Abstract
Laminin α chains have unique spatiotemporal expression patterns during development and defining their function is necessary to understand the regulation of epithelial morphogenesis. We investigated the function of laminin α5 in mouse submandibular glands (SMGs). Lama5-/- SMGs have a striking phenotype: epithelial clefting is delayed, although proliferation occurs; there is decreased FGFR1b and FGFR2b, but no difference in Lama1 expression; later in development, epithelial cell organization and lumen formation are disrupted. In wild-type SMGs α5 and α1 are present in epithelial clefts but as branching begins α5 expression increases while α1 decreases. Lama5 siRNA decreased branching, p42 MAPK phosphorylation, and FGFR expression, and branching was rescued by FGF10. FGFR siRNA decreased Lama5 suggesting FGFR signaling provides positive feedback for Lama5 expression. Anti-β1 integrin antibodies decreased FGFR and Lama5 expression, suggesting that β1 integrin signaling provides positive feedback for Lama5 and FGFR expression. Interestingly, the Itga3-/-:Itga6-/- SMGs have a similar phenotype to Lama5-/-. Our findings suggest that laminin α5 controls SMG epithelial morphogenesis through β1 integrin signaling by regulating FGFR expression, which also reciprocally regulates the expression of Lama5. These data link changes in basement membrane composition during branching morphogenesis with FGFR expression and signaling.
Keywords: Laminin α5, fibroblast growth factor receptors, salivary gland development, integrins α3, α6, β1, branching morphogenesis
Introduction
Basement membranes (BMs) separate cell types within tissues and are dynamic structures, being constantly synthesized and remodeled during tissue morphogenesis. Laminins are a major component of basement membranes that are essential for embryonic implantation, induction and maintenance of cell polarity, tissue morphogenesis, and organogenesis (Miner and Yurchenco, 2004; Sasaki et al., 2004). Laminins are heterotrimeric (α, β, γ) glycoproteins, and at least 15 isoforms have been identified with tissue-specific patterns at different stages during development (Aumailley et al., 2005; Ekblom et al., 1998; Miner and Yurchenco, 2004). Laminin-111 (α1β1γ1, previously termed laminin 1, see new nomenclature in (Aumailley et al., 2005) is the major embryonic laminin isoform and is necessary for epiblast differentiation, which requires cooperation between laminin polymerization and cell interactions with β1 integrins and other cell surface receptors (Li et al., 2003b). There is a hierarchy in BM formation, with laminin-111 polymerization acting as a scaffold for the recruitment of other components (Sasaki et al., 2004). Embryos lacking laminin α1 die at E7, well before organogenesis begins, while embryos lacking either laminin γ1 or β1 have no BMs and die within a day of implantation (Miner et al., 2004). Laminin α5, found in laminin-511 (α5β1γ1) and laminin-521 (α5β2γ1), is also widely expressed during development and in adult tissues (Miner et al., 1998). However, laminin α5 only partly compensates for the loss of α1 in embryonic BMs, suggesting α1 and α5 chains have nonoverlapping functions. Embryos with a deletion of the laminin α5 gene, Lama5, die late in embryogenesis with multiple defects, including exencephaly, syndactyly, small or absent kidneys and eyes, and defects in lung and tooth morphogenesis and hair growth (Fukumoto, 2006; Li et al., 2003a; Miner and Li, 2000; Nguyen et al., 2005). Analysis of the SMG phenotype in the Lama5-/- embryo allows investigation of the nonoverlapping functions of laminin α1 and α5 during branching morphogenesis. Laminin α1 and α5 are both present in the initiating SMG epithelial BM, but laminin α1 decreases during development leaving laminin α5 the predominant α chain in the adult SMG (Kadoya and Yamashina, 2005), suggesting they may have specific functions during development.
FGFR signaling is connected with basement membrane assembly during early embryo epithelial morphogenesis (Lonai, 2005), and embryoid bodies expressing an FGFR mutant fail to form a basement membrane (Li et al., 2001). Salivary gland development is particularly sensitive to levels of FGFR2b/FGF10 signaling. FGFR2b+/- and FGF10+/- mice have salivary gland hypoplasia but otherwise develop normally, but FGFR2b-/- and FGF10-/- mice have no salivary glands and also have multiple severe developmental problems (De Moerlooze et al., 2000; Jaskoll et al., 2005; Min et al., 1998; Ohuchi et al., 2000; Sekine et al., 1999). Therefore, identifying factors that regulate epithelial FGFR expression is critical to understanding salivary epithelial morphogenesis. The influence of laminin isoforms on FGFR-dependant salivary epithelial morphogenesis is unknown.
