Abstract
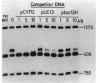
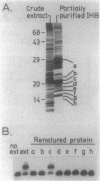
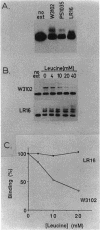
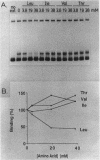
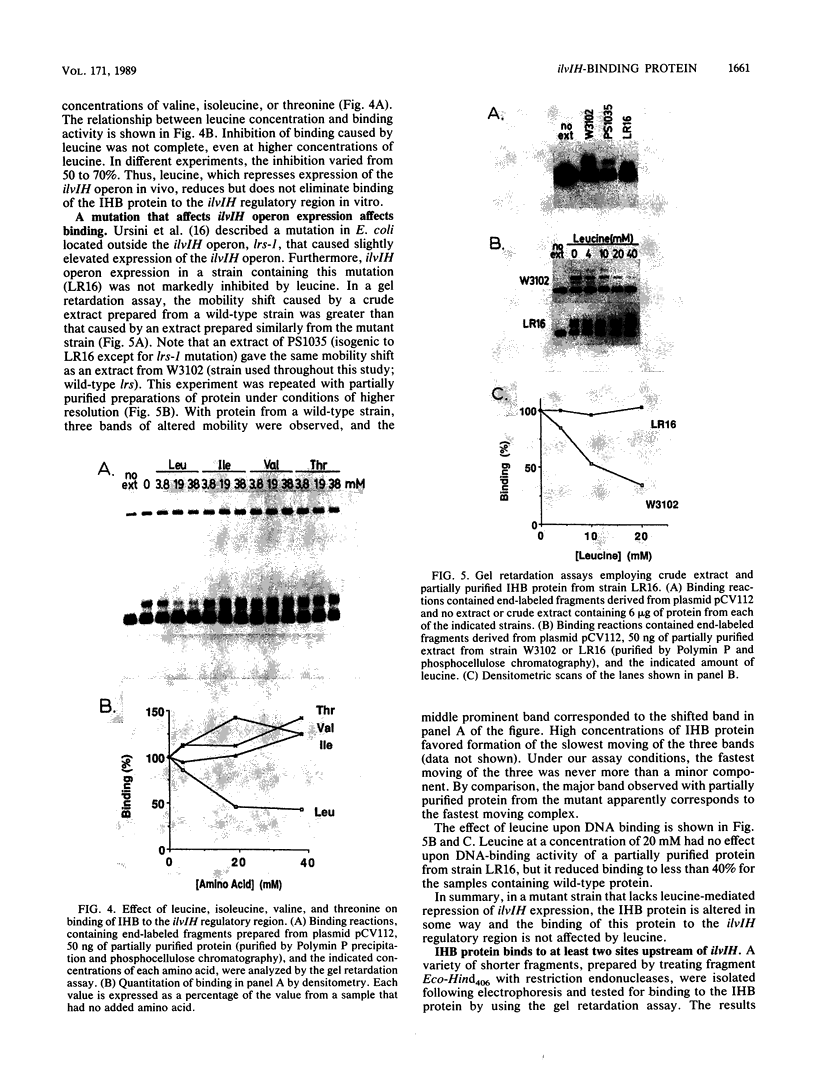
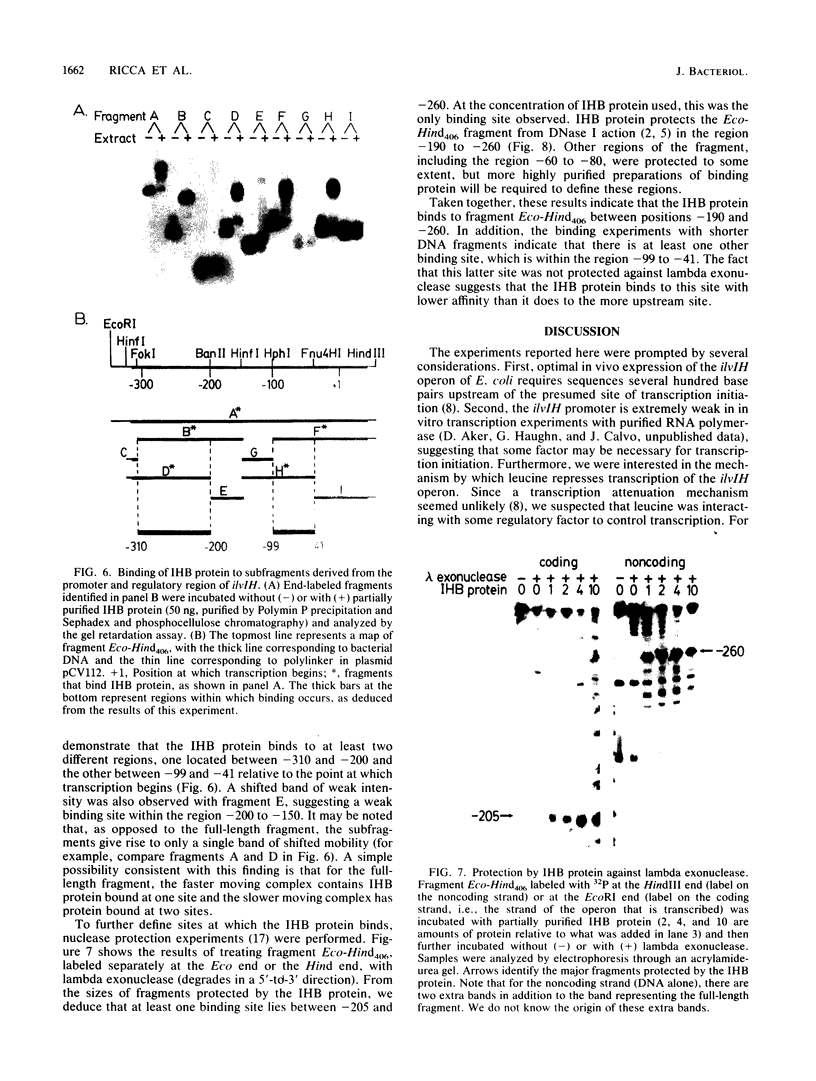
The ilvIH operon of Escherichia coli encodes acetohydroxyacid synthase III, an isoenzyme involved in branched-chain amino acid biosynthesis. Transcription of the ilvIH operon is repressed by growing cells in the presence of leucine (C.H. Squires, M. DeFelice, S.R. Wessler, and J.M. Calvo, J. Bacteriol. 147:797-804, 1981). A protein in crude extracts of E. coli, termed the ilvIH-binding (IHB) protein, bound specifically in vitro to DNA upstream of the ilvIH operon. The binding protein, partially purified by Polymin precipitation, gel filtration, and phosphocellulose chromatography, has a native molecular weight of 43,000 and is composed of two subunits of identical size. As determined by protection against lambda exonuclease and DNase I, the protein binds within a region -190 to -260 relative to the start point of transcription. In addition, the IHB protein binds to a site between positions -100 and -40. The following evidence suggests that binding of this protein to the region upstream of ilvIH is related to the regulation of this operon by leucine. Binding of the IHB protein to the ilvIH regulatory region in vitro was reduced by leucine but not by isoleucine, valine, or threonine. In a mutant strain isolated by M.V. Ursini, P. Arcari, and M. DeFelice (Mol. Gen. Genet. 