α1B-1, α2bδ, β1b), C11D8 (α1C-1, α2bδ, β2e), 5D12–20 (α1D, α2bδ, β3a), E52–3 (α1E-3, α2δ, β1b), and E58–19 (α1E-3, α2δ, β4a) cell lines, encoding P/Q, N, L, and R-type channels, respectively, have been reported (17). Transfected cells were cultured in DMEM containing 5.5% bovine calf serum, penicillin G (100 units/ml), streptomycin sulfate (100 μg/ml), geneticin (1 μg/ml), and zeocin (10 μg/ml for 5D12–20 line only).
K+-Stimulated Calcium Assay.
Cells were plated out into 96-well plates precoated with poly-l-lysine (10 μg/ml) at a density of 2–3 × 105 cells per well and were incubated overnight at 37°C. The cells then were washed extensively with Tyrode’s solution (137 mM NaCl/2.7 mM KCl/1 mM MgCl2/1.8 mM CaCl2/0.2 mM NaHPO4/12 mM NaHCO3/5.5 mM glucose) and were incubated with the fluorescent calcium-sensitive dye fluo-3AM (20 μM) for 1 h at room temperature. Excess dye was removed by further washing, and the cells were maintained in Tyrode’s solution (200 μl/well) for 30 min. Cells were depolarized by exposure to either KCl at final concentration of 70 mM for the 10–13, G1A1, C11D8, and 5D12–20 cell lines or KCl at a final concentration of 40 mM (E52–3 and E-58–19), and the fluorescence (F) was measured in real time by using the Labsystems Fluoroskan II (ThermoQuest, San Jose, CA). For each well, maximum fluorescence (Fmax) and minimum fluorescence (Fmin) were measured after sequential exposure to Nonidet P-40 (20 μl, 1%) and then EDTA (15 μl, 100 mM). Intracellular free calcium concentration [Ca2+]i then could be estimated from the fluorescence intensity values by using the equation [Ca2+] (nM) = [(F − Fmin)/(Fmax − F)]*Kd where Kd is the dissociation constant for Ca2+ binding (400 nM) (18). Calcium signals were quantitated by subtracting the basal [Ca2+]i from peak [Ca2+]i after the addition of KCl.
Preparation of IgG.
IgG was prepared from patients by the ethacridine lactate-ammonium sulfate method as described (19). We studied six patients with LEMS, of whom three had an associated SCLC. All patients had antibodies that immunoprecipitate 125I-ω-CTX MVIIC-labeled VDCCs, and two patients, both without associated SCLC, had antibodies to 125I-ω-CTX GVIA-labeled VDCCs. IgG also was prepared from two different pools of healthy controls, one individual healthy control, and three disease controls (SCLC without LEMS, myasthenia gravis, and Guillain–Barre syndrome).
Effect of IgG on K+-Stimulated Calcium Assay.
IgG from LEMS, healthy, or disease controls at a final concentration of 2 mg/ml was added to transfected HEK293 cells in 96-well plates and was incubated overnight at 37°C. The K+-evoked [Ca2+]i signal in cells exposed to IgG was compared with the signal in cells cultured in medium alone run on the same plate. Results are expressed as a percentage of calcium influx in cells cultured in medium alone. The use of selective toxins was used to confirm the pharmacology of the cell lines.
Neuronal Cell Culture.
Cerebellar Purkinje cells were cultured as described (10, 20). Cerebellar granule cells were prepared by using minor modifications to a procedure described by Courtney et al. (21). Cerebella were removed from Sprague–Dawley rat pups on postnatal day 7, were chopped, and then were incubated with trypsin (5 mg in 20 ml) for 20 min at 37°C before being plated onto poly-d-lysine coated (15 μg/ml) coverslips at a density of 3 × 105 cells/ml. Cultures were maintained in minimum essential media containing 25 mM KCl, 10 mM d-glucose, 10% fetal calf serum, 2 mM glutamine, and penicillin (100 units/ml) and streptomycin sulfate (100 μg/ml) at 37°C. After 24 h, fresh media containing cytosine arabinoside (final concentration of 10 μM) to inhibit astrocyte growth was added.
Electrophysiology.