The biological activity of laminins during epithelial morphogenesis is also mediated by interactions with cell surface receptors (De Arcangelis and Georges-Labouesse, 2000; Ekblom, 1996). The major laminin α5 chain receptors are integrins α3β1, α6β1, and α6β4 (Fukumoto, 2006; Kikkawa et al., 2000; Kikkawa et al., 1998; Nielsen and Yamada, 2001), dystroglycan (Shimizu et al., 1999), the Lutheran blood group glycoprotein (Hasenson et al., 2005; Moulson et al., 2001; Vainionpaa et al., 2005), and syndecans (Lin and Kurpakus-Wheater, 2002). The SMGs of integrin α3β1-/- embryos have defects in the apical-basal polarity of cells and altered expression patterns of E-cadherin, integrin α5β1, and fibronectin (Menko et al., 2001). Perturbation of integrin α6 function also decreases SMG branching morphogenesis in culture (Kadoya et al., 1995; Kashimata and Gresik, 1997; Sakai et al., 2003). These data suggest that the integrin receptors for laminin α5 are important for SMG morphogenesis.
Here we investigate the regulation of FGFR expression by laminin isoforms using SMGs from Lama5-/- embryos and siRNA-treated wild-type (WT) SMGs with reduced Lama5 expression, or WT SMGs treated with function-blocking antibodies to β1 integrins. All have decreased branching and reduced expression of FGFR1b, FGFR2b, and FGF1, with no change in Lama1. Decreasing FGFR expression in WT glands also decreases Lama5 expression but not Lama1 expression. Our findings suggest that laminin α5 levels control SMG epithelial morphogenesis through β1 integrin signaling by regulating FGFR expression, which also reciprocally regulates the expression of Lama5 independent of Lama1.
Materials and Methods
Breeding of mice
The generation of Lama5 mutant mice has been well described (Miner et al., 1998; Yin et al., 2003). Two strains with identical SMG phenotypes were used: a targeted null allele and a βgeo (β-galactosidase fused to neomycin phosphotransferase) gene-trap allele that encodes a nonfunctional laminin α5/βgeo fusion protein. Homozygotes were identified by the 100% penetrant syndactyly phenotype evident at E12.5. Staining for β-galactosidase activity with Xgal localized Lama5 expression and was used to genotype the SMGs after organ culture.
Histology and whole-mount immunofluorescence
Embryos were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. SMGs were also frozen in OCT, sectioned, and fixed in acetone, ethanol, or 2% paraformaldehyde. For whole-mount immunofluorescence, SMGs were fixed either with cold acetone:methanol (1:1) for 10 minutes or in 4% PFA for 1 hour and then blocked with 10% donkey serum, M.O.M. blocking reagent (Vector Laboratories, CA), and 1% BSA. Primary antibodies were in M.O.M. protein reagent, and Cy-labeled F(ab)2 secondary antibodies (Jackson Immunoresearch Labs, PA) were in PBS-Tween (Hoffman et al., 2002; Steinberg et al., 2005). Proliferation was detected using a BrdU Labeling and Detection Kit (Roche Molecular Biochemicals, IN). BrdU fluorescence was measured from both confocal sections through the entire gland and normalized to the whole gland area or from epithelial fluorescence normalized to epithelial area using Metamorph image analysis software (Molecular Devices Corporation, CA).
