Abstract
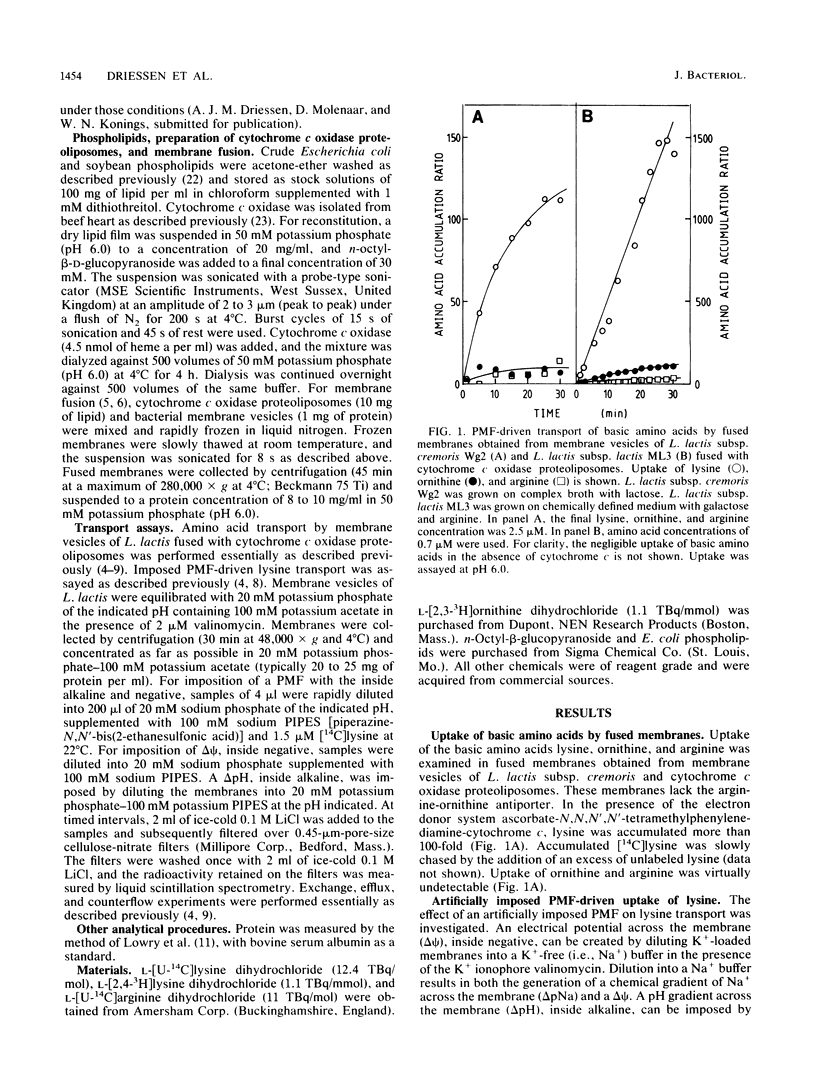
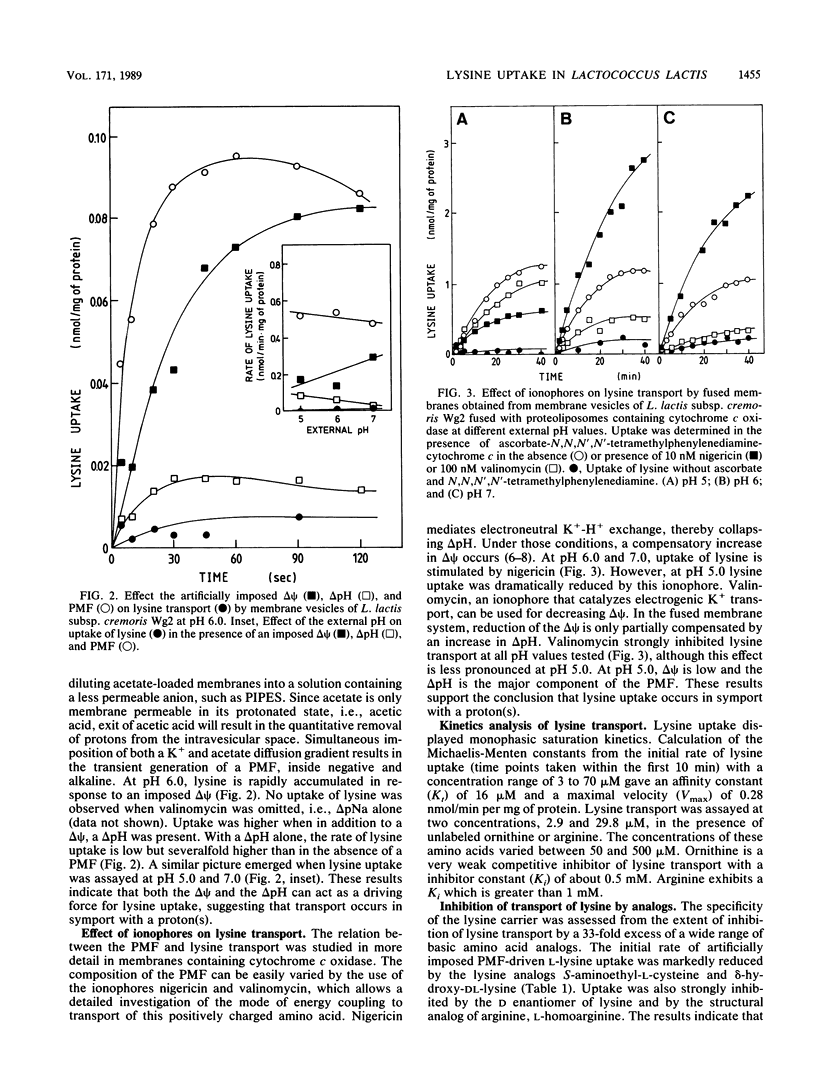
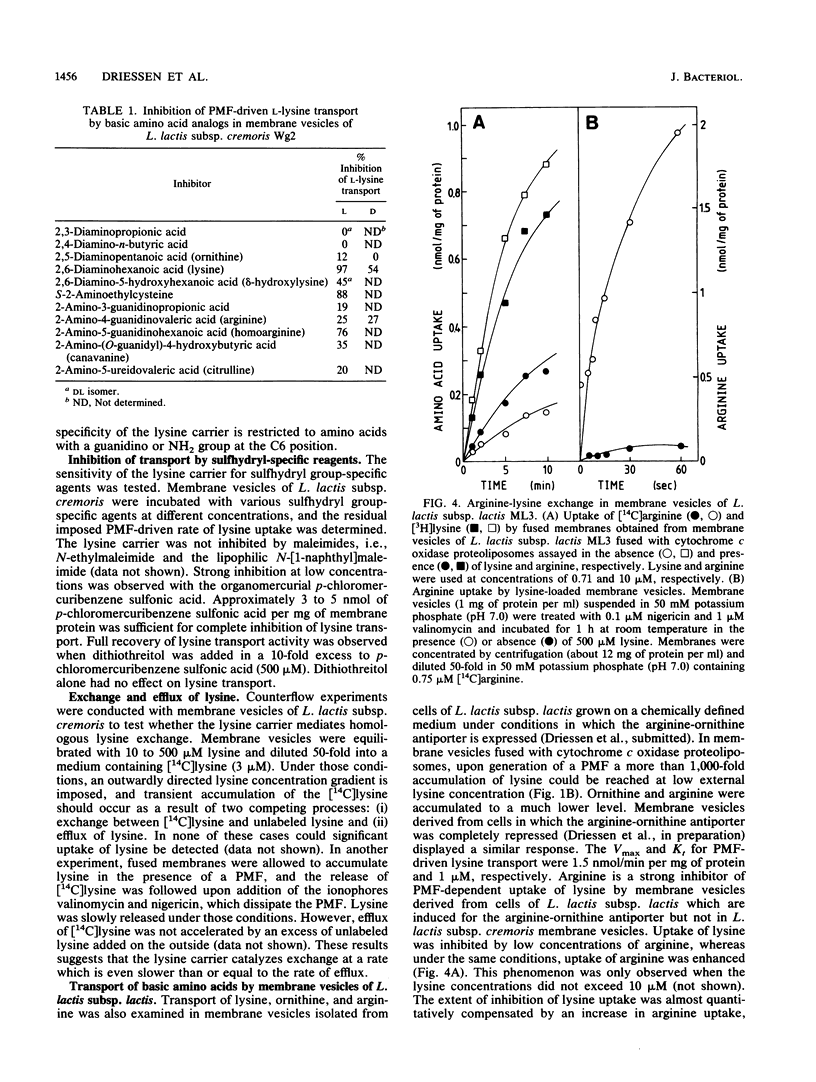
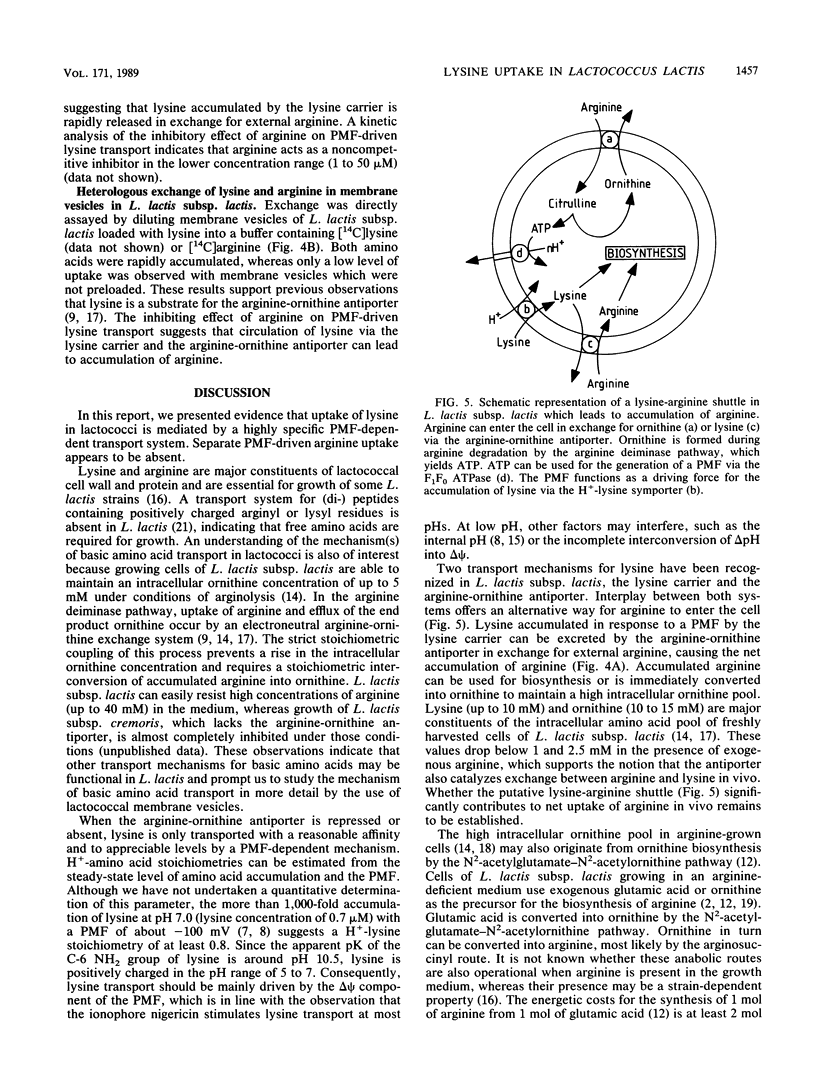
The uptake of the basic amino acids arginine, ornithine, and lysine was studied in membrane vesicles derived from cells of Lactococcus lactis which were fused with liposomes in which beef heart mitochondrial cytochrome c oxidase was incorporated as a proton motive force (PMF)-generating system. In the presence of ascorbate N,N,N'N'-tetramethylphenylenediamine-cytochrome c as the electron donor, these fused membranes accumulated lysine but not ornithine or arginine under aerobic conditions. The mechanism of energy coupling to lysine transport was examined in membrane vesicles of L. lactis subsp. cremoris upon imposition of an artificial electrical potential (delta psi) or pH gradient or both and in fused membranes of these vesicles with cytochrome c oxidase liposomes in which the delta psi and delta pH were manipulated with ionophores. Lysine uptake was shown to be coupled to the PMF and especially to the delta psi, suggesting a proton symport mechanism. The lysine carrier appeared to be specific for L and D isomers of amino acids