The whole-cell patch-clamp technique (22) was used to record transmembrane calcium currents in single cerebellar Purkinje or granule cell bodies at 20–22°C. Purkinje cells were voltage-clamped at a holding potential of −90 mV and was depolarized to a test potential of +10 mV for a duration of 50 msec every 30 sec. Granule cells were voltage-clamped at a holding potential of −80 mV and were depolarized to a test potential of 0 mV for a duration of 20 msec every 30 sec. Currents were recorded by using either a List EPC-7 (List Electronics, Darmstadt, Germany) or an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) and were filtered by an eight-pole, low pass Bessel filter (Axon Instruments, Foster City, CA) and were stored on computer. Leak currents and any uncompensated capacitance transients were subtracted from the current records by using standard hyperpolarizing P/4 protocols.
The pipette solution for voltage-clamp experiments contained 135 mM CsCl 1 mM MgCl2, 10 mM Hepes, 14 mM trisphosphocreatinine, 3.6 mM MgATP, and 50 units/ml creatinine phosphokinase adjusted to pH 7.1 with CsOH. Extracellular solution contained 143 mM tetraethylammonium chloride, 5 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, and 10 mM glucose adjusted to pH 7.4 with tetraethylammonium hydroxide. For experiments with granule cells, CaCl2 was replaced with BaCl2.
After stable whole-cell currents had been obtained, Purkinje cells were superfused with 50nM ω-Aga IVA, usually followed by 1 μM ω-CTX GVIA and 5 μM nifedipine. Because of faster run-down, granule cells were superfused with 30 nM ω-Aga IVA, 200 nM ω-Aga IVA, 1 μM ω-CTX GVIA, or 5 μM nifedipine. Reductions were measured as a percentage of the control current, and population data were expressed as the mean ± SEM.
Incubation with LEMS Antibodies.
Cultures were incubated with 2 mg/ml LEMS IgG or pooled healthy control IgG for 15–22 h. The effects of IgG incubation were evaluated in Purkinje cells between 9 and 16 days in vitro and in granule cells between 6 and 9 days in vitro.
RESULTS
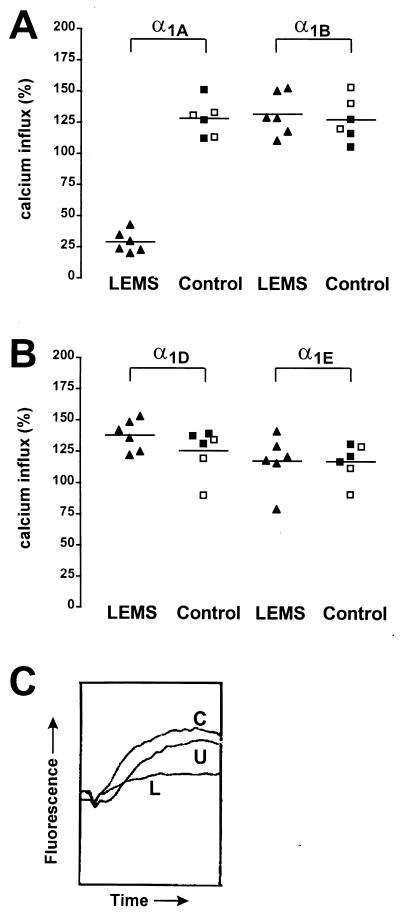
Overnight incubation with LEMS IgGs at 2 mg/ml reduced the K+-stimulated [Ca2+]i signal in the α1A (10–13) cell line to 29 ± 4% of the influx in cells incubated in medium alone whereas there was an increase in cells incubated with healthy and disease control IgGs (128 ± 6%, P < 0.0001; Fig. 1A). By contrast, there was no significant difference between LEMS IgGs and control IgGs in the α1B (G1A1) cell line, even though two LEMS patients had antibodies to 125I-ω-CTX GVIA-labeled VDCCs (132 ± 7% vs. 127 ± 7%, P > 0.05, Fig. 1A). Similarly, there was no significant difference between LEMS and control IgG treated cells in the α1D (5D12–20) and α1E (E52–3) cell lines (Fig. 1B) or in the α1C cell line (111 ± 7% vs. 107 ± 11%, P > 0.05). Furthermore, the α1E-containing cell line E58–19 (α1E-3, α2bδ, β4a), which, like the LEMS IgG-sensitive 10–13 cell line contains the β4a subunit, also was unaffected by overnight exposure to LEMS IgG (102 ± 3% vs. 114 ± 10%, P > 0.05). These results suggest that antibodies to the α1A subunit are responsible for the reduction in calcium influx seen in the 10–13 cell line rather than antibodies directed against the β4a or α2bδ subunits.