Antibodies
Laminin α5 rabbit antiserum was generated as previously described (Miner et al., 1997). Anti-laminin α1 and anti-laminin β2 rabbit polyclonals, gifts from Dr T. Sasaki (Abrahamson et al., 1989; Sasaki et al., 2002), and anti-laminin α1 (8B3) and anti-laminin β1 (5A2), gifts from Dr D. Abrahamson (Abrahamson et al., 1989), were used for cryosections. Anti-α3 integrin rabbit polyclonal antiserum was a gift from Dr M. DiPersio (DiPersio et al., 1997). Laminin γ1 (MAB1914), perlecan (MAB1948), integrin α6 (GoH3) (Chemicon International, CA), laminin α2 rat Mab (Alexis Biochemical Corp., CA), troma-1 cytokeratin antibody (Developmental Studies Hybridoma Bank, University of Iowa), Rabbit anti-ZO-1 (Zymed, Invitrogen, CA), and Alexa 546 phalloidin (Molecular Probes, OR) are commercially available. Preservative-free anti-β1 integrin (Ha2/5), hamster IgG (BD Biosciences, CA), anti-α6 integrin (GoH3), and rat IgG (both Serotec) were also used for function-blocking studies and immunostaining. Antibodies used for Western blots include phospho-p42/44 MAPK (Erk1/2), p42/44 MAPK (Erk1/2) (Cell Signaling Technology, MA) and anti-GAPDH (Research Diagnostics Inc., NJ).
Gene expression analysis
SMGs were combined from at least five mouse embryos/stage at embryonic day 12, 13, 14, 15, 17, and postnatal days 1, 5, and adult, and three independent sets of cDNA were made at each stage. The epithelium and mesenchyme of E13 SMGs were separated after dispase treatment, and gene expression was measured after SMG culture, as previously described (Steinberg et al., 2005). DNase-free RNA was prepared using an RNAqueous-4PCR kit (Ambion, Inc., TX), TaqMan™ reverse transcription reagents (Applied Biosystems, CA) were used to make cDNA, and real-time PCR was performed using SYBR Green PCR Master Mix (Biorad, CA) with a Biorad I-cycler thermocycler. cDNA (1-10 ng) was amplified with 40 cycles of 94 °C for 10 seconds and 62 °C for 30 seconds. Primer sequences (available on request) were designed with similar parameters (Tms 65 +/- 3 °C) using Beacon Designer software (Premier Biosoft, CA) with amplicons between 75 and 150 bp. Melt-curve analysis and serial dilution of control cDNA confirmed that single products were amplified with similar efficiencies. Gene expression was normalized to 29S, reactions run in triplicate, and experiments repeated at least three times.
Ex vivo SMG organ culture
The heads of the embryos were shipped overnight between laboratories (from JHM to MPH labs) in DMEM/F12 on wet ice. The SMGs were dissected and cultured on Nuclepore Track-etch filters (VWR, IL) and floated on 200 μl of DMEM/F12 in 50-mm glass-bottom microwell dishes (MatTek, MA) as previously described (Steinberg et al., 2005). The medium contained 100 U/ml penicillin, 100 μg/ml streptomycin, 150 μg/ml vitamin C, and 50 μg/ml transferrin. SMGs were cultured at 37 °C in a humidified 5% CO2/95% air atmosphere. At least four SMGs per group were cultured, and each experiment was repeated at least three times.
RNA interference and growth factor rescue
RNA interference sequences for FGFR1 (R1.6, accgaattggaggctacaag) and FGFR2 (R2.5, atgtatccatcgagattt) were designed using Oligoengine-2 software (Oligoengine, WA). Lama5 siRNA (lama5.1, cgactcacctcatgtctgt) and nonsilencing siRNA were also used (Dharmacon, CO). At least three siRNAs were tested for each gene, and gene knockdown was measured using at least two siRNAs for each gene. SMGs were transfected with 400 nM of siRNA in 200 μl of culture medium using Oligofectamine (Invitrogen, CA) as previously described (Sakai et al., 2003) or RNAiFect (Qiagen, CA). Total RNA was purified after 36 or 48 hours of culture, and gene knockdown was measured by RT-PCR analysis. SMGs were also fixed after siRNA treatment, and decreased protein expression was measured by immunofluorescent staining for laminin α5, normalizing to either E-cadherin staining. The decreased branching with Lama5 knockdown was rescued by activating both FGFR1 and FGFR2 signaling with FGFs. FGF1 (10 and 20 ng/ml), FGF2 (10 and 20 ng/ml), and FGF10 (50 and 100 ng/ml) were added to the culture media 1 hour after the siRNA transfection. Western analysis was previously described (Larsen et al., 2003); SMGs were solubilized in RIPA buffer, and 5 μg of protein per lane was analyzed by mini SDS-PAGE. After transfer, membranes were blocked, incubated with primary and secondary antibodies for 1 h, developed (Super Signal West Dura; Pierce), and exposed to film. Bands were quantitated using an AlphaImager 3400 (Alpha Innotech, CA), and the phospho-p42/44 MAPK bands were normalized to total p42/44 MAPK.
