Abstract
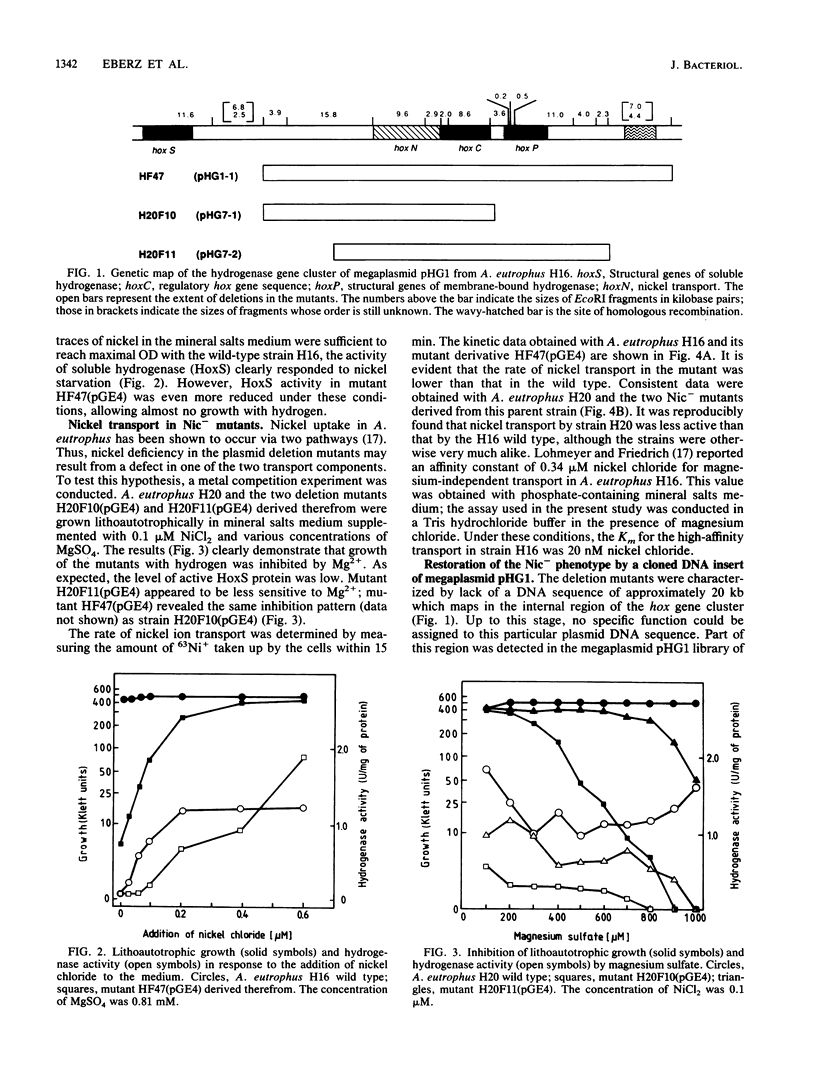
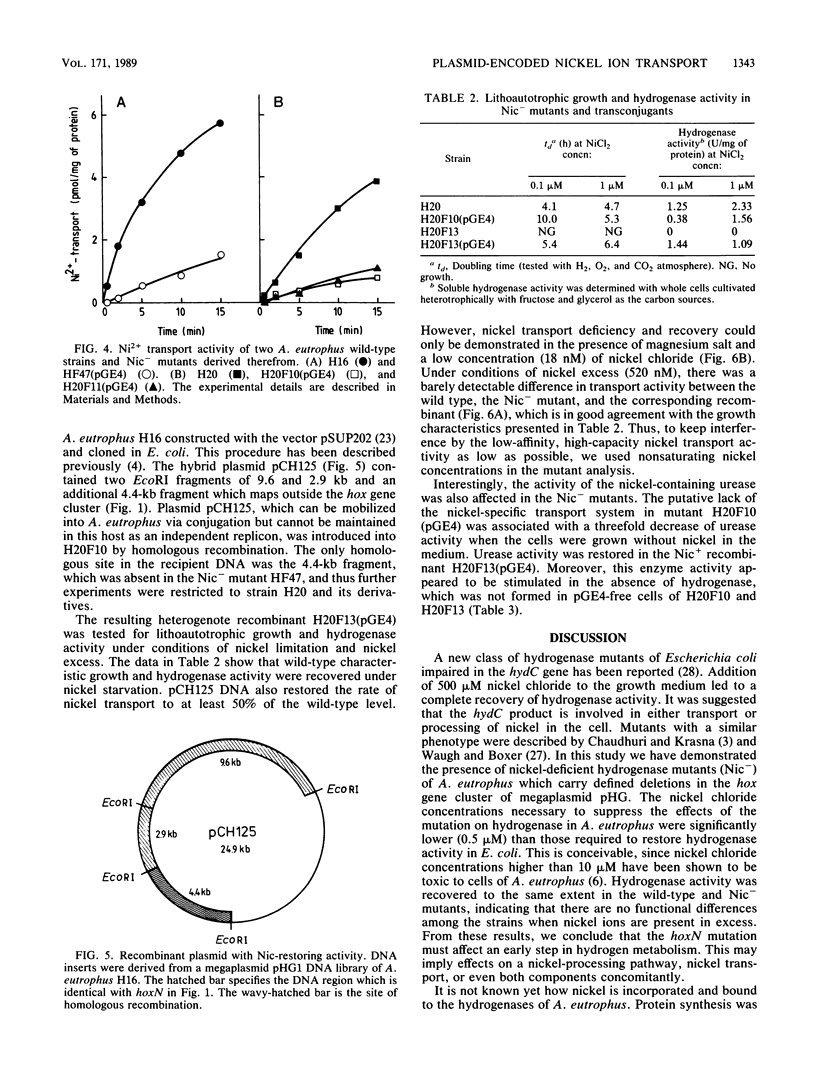
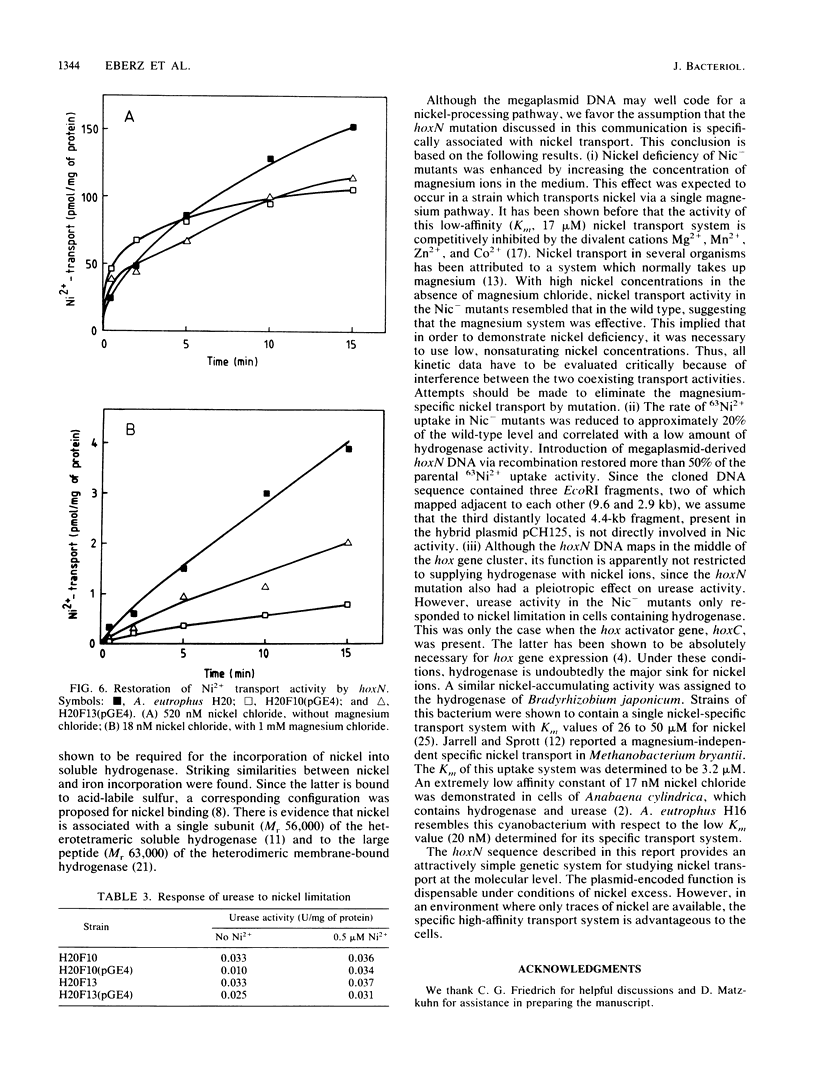
Nickel-deficient (Nic-) mutants of Alcaligenes eutrophus requiring high levels of nickel ions for autotrophic growth with hydrogen were characterized. The Nic- mutants carried defined deletions in the hydrogenase gene cluster of the indigenous pHG megaplasmid. Nickel deficiency correlated with a low level of the nickel-containing hydrogenase activity, a slow rate of nickel transport, and reduced activity of urease. The Nic+ phenotype was restored by a cloned DNA sequence (hoxN) of a megaplasmid pHG1 DNA library of A. eutrophus H16. hoxN is part of the hydrogenase gene cluster. The nickel requirement of Nic- mutants was enhanced by increasing the concentration of magnesium. This suggests that the Nic- mutants are impaired in the nickel-specific transport system and thus depend on the second transport activity which normally mediates the uptake of magnesium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. M., Smith G. D. Transport and accumulation of nickel ions in the cyanobacterium Anabaena cylindrica. Arch Biochem Biophys. 1986 Feb 1;244(2):470–477. doi: 10.1016/0003-9861(86)90615-6. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Krasna A. I. Isolation of genes required for hydrogenase synthesis in Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3289–3298. doi: 10.1099/00221287-133-12-3289. [DOI] [PubMed] [Google Scholar]