Figure 1.
Effect of LEMS and control IgGs on K+-stimulated [Ca2+]i signals in transfected HEK293 cells. Results are expressed as a percentage of the response in cells incubated in medium alone which was normalized to 100%. The mean results from six LEMS IgGs (▴) are compared with three healthy control IgGs (□) and three disease control IgGs (▪). Each IgG sample was studied in three separate experiments each of six replicates. (A) LEMS IgGs cause a significant reduction in the K+-stimulated calcium influx in the α1A cell line compared with control IgG (P < 0.0001). There was no significant reduction in the α1B cell line. (B) LEMS IgGs caused no significant reduction in calcium influx in the α1D and α1E cell lines. (C) Representative real-time fluorescence traces after K+-stimulated depolarization in the α1A cell line. Incubation with LEMS IgG (L) causes a marked reduction in the rise in fluorescence compared with untreated (U) and control IgG (C) treated cells.
The effect of LEMS IgG was dose-dependent, with a significant reduction in calcium influx at 0.5 mg/ml to 54 ± 5% of influx in cells incubated in medium alone and a maximum reduction to 25 ± 3% at 4 mg/ml. There was no significant effect of LEMS IgG at 0.1 mg/ml (102% ± 5%, P > 0.05). LEMS IgG from all six patients appeared to be approximately equally potent at inhibiting calcium influx in the α1A cell line.
Technical difficulties were encountered in obtaining maximal fluorescence values for calibrating calcium influx when IgGs (LEMS and control) were added directly to the Tyrode’s solution after dye loading. However, there was no apparent effect of LEMS IgG on calcium influx after 20 min incubation (data not shown). In contrast, 6-hour incubation with LEMS IgG at 2 mg/ml led to a reduction to 50 ± 1% of the calcium influx of cells grown in medium alone.
VDCC Currents in Purkinje Neurons.
After 8 days in vitro, most of the large cerebellar cells assumed the morphological and immunocytochemical features ascribed to Purkinje neurons (10). VDCC currents in these cells were reduced by 58 ± 3% by 50nM ω-Aga IVA (P-type), 22 ± 2% by 1 μM ω-CTX GVIA (N-type), and 6 ± 1% by 5 μM nifedipine (L-type). The remaining 14% of the whole-cell calcium current was resistant (R-type) to the above pharmacological blockers. Little evidence for a Q-type component was found in these cells because concentrations of ω-Aga IVA higher than 50nM produced no additional steady state inhibition of the current (IC50 for ω-Aga IVA block was ≈6 nM).