Results
Lama5-/- SMGs are developmentally delayed at E13, and epithelial cell organization and lumen formation are disrupted by E17
E13 SMGs from Lama5/βgeo gene-trap mice were stained for β-gal activity with Xgal (Fig. 1A); βgeo expression is driven by the endogenous Lama5 promoter, so β-gal activity serves as a reporter for Lama5 expression (Yin et al., 2003). The entire SMG and sublingual (SLG) epithelium, including ducts and end buds, expressed laminin α5 (also confirmed by the PCR data Fig. 2C). The E13 Lama5-/- SMG was developmentally delayed by ∼ 1 day: the first round of cleft formation and branching had not occurred although the end bud was enlarged, and the SLG had not formed (Fig. 1A). The contralateral SMG was stained undissected from the mandible, and a slight thickening of the oral epithelium was observed in place of the SLG (Fig. 1B, red arrowhead). The phenotype with no SLG is ∼83% penetrant; the absence of SLGs did not correlate with any observable phenotype, such as exencephaly. These results suggest that laminin α5 has specific functions in the initiation of cleft formation before the first round of branching and for sublingual gland initiation.
Fig. 1.
Lama5-/- SMGs are developmentally delayed and smaller than WT, lack sublingual glands, and by E17, epithelial cell organization and lumen formation are disrupted. A. Localization of Lama5 expression in E13 salivary glands using a lacZ insertion in the Lama5 locus. After β-galactosidase staining, the SMGs were dissected out of the embryos and photographed. The salivary epithelium is outlined in the dark-field view of the WT gland. The heterozygous Lama5+/- shows laminin α5 expression throughout the submandibular and sublingual epithelium. The Lama5-/- SMG is delayed, appearing as a single bud, and the sublingual gland (SLG) is absent. B. The contralateral Lama5-/- gland is shown in vivo in the mandible and clearly shows agenesis of the SLG (red arrowhead). C. Histological analysis of E14 Lama5-/- SMGs, which have fewer epithelial end buds than WT. D. At higher magnification, the cells in the E14 Lama5-/- SMGs appear similar to WT. E. At E17 the SMGs from Lama5-/- mice are ∼1/3 the size of WT, and the SLG is absent. F. At higher magnification, the cells in the E17 Lama5-/- SMGs appear disorganized, the nuclei are not basolaterally localized, and lumen formation is reduced compared to WT cells. Paraffin sections were stained with hematoxylin and eosin. G. Immunostaining of E17 SMGs with ZO-1 (green), a marker of lumen formation, phalloidin (red) to stain actin, and a merged image with nuclear staining (blue) which also highlights colocalization of ZO-1 and actin at well-organized lumens (yellow) in the WT. Some ZO-1 staining is present in the Lama5-/- SMGs, but the terminal buds are not well polarized, lacking obvious lumens.
Fig. 2.
Localization of laminin chains in WT and Lama5-/- E14.5 SMGs and expression of laminin chains during SMG development and in E13 epithelium and mesenchyme. A. Immunostaining of WT and Lama5-/- SMGs with laminin chain-specific antibodies does not show increased staining of other laminins, although there is increased cytoplasmic localization of β1 and γ1 in the Lama5-/- SMGs. Frozen serial sections of SMGs were stained with troma-1, a cytokeratin antibody that stains SMG epithelium. Laminins α1, α2, α5, β1, and γ1 are localized in the subepithelial basement membranes. Laminin β2 is around endothelial cells and within the cytoplasm of the duct cells. B. Laminin chain expression is developmentally regulated during SMG organogenesis, and laminin α1 and α5 are the major epithelial α chains in E13 SMGs. The relative expression of laminin chains was analyzed by real-time PCR and expressed as fold change in expression compared to E12 glands. The Lama1, Lamb1, Lamb2, and Lamc1 chains are expressed most highly in the first few days of development. Lama1 expression decreases during early development while other chains increase. The Lamb3 and Lamc3 chains are expressed most highly in adult glands. Lower CT values correlate to higher relative expression (see Materials and Methods). C. Lama1 and Lama5 are the major epithelial α chains in early SMG development. The epithelium and mesenchyme of E13 SMGs were separated, and the relative expression of laminin chains was compared in epithelium and mesenchyme. The epithelium expresses Lama1, Lama5, and comparatively low levels of Lama3, while the mesenchyme expresses Lama1, Lama2, and Lama4.
