Abstract
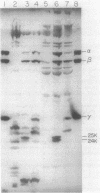
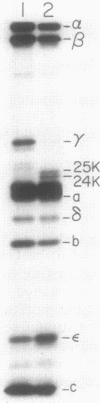
A strain of Escherichia coli which was derived from a gentamicin-resistant clinical isolate was found to be cross-resistant to neomycin and streptomycin. The molecular nature of the genetic defect was found to be an insertion of two GC base pairs in the uncG gene of the mutant. The insertion led to the production of a truncated gamma subunit of 247 amino acids in length instead of the 286 amino acids that are present in the normal gamma subunit. A plasmid which carried the ATP synthase genes from the mutant produced resistance to aminoglycoside antibiotics when it was introduced into a strain with a chromosomal deletion of the ATP synthase genes. Removal of the genes coding for the beta and epsilon subunits abolished antibiotic resistance coded by the mutant plasmid. The relationship between antibiotic resistance and the gamma subunit was investigated by testing the antibiotic resistance of plasmids carrying various combinations of unc genes. The presence of genes for the F0 portion of the ATP synthase in the presence or absence of genes for the gamma subunit was not sufficient to cause antibiotic resistance. alpha, beta, and truncated gamma subunits were detected on washed membranes of the mutant by immunoblotting. The first 247 amino acid residues of the gamma subunit may be sufficient to allow its association with other F1 subunits in such a way that the proton gate of F0 is held open by the mutant F1.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Harold F. M., Simoni R. D. Impairment and restoration of the energized state in membrane vesicles of a mutant of Escherichia coli lacking adenosine triphosphatase. J Biol Chem. 1974 Jul 25;249(14):4587–4593. [PubMed] [Google Scholar]
- Aris J. P., Klionsky D. J., Simoni R. D. The Fo subunits of the Escherichia coli F1Fo-ATP synthase are sufficient to form a functional proton pore. J Biol Chem. 1985 Sep 15;260(20):11207–11215. [PubMed] [Google Scholar]
- Aris J. P., Simoni R. D. The beta subunit of the Escherichia coli ATP synthase exhibits a tight membrane binding property. Biochem Biophys Res Commun. 1985 Apr 16;128(1):155–162. doi: 10.1016/0006-291x(85)91658-4. [DOI] [PubMed] [Google Scholar]
- Brown S., Fournier M. J. The 4.5 S RNA gene of Escherichia coli is essential for cell growth. J Mol Biol. 1984 Sep 25;178(3):533–550. doi: 10.1016/0022-2836(84)90237-7. [DOI] [PubMed] [Google Scholar]
- Brusilow W. S. Proton leakiness caused by cloned genes for the F0 sector of the proton-translocating ATPase of Escherichia coli: requirement for F1 genes. J Bacteriol. 1987 Nov;169(11):4984–4990. doi: 10.1128/jb.169.11.4984-4990.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Downie J. A., Langman L., Senior A. E., Ash G., Fayle D. R., Gibson F. Assembly of the adenosine triphosphatase complex in Escherichia coli: assembly of F0 is dependent on the formation of specific F1 subunits. J Bacteriol. 1981 Oct;148(1):30–42. doi: 10.1128/jb.148.1.30-42.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens A. L., Walker J. E. The organization and sequence of the genes for ATP synthase subunits in the cyanobacterium Synechococcus 6301. Support for an endosymbiotic origin of chloroplasts. J Mol Biol. 1987 Apr 5;194(3):359–383. doi: 10.1016/0022-2836(87)90667-x. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Langman L., Cox G. B., Yanofsky C., Gibson F. Subunits of the adenosine triphosphatase complex translated in vitro from the Escherichia coli unc operon. J Bacteriol. 1980 Jul;143(1):8–17. doi: 10.1128/jb.143.1.8-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Dunn S. D., Futai M. Reconstitution of a functional coupling factor from the isolated subunits of Escherichia coli F1 ATPase. J Biol Chem. 1980 Jan 10;255(1):113–118. [PubMed] [Google Scholar]
- Dunn S. D., Tozer R. G., Antczak D. F., Heppel L. A. Monoclonal antibodies to Escherichia coli F1-ATPase. Correlation of binding site location with interspecies cross-reactivity and effects on enzyme activity. J Biol Chem. 1985 Sep 5;260(19):10418–10425. [PubMed] [Google Scholar]