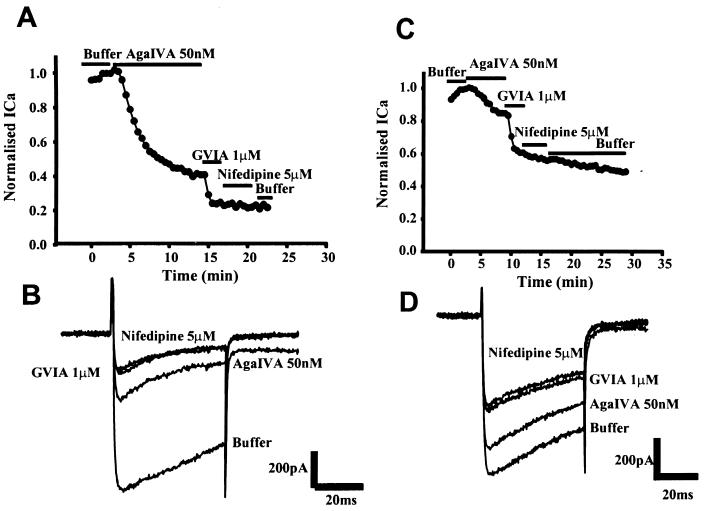
LEMS IgGs from two patients were tested on the Purkinje cells. Neither of these patients had detectable antibodies to N-type VDCCs on radioimmunoassay. Overnight incubation (15–22 h) with 2 mg/ml LEMS IgG or healthy control IgG did not affect the morphology or viability of Purkinje cells in culture. The magnitude of whole-cell currents and current density in cells incubated with LEMS IgGs was not significantly different from cells incubated with healthy control IgG [43 ± 3 pA/pF (n = 8), 34 ± 4pA/pF (n = 4) vs. 41 ± 4 pA/pF (n = 10)]. However, pharmacological analysis of toxin inhibitions revealed that there was a large reduction in the ω-Aga IVA-sensitive component of the whole-cell current in cells incubated for 15–22 h with LEMS IgGs compared with cells incubated with healthy control IgG (Fig. 2). Thus, 50nM ω-Aga IVA reduced the whole-cell calcium current by 11 ± 4% (n = 4; P < 0.001) and 19 ± 5% (n = 8; P < 0.001) in cells incubated with the two LEMS IgGs compared with 60 ± 6% (n = 10) in cells incubated with healthy control IgG (Fig. 3). In parallel, there was a significant increase in the proportion of residual current observed in the LEMS IgG-treated cells compared with control IgG-treated cells. The residual current (resistant to sequential application of ω-Aga IVA, ω-CTX GVIA, and nifedipine) accounted for 27 ± 2% (n = 6) of the whole-cell current in cells incubated with control IgG compared with 49 ± 3% (n = 5; P < 0.01) and 57 ± 5% (n = 4; P < 0.01) in cells incubated with LEMS IgGs.
Figure 2.
The effect of control and LEMS IgG on the whole-cell calcium currents in cultured Purkinje cells (13 days in vitro). Representative time course and traces from Purkinje cells treated with ω-Aga IVA, ω-CTX GVIA, and nifedipine are shown. (A and B) Control IgG. (C and D) LEMS IgG (patient 2). Treatment with LEMS IgG causes a marked reduction in the ω-Aga IVA-sensitive calcium current.
Figure 3.
The effect of LEMS and control IgG on the whole-cell calcium currents in cultured Purkinje cells (9–13 days in vitro). Results represent the mean from between 4 and 10 cells ± SEM. ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001 (Student’s t test).
Bath application of 2 mg/ml LEMS IgG for 14–16 min during whole-cell patch-clamp recordings had little effect on the whole-cell calcium current, reducing it by only 3 ± 1% (n = 3). This finding is consistent with data from the α1A cell line and suggests that LEMS IgG does not cause a pharmacological block of the channel. LEMS IgG did not prevent binding of ω-Aga IVA to the P-type VDCC because ω-Aga IVA reduced whole-cell calcium currents by 59 ± 8% (n = 3) in cells treated with LEMS IgG for 40 min immediately before recording.
VDCC Currents in Cerebellar Granule Cells.
Pharmacological dissection of whole-cell barium currents was carried out by using selective peptide neurotoxins and drugs to identify the contribution of different subtypes of VDCC. Application of 1 μM ω-CTX GVIA led to a rapid and irreversible block of 29 ± 2% (n = 10) of the whole-cell barium current. Perfusion with 5 μM nifedipine produced 26 ± 4% (n = 5) inhibition of the whole-cell barium current. The remaining high-voltage activated current could be subdivided into two current types based on differential sensitivity to increasing concentrations of ω-Aga IVA and differences in inactivation kinetics. Addition of 30 or 200 nM ω-Aga IVA produced an inhibition of 21 ± 1% and 52 ± 4% (n = 8) of the whole-cell barium currents, respectively. These results are consistent with those of Randall and Tsien (9).