E14.5 Lama5-/- SMGs had larger terminal lobules than WT SMGs (Fig. 1C), but the epithelial cell morphology appeared similar to the WT (Fig. 1D), as seen in H and E-stained sections. At E17.5, the Lama5-/- SMGs were ∼1/3 the size of WT SMGs (Fig. 1E) and the SLGs are often absent. The ductal and terminal lobule epithelial cells appeared disorganized (Fig. 1F): the nuclei were not basolaterally localized and lumen formation was reduced. ZO-1 staining (Fig. 1G, green), which highlights lumen formation, and phalloidin staining (Fig. 1G, red) colocalized at well-organized lumens in the WT (Fig. 1G, yellow). Some ZO-1 staining was present in the Lama5-/- SMGs but the terminal buds lack lumens. We see from these histology results that although cleft formation is delayed, the epithelium eventually begins to branch, possibly from compensation by another laminin isoform such as Lama3, which is increasing in expression at E14 and is produced by epithelial cells (Fig. 2B and C). The cellular organization within the E14.5 bud appeared normal, with basally located nuclei in the outer layer of cells, and laminin α1 likely functions to maintain this. However, by E17.5, the loss of laminin α5 resulted in smaller SMGs than WT glands, with abnormal cellular differentiation and reduced lumen formation (Fig. 1F and G). From these data, we suggest laminin α5 has a role in maintaining epithelial cell organization and is important for lumen formation.
Other laminins do not increase in Lama5-/- glands, although there is intracellular accumulation of laminin β1 and γ1 chains
There was no increase in immunolocalization of other laminin chain isoforms (i.e., α1 or α2 chains) in the subepithelial BM of E14.5 Lama5-/- SMGs (Fig. 2A); however this does not mean other isoforms cannot functionally compensate even though their expression does not change. The cytokeratin antibody, troma-1, identified the SMG epithelium in the sections. The Lama5-/- epithelium had increased intracellular β1 and γ1 chain cytoplasmic staining. The lack of laminin β2 in the epithelial BM suggests that laminin-511, not laminin-521, is present in the subepithelial BM. Laminin β2 was also highly expressed around blood vessels and has some expression in the epithelium (Fig. 2C) which appeared in the epithelial cells near the duct lumen (Fig. 2A). These data suggest that Lama5 does not regulate the expression of other isoforms, but the secretion of laminin β1 and γ1 may be disrupted, resulting in intracellular accumulation.
Laminin chain expression is developmentally regulated, and α1 and α5 chains are the major epithelial α chains in early SMG development
Since the localization of other laminin chains was not altered at E14.5, we did a comprehensive analysis of laminin chain gene expression at 8 stages of WT SMG development (Fig. 2B) and within the epithelium or mesenchyme at E13 (Fig. 2C) by real-time PCR. The profiles (Fig. 2B) highlight the dynamic and dramatic changes in laminin chain expression throughout development. The most highly expressed laminin chains at E12 (low Ct values from PCR) were α1 and β1 but β2 and γ1 were also highly expressed in the first few days of development. Laminin α1 chain gene expression decreased during early development while other laminin α chains increased, which is consistent with previous immunostaining results (Kadoya et al., 1995). Since the SMG lysates include multiple cell types in the mesenchyme, particularly endothelial and neuronal cells (Patel et al., 2006), the epithelium was separated from the mesenchyme at E13 to determine the expression level of epithelial laminin chains (Fig. 2C). Importantly, laminin α1 and α5 were the most highly expressed α chains in the epithelium at E13 (Fig. 2C). The α3 chain, which is also expressed in the epithelium, had peak expression at E15 but very low expression in the E13 SMGs relative to the other α chains. Expression of the γ2 chain was similar to α3 and was also expressed in the epithelium. Laminin α2 chain expression peaked at E14 and again at P5 and was expressed in the mesenchyme at E13 (Fig. 2C), and it was also present in the BM along differentiating ducts (Kadoya and Yamashina, 2005). Laminin α4 chain expression was highest at E13, but it was expressed in the mesenchyme (Fig. 2C) and is likely associated with endothelial cells (Lefebvre et al., 1999). Laminin γ2 was expressed highly throughout development and had high expression in the epithelium at E13. The β3 and γ3 chains were expressed most highly in the adult gland, although at E13 the β3 chain was expressed in both epithelium and mesenchyme and the γ3 chain mainly in the mesenchyme. We conclude that as α1 chain expression decreases the epithelial expression of the α5 chain increases (Fig. 2C). We therefore focused on the function of laminin α5 in the early developing gland, by comparing the localization of α5 to α1 by whole-mount immunofluorescence.