181:491-496, 1981), transcription was not repressed by leucine. A protein in extracts of this mutant strain bound to the ilvIH regulatory region, but the complex migrated through agarose gels with a mobility different from that of the complex formed by wild-type protein. Furthermore, a concentration of leucine that substantially reduced binding of the wild-type to DNA did not affect binding of the protein from the mutant strain. A simple model consistent with these findings is that transcription from the ilvIH promoter is stimulated by binding the IHB protein to one or more sites upstream of the promoter and that leucine interferes with this binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Nash H. A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984 Dec;39(3 Pt 2):707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Haughn G. W., Squires C. H., De Felice M., Largo C. T., Calvo J. M. Unusual organization of the ilvIH promoter of Escherichia coli. J Bacteriol. 1985 Jul;163(1):186–198. doi: 10.1128/jb.163.1.186-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Wessler S. R., Calvo J. M. Physical characterization of the ilvHI operon of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):797–804. doi: 10.1128/jb.147.3.797-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini M. V., Arcari P., De Felice M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K-12: a trans-acting regulatory locus of ilvHI gene expression. Mol Gen Genet. 1981;181(4):491–496. doi: 10.1007/BF00428741. [DOI] [PubMed] [Google Scholar]
- Wu C. An exonuclease protection assay reveals heat-shock element and TATA box DNA-binding proteins in crude nuclear extracts. Nature. 1985 Sep 5;317(6032):84–87. doi: 10.1038/317084a0. [DOI] [PubMed] [Google Scholar]
- de Felice M., Lago C. T., Squires C. H., Calvo J. M. Acetohydroxy acid synthase isoenzymes of Escherichia coli K12 and Salmonella typhimurium. Ann Microbiol (Paris) 1982 Mar-Apr;133(2):251–256. [PubMed] [Google Scholar]