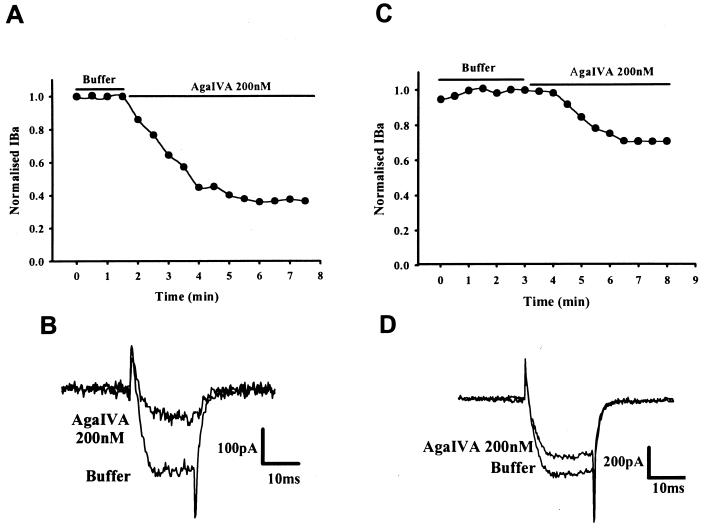
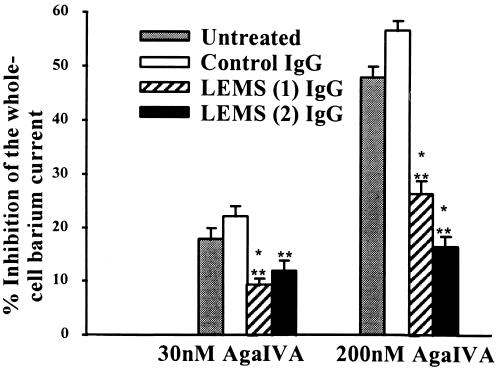
Granule cells were incubated overnight (15 h) with IgG at 2 mg/ml from two LEMS patients or from pooled healthy control IgG (Fig. 4). Mean current densities were not significantly different between LEMS and control IgG treated cells [45 ± 5 pA/pF (n = 28) vs. 38 ± 5 pA/pF (n = 23)]. Incubation with both samples of LEMS IgG led to a significant reduction in the current inhibition produced by 30 nM ω-Aga IVA, compared with control IgG [9 ± 1%; n = 7 and 12 ± 2%; n = 4 (LEMS IgGs) vs. 22 ± 2%; n = 4 (control IgG), P < 0.001 and P < 0.05 respectively]. Similarly, preincubation with LEMS IgG significantly reduced the extent of current block observed with 200 nM ω-Aga IVA compared with control IgG [27 ± 2% and 17 ± 2% (LEMS IgGs) vs. 57 ± 2% (control IgG), P < 0.01 for both patients] (Fig. 5). LEMS IgGs did not effect the ω-CTX GVIA-sensitive current in granule cells. Thus, 1 μM ω-CTX GVIA reduced the whole-cell barium current by 36 ± 6% (n = 3) in cells treated with LEMS IgG (patient 1) compared with 29 ± 2% (n = 10) in untreated controls. Taken together, these results suggest that LEMS IgG leads to a parallel reduction in both P-type and Q-type VDCC currents in cerebellar granule cells.
Figure 4.
The effect of control and LEMS IgG on the whole-cell barium currents in cultured rat cerebellar granule cells. Representative time course and traces from granule cells treated with 200nM ω-Aga IVA. (A and B) Control IgG. (C and D) LEMS IgG (patient 1). Treatment with LEMS IgG causes a marked reduction in the ω-Aga IVA-sensitive barium current.
Figure 5.
The effect of LEMS and control IgG on the whole-cell barium currents in cultured rat cerebellar granule cells. Results are the mean from between four and nine cells ± SEM. ∗∗, P < 0.01 and ∗∗∗, P < 0.001 (Student’s t test).