Laminin α1 and α5 are present in regions of early cleft formation
Both laminin α1 and α5 chains were immunolocalized over the entire epithelial BM in E13 SMGs (Fig. 3A and B; projection of confocal sections). The projections show the surface topography of the epithelium beginning to cleft, highlighting the accumulation of both laminin chains in discrete areas in the BM along the ducts and the presence of both chains where clefts are forming. Single sections through an end bud show that both laminin chains are present in early epithelial clefts, which have basement membrane on both epithelial surfaces in the cleft (Fig. 3C and D).
Fig. 3.

Laminin α1 (A and C) and laminin α5 (B and D) are present over the entire epithelial basement membrane and accumulate in discrete areas in the basement membrane along the ducts and are present in regions of cleft formation (white arrowheads). A and B are single projections of multiple confocal sections after whole-mount immunostaining and highlight the surface topography of E13 epithelial buds. C and D are single confocal sections of the end bud showing the presence of laminin α1 (C) and laminin α5 (D).
The defects in branching morphogenesis begin early in cleft formation during ex vivo culture of Lama5-/- SMGs
Lama5-/- glands were developmentally delayed and by E17.5 were 1/3 the WT size, with disrupted epithelial cell organization and lumen formation; therefore, we used ex vivo organ culture to investigate the mechanisms involved in the defect in branching. The developmental delay of Lama5-/- SMGs was obvious at E13.5 (Fig. 4A): the SMGs had only a single bud, similar to an E12 WT gland, whereas the heterozygote and WT glands had ∼8 buds. The WT and heterozygous glands appeared the same. Strikingly, the E13.5 Lama5-/- SMGs continued to increase in size but did not form clefts in the epithelium for the first 20 hours of culture. In contrast, WT E12 SMGs form clefts from the single-bud stage within 6 hours (Sakai et al., 2003). The heterozygous glands also showed a decrease in branching compared to WT glands within the first 20 hours of culture (Fig. 4B), although their morphology was similar to WT (Fig. 4A). This result is important because it suggests that decreased levels of laminin α5 have a functional consequence on branching morphogenesis and provides a rationale for knocking down Lama5 expression in WT glands to investigate laminin α5 functions. After 42 hours there was no significant difference in the number of end buds (expressed as a ratio of the number of end buds at 42 hours/the number at 2 hours), but the Lama5-/- SMGs had larger epithelial buds and did not resemble the wildtype or heterozygous SMGs (Fig. 4A). The defect in branching morphogenesis of the Lama5-/- glands was not only a delay in branching that began during early cleft formation, but also involved abnormal morphogenesis resulting in glands that did not resemble the wildtype SMGs after further culture. There was a disruption in the patterning of the first five buds (see Lama5-/- in Figs. 4A and C): the lobules did not septate normally, and they had wider ducts and enlarged end buds, which were apparent in the histology of E14.5 SMGs in vivo (Fig. 1C). The initial clefting and branching of E13 Lama5-/- glands were still delayed compared to size-matched E12 WT glands, which also have a single epithelial bud but cleft within 6 hours (data not shown, also see Fig. 5A). The βgeo insertion allowed genotyping of the glands after organ culture with β-galactosidase staining (Fig. 4C), which confirmed that the expression level of Lama5 in the Lama5+/- SMG was reduced and that Lama5 was expressed throughout the ducts and end bud epithelium (Fig. 4C). It is apparent from the ex vivo organ culture that laminin α5 has a unique role during branching morphogenesis that is evident when cleft formation begins.
Fig. 4.

