Abstract
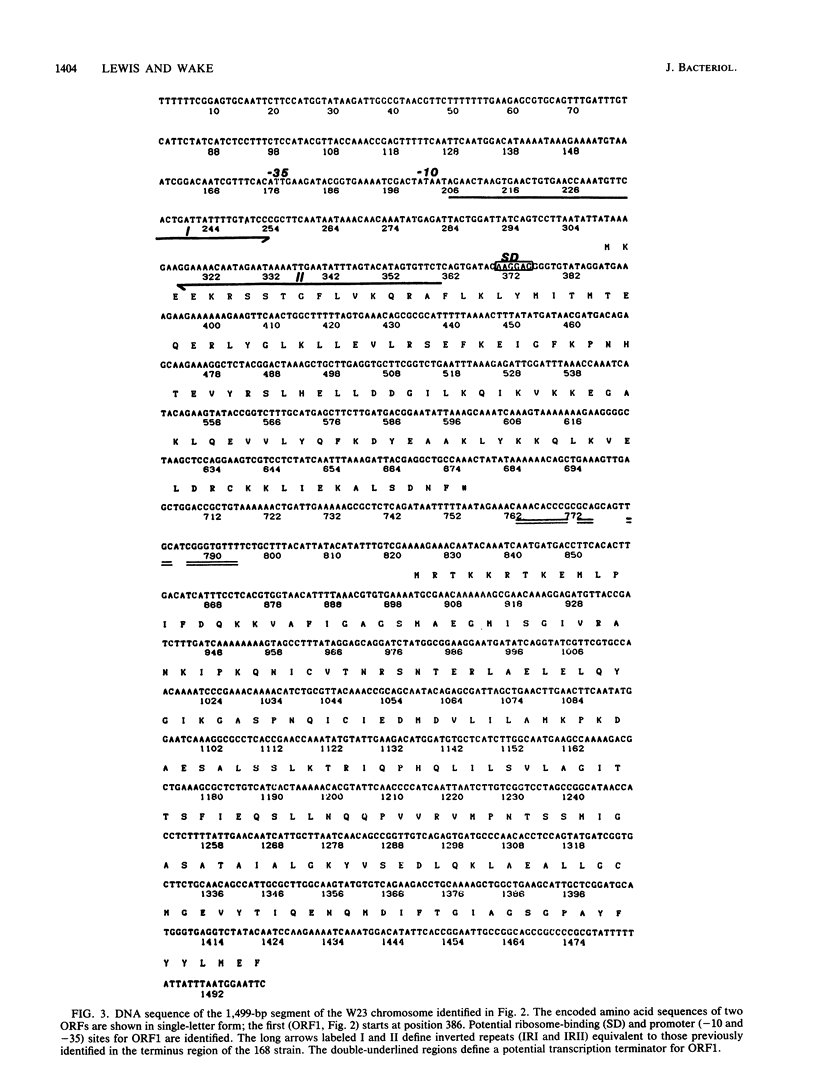
Cloned DNA from the replication terminus region of Bacillus subtilis 168 was used to identify and construct a restriction map of the homologous region in B. subtilis W23. With this information, DNA from the terminus region of W23 was cloned and the sequence was determined for a 1,499-base-pair segment spanning the expected terC site. The position of the site was then located more precisely. Use of the cloned DNA from strain W23 as a probe for digests of DNA from exponentially growing cells of the same strain established the presence of the slowly migrating replication termination intermediate (forked DNA). The orientation and dimensions of the forked molecule were consistent with arrest of the clockwise fork at the terC site in W23, as has been shown to occur in strain 168. Thus, despite significant differences between the two strains, the same termination mechanism appears to be used. The DNA sequences spanning the terC site in strains 168 and W23 showed a high level of homology (90.2%) close to the site but very little at a distance of approximately 250 base pairs from the site in one particular direction. The overall sequence comparison emphasised the importance of the open reading frame for a 122-amino-acid protein adjacent to terC. Although there were 22 base differences in the open reading frames between the strains, the amino acid sequence of the encoded protein was completely conserved. It is suggested that the amino acid sequence conservation reflects a role for the protein in the clockwise fork arrest mechanism as proposed earlier (M.T. Smith and R.G. Wake, J. Bacteriol. 170:4083-4090, 1988).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrigan C. M., Haarsma J. A., Smith M. T., Wake R. G. Sequence features of the replication terminus of the Bacillus subtilis chromosome. Nucleic Acids Res. 1987 Oct 26;15(20):8501–8509. doi: 10.1093/nar/15.20.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley P. J., Carrigan C. M., Rowe D. B., Wake R. G. Breakdown and quantitation of the forked termination of replication intermediate of Bacillus subtilis. J Mol Biol. 1987 Aug 5;196(3):721–727. doi: 10.1016/0022-2836(87)90043-x. [DOI] [PubMed] [Google Scholar]
- Harford N. Bidirectional chromosome replication in Bacillus subtilis 168. J Bacteriol. 1975 Mar;121(3):835–847. doi: 10.1128/jb.121.3.835-847.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iismaa T. P., Wake R. G. The normal replication terminus of the Bacillus subtilis chromosome, terC, is dispensable for vegetative growth and sporulation. J Mol Biol. 1987 May 20;195(2):299–310. doi: 10.1016/0022-2836(87)90651-6. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. T., Aynsley C., Wake R. G. Cloning and localization of the Bacillus subtilis chromosome replication terminus, terC. Gene. 1985;38(1-3):9–17. doi: 10.1016/0378-1119(85)90198-2. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Wake R. G. DNA sequence requirements for replication fork arrest at terC in Bacillus subtilis. J Bacteriol. 1988 Sep;170(9):4083–4090. doi: 10.1128/jb.170.9.4083-4090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. A unique DNA intermediate associated with termination of chromosome replication in Bacillus subtilis. Cell. 1984 Dec;39(3 Pt 2):683–689. doi: 10.1016/0092-8674(84)90475-6. [DOI] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. Impediment to replication fork movement in the terminus region of the Bacillus subtilis chromosome. J Mol Biol. 1984 Nov 15;179(4):745–750. doi: 10.1016/0022-2836(84)90165-7. [DOI] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G., Inman R. B. An immobilized fork as a termination of replication intermediate in Bacillus subtilis. J Mol Biol. 1986 Mar 20;188(2):199–205. doi: 10.1016/0022-2836(86)90304-9. [DOI] [PubMed] [Google Scholar]
- Weiss A. S., Wake R. G. Restriction map of DNA spanning the replication terminus of the Bacillus subtilis chromosome. J Mol Biol. 1983 Dec 5;171(2):119–137. doi: 10.1016/s0022-2836(83)80349-0. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]