DISCUSSION
LEMS IgG causes a highly significant dose-dependent reduction in K+-stimulated calcium influx in the α1A (10–13) cell line and reduces both P-type and Q-type calcium currents in cerebellar neurons. Passive transfer of LEMS IgG to mice has been shown to cause a reduction in the quantal release of acetylcholine at the neuromuscular junction, a process that is believed to be mediated by the P/Q-type VDCC (19, 23, 24). In addition, recent studies have demonstrated presynaptic expression of the α1A subunit at the human neuromuscular junction (25). Taken in conjunction with these published studies, our data provide strong evidence that antibodies directed against P/Q-type VDCCs are responsible for the impairment of neuromuscular transmission in LEMS. These findings also are consistent with the study of Waterman et al. (26) in which passive transfer of LEMS IgG blocked the component of neurotransmitter release subserved by both P-type and Q-type VDCCs in the autonomic neurons of mice.
No inhibition of calcium influx was observed in the α1B (G1A1) cell line with IgGs from any of the six LEMS patients tested. Two of six patients had antibodies to N-type VDCCs on radioimmunoassay. Both of these LEMS patients also had antibodies to the intracellular β subunit of the VDCC complex, which may account for their ability to immunoprecipitate 125I-ω-CTX GVIA-labeled VDCCs (27). Antibodies to this intracellular subunit would not be expected to cause functional effects on VDCCs in living cells. Previous workers (28–30) have shown that LEMS IgG blocks both P-type and N-type VDCCs in a human SCLC cell line and N-type and L-type VDCCs in human neuroblastoma cell lines. However, the data presented here suggest that the α1B subunit is not a general target of antibodies in a clinically representative sample of six LEMS patients. There are several explanations for the difference between our results and those of other workers. First, IgGs from a minority of LEMS patients may have a functional effect on the α1B (N-type) VDCC, but these IgGs were not represented in our panel of patient IgGs. Second, different α1B splice variants have been identified and may result in altered sensitivity to LEMS IgG. LEMS IgG did not reduce calcium influx in α1C, α1D, or α1E cell lines. These data suggest that LEMS IgG antibodies directed against the α1A subunit lead to inhibition of calcium influx in the transfected cell lines. Taken together, the data presented here establish unequivocally the importance of antibodies to P/Q-type VDCCs in the pathogenesis of LEMS. Moreover, it appears that antibodies to the α1A subunit are the principal factor underlying the impairment of neurotransmission seen in LEMS.
We have used the marked specificity of LEMS IgGs for the α1A subunit in transfected cell lines to identify the pore-forming subunit of native P-type and Q-type VDCCs in rat cerebellar neurones. P-type VDCCs in Purkinje cells are highly sensitive to low nanomolar concentrations of ω-Aga IVA (IC50 ≈ 1–2 nM; ref. 8). However, expression of α1A mRNA in Xenopus oocytes gives rise to currents that are relatively insensitive to ω-Aga IVA (IC50 ≈ 200 nM), raising the question as to whether this gene encodes the P-type VDCC in Purkinje neurones (31). Recent studies in which cultured Purkinje cells were incubated with antisense oligonucleotides against the α1A subunit has provided evidence that the α1A gene encodes the P-type VDCC in these neurones (10). Moreover, expression of the α1A gene in combination with certain β subunits in mammalian cells gives rise to currents that more closely resemble P-type currents (32, 33). Finally, a truncated form of the α1A subunit has been identified that may possess different pharmacological characteristics from the full-length subunit (34). In this study, overnight incubation of Purkinje cells with LEMS IgG led to a highly significant reduction in the ω-Aga IVA-sensitive component of whole-cell calcium current. This effect was specific for LEMS IgG because only the ω-Aga IVA-sensitive component was reduced, and the ω-Aga IVA-sensitive currents of control IgG treated cells were not significantly different from untreated cells. Of interest, incubation with LEMS IgG led to a significant increase in the absolute magnitude of the residual current compared with control IgG treated cells. The mechanism and significance of this effect is not clear. These results imply that LEMS IgG specifically reduces P-type currents in cultured Purkinje cells and provide further evidence that the α1A gene encodes for the P-type VDCC.
Incubation of cultured rat cerebellar granule cells with LEMS IgG caused a reduction in sensitivity to both low (30 nM) and high concentrations (200 nM) of ω-Aga IVA that are thought to represent the P-type and the combined P and Q-type components of calcium currents in these neurones. This effect is specific to LEMS IgG and was not seen in granule cells treated with control IgG. Thus, LEMS IgG specific for the α1A subunit reduced both P-type and Q-type currents in rat granule cells.