- Eberz G., Hogrefe C., Kortlüke C., Kamienski A., Friedrich B. Molecular cloning of structural and regulatory hydrogenase (hox) genes of Alcaligenes eutrophus H16. J Bacteriol. 1986 Nov;168(2):636–641. doi: 10.1128/jb.168.2.636-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAWCETT J. K., SCOTT J. E. A rapid and precise method for the determination of urea. J Clin Pathol. 1960 Mar;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B., Heine E., Finck A., Friedrich C. G. Nickel requirement for active hydrogenase formation in Alcaligenes eutrophus. J Bacteriol. 1981 Mar;145(3):1144–1149. doi: 10.1128/jb.145.3.1144-1149.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich C. G., Schneider K., Friedrich B. Nickel in the catalytically active hydrogenase of Alcaligenes eutrophus. J Bacteriol. 1982 Oct;152(1):42–48. doi: 10.1128/jb.152.1.42-48.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogrefe C., Römermann D., Friedrich B. Alcaligenes eutrophus hydrogenase genes (Hox). J Bacteriol. 1984 Apr;158(1):43–48. doi: 10.1128/jb.158.1.43-48.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornhardt S., Schneider K., Schlegel H. G. Characterization of a native subunit of the NAD-linked hydrogenase isolated from a mutant of Alcaligenes eutrophus H16. Biochimie. 1986 Jan;68(1):15–24. doi: 10.1016/s0300-9084(86)81063-x. [DOI] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982 Sep;151(3):1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Krämer J., Kaltwasser H., Schlegel H. G. Die Bedutung der Ureaserepression für die taxonomische Klassifizierung von Bakterine. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1967;121(4):414–423. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SCHLEGEL H. G., KALTWASSER H., GOTTSCHALK G. [A submersion method for culture of hydrogen-oxidizing bacteria: growth physiological studies]. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G., Jochim K. Effect of nickel on activity and subunit composition of purified hydrogenase from Nocardia opaca 1 b. Eur J Biochem. 1984 Feb 1;138(3):533–541. doi: 10.1111/j.1432-1033.1984.tb07948.x. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Urban M., Friedrich B. Mutagenesis of Alcaligenes eutrophus by insertion of the drug-resistance transposon Tn5. Arch Microbiol. 1982 May;131(3):203–207. doi: 10.1007/BF00405879. [DOI] [PubMed] [Google Scholar]
- Stults L. W., Mallick S., Maier R. J. Nickel uptake in Bradyrhizobium japonicum. J Bacteriol. 1987 Apr;169(4):1398–1402. doi: 10.1128/jb.169.4.1398-1402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabillion R., Kaltwasser H. Energieabhängige 63Ni-Aufnahme bei Alcaligenes eutrophus Stamm H1 und H16. Arch Microbiol. 1977 May 13;113(1-2):145–151. doi: 10.1007/BF00428595. [DOI] [PubMed] [Google Scholar]
- Waugh R., Boxer D. H. Pleiotropic hydrogenase mutants of Escherichia coli K12: growth in the presence of nickel can restore hydrogenase activity. Biochimie. 1986 Jan;68(1):157–166. doi: 10.1016/s0300-9084(86)81080-x. [DOI] [PubMed] [Google Scholar]
- Wu L. F., Mandrand-Berthelot M. A. Genetic and physiological characterization of new Escherichia coli mutants impaired in hydrogenase activity. Biochimie. 1986 Jan;68(1):167–179. doi: 10.1016/s0300-9084(86)81081-1. [DOI] [PubMed] [Google Scholar]