with a guanidine or NH2 group at the C6 position of the side chain. Uptake of lysine was blocked by p-chloromercuribenzene sulfonic acid but not by maleimides. Counterflow of lysine could not be detected in L. lactis subsp. cremoris, but in the arginine-ornithine antiporter-containing L. lactis subsp. lactis, rapid counterflow occurred. Homologous exchange of lysine and heterologous exchange of arginine and lysine were mediated by this antiporter. PMF-driven lysine transport in these membranes was noncompetitively inhibited by arginine, whereas the uptake of arginine was enhanced by lysine. These observations are compatible with a model in which circulation of lysine via the lysine carrier and the arginine-ornithine antiporter leads to accumulation of arginine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Glansdorff N., Piérard A., Stalon V. Biosynthesis and metabolism of arginine in bacteria. Microbiol Rev. 1986 Sep;50(3):314–352. doi: 10.1128/mr.50.3.314-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Hellingwerf K. J., Konings W. N. Mechanism of energy coupling to entry and exit of neutral and branched chain amino acids in membrane vesicles of Streptococcus cremoris. J Biol Chem. 1987 Sep 15;262(26):12438–12443. [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Poolman B., Kiewiet R., Konings W. Arginine transport in Streptococcus lactis is catalyzed by a cationic exchanger. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6093–6097. doi: 10.1073/pnas.84.17.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Smid E. J., Konings W. N. Transport of diamines by Enterococcus faecalis is mediated by an agmatine-putrescine antiporter. J Bacteriol. 1988 Oct;170(10):4522–4527. doi: 10.1128/jb.170.10.4522-4527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Jong S., Konings W. N. Transport of branched-chain amino acids in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1987 Nov;169(11):5193–5200. doi: 10.1128/jb.169.11.5193-5200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Functional incorporation of beef-heart cytochrome c oxidase into membranes of Streptococcus cremoris. Eur J Biochem. 1986 Feb 3;154(3):617–624. doi: 10.1111/j.1432-1033.1986.tb09443.x. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller S. P., Thompson J. Biosynthesis and stereochemical configuration of N5-(1-carboxyethyl)ornithine. An unusual amino acid produced by Streptococcus lactis. J Biol Chem. 1987 Nov 25;262(33):16109–16115. [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of solute transport in streptococci by external and internal pH values. Microbiol Rev. 1987 Dec;51(4):498–508. doi: 10.1128/mr.51.4.498-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smid E. J., Driessen A. J., Konings W. N. Mechanism and energetics of dipeptide transport in membrane vesicles of Lactococcus lactis. J Bacteriol. 1989 Jan;171(1):292–298. doi: 10.1128/jb.171.1.292-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Curtis M. A., Miller S. P. N5-(1-carboxyethyl)-ornithine, a new amino acid from the intracellular pool of Streptococcus lactis. J Bacteriol. 1986 Aug;167(2):522–529. doi: 10.1128/jb.167.2.522-529.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Miller S. P. N6-(1-carboxyethyl)lysine formation by Streptococcus lactis. Purification, synthesis, and stereochemical structure. J Biol Chem. 1988 Feb 5;263(4):2064–2069. [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Yu C., Yu L., King T. E. Studies on cytochrome oxidase. Interactions of the cytochrome oxidase protein with phospholipids and cytochrome c. J Biol Chem. 1975 Feb 25;250(4):1383–1392. [PubMed] [Google Scholar]