- Ehrig K., Hoppe J., Friedl P., Schairer H. U. An antibody-binding site in the native enzyme between amino acid residues 205-287 of the gamma-subunit of F1 from Escherichia coli. Biochem Biophys Res Commun. 1986 May 29;137(1):468–473. doi: 10.1016/0006-291x(86)91233-7. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Porter B., Hermolin J., White L. K. Synthesis of a functional F0 sector of the Escherichia coli H+-ATPase does not require synthesis of the alpha or beta subunits of F1. J Bacteriol. 1986 Jan;165(1):244–251. doi: 10.1128/jb.165.1.244-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Brusilow W. S., Simoni R. D. Gene order and gene-polypeptide relationships of the proton-translocating ATPase operon (unc) of Escherichia coli. Proc Natl Acad Sci U S A. 1982 Jan;79(2):320–324. doi: 10.1073/pnas.79.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert R., Brusilow W. S., Gunsalus R. P., Klionsky D. J., Simoni R. D. Escherichia coli mutants defective in the uncH gene. J Bacteriol. 1983 Jan;153(1):416–422. doi: 10.1128/jb.153.1.416-422.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Hama H., Rosen B. P., Futai M. Deletion of seven amino acid residues from the gamma subunit of Escherichia coli H+-ATPase causes total loss of F1 assembly on membranes. Arch Biochem Biophys. 1985 Sep;241(2):364–370. doi: 10.1016/0003-9861(85)90558-2. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Noumi T., Futai M., Nitta T. Escherichia coli mutants defective in the gamma subunit of proton-translocating ATPase: intracistronic mapping of the defective site and the biochemical properties of the mutants. Arch Biochem Biophys. 1983 Jun;223(2):521–532. doi: 10.1016/0003-9861(83)90617-3. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. Assembly of a functional F0 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1983 Aug 25;258(16):10136–10143. [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Simoni R. D. Assembly of a functional F1 of the proton-translocating ATPase of Escherichia coli. J Biol Chem. 1985 Sep 15;260(20):11200–11206. [PubMed] [Google Scholar]
- Kumamoto C. A., Simoni R. D. Genetic evidence for interaction between the a and b subunits of the F0 portion of the Escherichia coli proton translocating ATPase. J Biol Chem. 1986 Aug 5;261(22):10037–10042. [PubMed] [Google Scholar]
- Maeda M., Futai M., Anraku Y. Biochemical characterization of the uncA phenotype of Escherichia coli. Biochem Biophys Res Commun. 1976 May 23;76(2):331–338. doi: 10.1016/0006-291x(77)90729-x. [DOI] [PubMed] [Google Scholar]
- Mates S. M., Eisenberg E. S., Mandel L. J., Patel L., Kaback H. R., Miller M. H. Membrane potential and gentamicin uptake in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6693–6697. doi: 10.1073/pnas.79.21.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mates S. M., Patel L., Kaback H. R., Miller M. H. Membrane potential in anaerobically growing Staphylococcus aureus and its relationship to gentamicin uptake. Antimicrob Agents Chemother. 1983 Apr;23(4):526–530. doi: 10.1128/aac.23.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki J., Takeyama M., Noumi T., Kanazawa H., Maeda M., Futai M. Escherichia coli H+-ATPase: loss of the carboxyl terminal region of the gamma subunit causes defective assembly of the F1 portion. Arch Biochem Biophys. 1986 Dec;251(2):458–464. doi: 10.1016/0003-9861(86)90352-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mosher M. E., Peters L. K., Fillingame R. H. Use of lambda unc transducing bacteriophages in genetic and biochemical characterization of H+-ATPase mutants of Escherichia coli. J Bacteriol. 1983 Dec;156(3):1078–1092. doi: 10.1128/jb.156.3.1078-1092.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir M. E., Wallace B. J. Isolation of mutants of Escherichia coli uncoupled in oxidative phosphorylation using hypersensitivity to streptomycin. Biochim Biophys Acta. 1979 Aug 14;547(2):218–229. doi: 10.1016/0005-2728(79)90005-7. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Rosen B. P. Restoration of active transport in an Mg2+-adenosine triphosphatase-deficient mutant of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1124–1129. doi: 10.1128/jb.116.3.1124-1129.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. All three subunits are required for the reconstitution of an active proton channel (F0) of Escherichia coli ATP synthase (F1F0). EMBO J. 1985 Feb;4(2):515–518. doi: 10.1002/j.1460-2075.1985.tb03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon K. A., Brusilow W. S. Effect of an uncE ribosome-binding site mutation on the synthesis and assembly of the Escherichia coli proton-translocating ATPase. J Biol Chem. 1988 Apr 15;263(11):5402–5407. [PubMed] [Google Scholar]
- Taber H. W., Mueller J. P., Miller P. F., Arrow A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987 Dec;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. H. Letter: Aminoglycoside-resistant Esch. coli. N Engl J Med. 1976 Jul 22;295(4):225–226. doi: 10.1056/NEJM197607222950417. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Okamoto H., Sone N., Hirata H., Kagawa Y. Reconstitution of thermostable ATPase capable of energy coupling from its purified subunits. Proc Natl Acad Sci U S A. 1977 Mar;74(3):936–940. doi: 10.1073/pnas.74.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]