LEMS IgG does not appear to cause a direct pharmacological block of the P/Q-type VDCC either in transfected HEK293 cells or in rat Purkinje cells, which is in agreement with published studies (35, 36). Divalent F(ab)2 but not monovalent Fab fragments from LEMS IgG cause reduction in nerve-evoked release of acetylcholine and disruption of active zone particles at the presynaptic terminal of the neuromuscular junction, suggesting that LEMS IgG acts by cross-linking and down-regulating calcium channel numbers (37, 38). Such a mechanism might explain the inhibition of VDCC function in our studies on transfected HEK293 cells and cerebellar neurones.
LEMS IgG antibodies specific for the α1A subunit block both P-type and Q-type VDCCs in cerebellar neurones. The simplest explanation of these results is that the α1A gene encodes for both P-type and Q-type VDCCs in cerebellar neurones. We cannot, however, exclude the possibility that LEMS IgG cross-reacts with an antigenically similar but as yet genetically undefined α1 pore-forming subunit that encodes for either a P-type or Q-type VDCC.
Acknowledgments
We thank Dr. A. Vincent for helpful comments on the manuscript and the Wellcome Trust (A.P.), Sir Jules Thorne Charitable Trust (B.L.), and the Medical Research Council of the United Kingdom (J.N.D. and B.L.) for funding and support.
ABBREVIATIONS
- VDCC
voltage-dependent calcium channel
- LEMS
Lambert–Eaton myasthenic syndrome
- SCLC
small cell lung carcinoma
- HEK
human embryonic kidney
- F
fluorescence
- Aga
agotoxin
- CTX
conotoxin
References
- 1.Dunlap K, Luebke J I, Turner T J. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 2.Birnbaumer L, Campbell K P, Catterall W A, Harpold M M, Hofmann F, Horne W A, Mori Y, Schwartz A, Snutch T P, Tanabe T, et al. Neuron. 1994;13:505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 3.Catterall W A. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann F, Biel M, Flockerzi V. Annu Rev Neurosci. 1994;17:399–418. doi: 10.1146/annurev.ne.17.030194.002151. [DOI] [PubMed] [Google Scholar]
- 5.Williams M E, Brust P F, Feldman D H, Patthi S, Simerson S, Maroufi A, McCue A F, Velicelebi G, Ellis S B, Harpold M M. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- 6.Williams M E, Feldman D H, McCue A F, Brenner R, Velicelebi G, Ellis S B, Harpold M M. Neuron. 1992;8:71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe T, Mikami A, Numa S, Beam K G. Nature (London) 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- 8.Mintz I M, Adams M E, Bean B P. Neuron. 1992;9:85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- 9.Randall A, Tsien R W. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillard S E, Volsen S G, Smith W, Beattie R E, Bleakman D, Lodge D. Neuropharmacology. 1997;36:405–409. doi: 10.1016/s0028-3908(97)00046-4. [DOI] [PubMed] [Google Scholar]
- 11.Lang B, Newsom-Davis J. Springer Semin Immunopathol. 1995;17:3–15. doi: 10.1007/BF00194096. [DOI] [PubMed] [Google Scholar]
- 12.Roberts A, Perera S, Lang B, Vincent A, Newsom-Davis J. Nature (London) 1985;317:737–739. doi: 10.1038/317737a0. [DOI] [PubMed] [Google Scholar]
- 13.Motomura M, Lang B, Johnston I, Palace J, Vincent A, Newsom Davis J. J Neurol Sci. 1997;147:35–42. doi: 10.1016/s0022-510x(96)05303-8. [DOI] [PubMed] [Google Scholar]
- 14.Lennon V A, Kryzer T J, Griesmann G E, O’Suilleabhain P E, Windebank A J, Woppmann A, Miljanich G P, Lambert E H. N Engl J Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- 15.Williams M E, Marubio L M, Deal C R, Hans M, Brust P F, Philipson L H, Miller R J, Johnson E C, Harpold M M, Ellis S B. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 16.Bleakman D, Bowman D, Bath C P, Brust P F, Deal C P, Johnson E C, Miller R J, Hans M, Ellis S B, Harpold M M, Grantham C. Neuropharmacology. 1995;34:753–765. doi: 10.1016/0028-3908(95)00078-k. [DOI] [PubMed] [Google Scholar]
- 17.Boot, J. R., O’Brien, A. & Tran, S. (1997) Brit. J. Pharmacol. 122, Suppl. 1, 89P (abstr.).
- 18.Kao J P, Harootunian A T, Tsien R Y. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- 19.Lang B, Newsom-Davis J, Prior C, Wray D. J Physiol (London) 1983;344:335–345. doi: 10.1113/jphysiol.1983.sp014943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brorson J R, Bleakman D, Gibbons S J, Miller R J. J Neurosci. 1991;11:4024–4043. doi: 10.1523/JNEUROSCI.11-12-04024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courtney M J, Lambert J J, Nicholls D G. J Neurosci. 1990;10:3873–3879. doi: 10.1523/JNEUROSCI.10-12-03873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 23.Hong S J, Chang C C. J Physiol (London) 1995;482:283–290. doi: 10.1113/jphysiol.1995.sp020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Protti D A, Reisin R, Mackinley T A, Uchitel O D. Neurology. 1996;46:1391–1396. doi: 10.1212/wnl.46.5.1391. [DOI] [PubMed] [Google Scholar]
- 25.Day N C, Wood S J, Ince P G, Volsen S G, Smith W, Slater C R, Shaw P J. J Neurosci. 1997;17:6226–6235. doi: 10.1523/JNEUROSCI.17-16-06226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterman S A, Lang B, Newsom-Davis J. Ann Neurol. 1997;42:147–156. doi: 10.1002/ana.410420204. [DOI] [PubMed] [Google Scholar]
- 27.Verschuuren J J, Dalmau J, Tunkel R, Lang B, Graus F, Schramm L, Posner J B, Newsom-Davis J, Rosenfeld M R. Neurology. 1998;50:475–479. doi: 10.1212/wnl.50.2.475. [DOI] [PubMed] [Google Scholar]
- 28.Grassi C, Magnelli V, Carabelli V, Sher E, Carbone E. Neurosci Lett. 1994;181:50–56. doi: 10.1016/0304-3940(94)90558-4. [DOI] [PubMed] [Google Scholar]
- 29.Meriney S D, Hulsizer S C, Lennon V A, Grinnell A D. Ann Neurol. 1996;40:739–749. doi: 10.1002/ana.410400510. [DOI] [PubMed] [Google Scholar]
- 30.Peers C, Lang B, Newsom-Davis J, Wray D W. J Physiol (London) 1990;421:293–308. doi: 10.1113/jphysiol.1990.sp017945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sather W A, Tanabe T, Zhang J F, Mori Y, Adams M E, Tsien R W. Neuron. 1993;11:291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- 32.Berrow N S, Brice N L, Tedder I, Page K M, Dolphin A C. Eur J Neurosci. 1997;9:739–748. doi: 10.1111/j.1460-9568.1997.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 33.Moreno H, Rudy B, Llinas R. Proc Natl Acad Sci USA. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott V E, Felix R, Arikkath J, Campbell K P. J Neurosci. 1998;18:641–647. doi: 10.1523/JNEUROSCI.18-02-00641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y I, Sanders D B, Johns T R, Phillips L H, Smith R E. J Neurol Sci. 1988;87:1–13. doi: 10.1016/0022-510x(88)90049-4. [DOI] [PubMed] [Google Scholar]
- 36.Magnelli V, Grassi C, Parlatore E, Sher E, Carbone E. FEBS Lett. 1996;387:47–52. doi: 10.1016/0014-5793(96)00465-6. [DOI] [PubMed] [Google Scholar]
- 37.Nagel A, Engel A G, Lang B, Newsom-Davis J, Fukuoka T. Ann Neurol. 1988;24:552–558. doi: 10.1002/ana.410240412. [DOI] [PubMed] [Google Scholar]
- 38.Peers C, Johnston I, Lang B, Wray D. Neurosci Lett. 1993;153:45–48. doi: 10.1016/0304-3940(93)90073-t. [DOI] [PubMed] [Google Scholar]