There is delayed cleft formation in Lama5-/- SMGs and decreased branching morphogenesis of both Lama5+/- and Lama5-/- SMGs after 20 hours of culture compared with WT. A. The Lama5-/- SMGs are developmentally delayed compared to Lama5+/- and WT SMGs. Within the first 20 hours of culture, the single epithelial end bud enlarges but fails to cleft (red outline); however, after 42 hours of culture, the gland starts to cleft and form new branches but with less development of ducts compared to the controls at this time point. B. Quantitation of the number of end buds (expressed as a ratio of the number at 20 hours/ the number at 2 hours) shows that the Lama5+/- (n=15) and Lama5-/- (n=9) SMGs have reduced branching morphogenesis compared to WT (n=12). However, after 42 hours there is no significant difference in branching morphogenesis (expressed as a ratio of the number at 42 hours/the number at 2 hours). P values are ANOVA compared to WT, *p< 0.05, **p< 0.01. C. After culture the Lama5/βgeo gene-trap SMGs were stained for β-gal activity, photographed, then sectioned and counterstained with eosin, highlighting the fact that Lama5 is expressed in all epithelial cells of the ducts and buds and that there is more expression (i.e., less β-gal activity) in Lama5+/- compared to Lama5-/-.
Fig. 5.

There is no difference in proliferation after 24 hours of culture, but expression of FGFR1b, FGFR2b, and FGF1 is reduced in Lama5-/- compared to WT SMGs. A. Light micrograph and single projection of confocal sections of the whole-mount BrdU-labeled SMGs are shown. The E13 Lama5-/- SMG is compared to its WT littermate and also compared to E12 size-matched WT glands. The size-matched E12 WT gland has gone through the first round of branching after 24 hours, whereas the E13 Lama5-/- SMG is just beginning to cleft. B. Quantitation of whole-mount BrdU staining. There is no statistical difference between the level of proliferation in the E13 Lama5-/- SMG compared to WT littermate or to a size-matched E12 WT gland. BrdU labeling was expressed as the average pixel intensity of the whole gland/whole gland area (AU X106) or as the average pixel intensity of the epithelium/epithelial area (AU X106). At least 3 glands/condition were used for quantitation, and the experiments were repeated and results combined. C. Comparison of FGFR and FGF gene expression by real-time PCR, normalized to 29S expression, reveals a 53% decrease in FGFR1b, a 37% decrease in FGFR2b, and a 75% decrease in FGF1 levels in E14 Lama5-/- SMGs compared to WT, but no change in Lama1 expression. P values are unpaired T-tests compared to 29S expression levels ** P < 0.01, * P < 0.05.
The delay in clefting is not caused by decreased cell proliferation
Cell proliferation was measured by BrdU incorporation after 24 hours in culture (Fig. 5A). The E13 Lama5-/- SMGs were smaller than the WT littermates; therefore, we also compared proliferation to size-matched E12 WT SMGs. After 24 hours of culture, the E12 SMGs had clefted and gone through the first round of branching, whereas the E13 Lama5-/- SMGs were just beginning to cleft. The amount of total or epithelial proliferation was measured from the confocal stacks using an image analysis program and normalized to either the total gland area or the epithelial area of the gland (Fig. 5B). There was no significant difference in proliferation between the groups in terms of total or epithelial proliferation. Therefore, laminin α5 does not affect proliferation during the first 20 hours of culture when there is delayed epithelial clefting. However, because of the decreased size of the Lama5-/- SMGs at E17.5 we speculate that the lack of laminin α5 later in development may influence cell proliferation, which may be associated with the disruption in epithelial cell organization.
There is decreased FGFR1b, FGFR2b, and FGF1 expression in Lama5-/- SMGs
We previously reported that FGFR1b, FGFR2b, and FGF1 are expressed in the salivary epithelium and regulate SMG epithelial proliferation and morphogenesis (Hoffman et al., 2002; Steinberg et al., 2005). Here we analyzed the expression of FGFs and FGFRs expressed in E14 Lama5-/-glands compared to E14 WT glands. There was a 53% decrease in FGFR1b, a 37% decrease of FGFR2b, and a 75% decrease in FGF1, but no difference in Lama1 expression in Lama5-/- versus WT glands (Fig. 5C). There was no significant decrease in FGF7, FGF10, and FGF2, other FGFs shown to be present in SMGs at E13. Thus, downstream signaling from laminin α5 influences FGFR1b, FGFR2b, and FGF1 expression; however, the reduction in FGFR expression in the absence of Lama5 does not affect epithelial proliferation for the first 20 hours of culture.
Lama5-siRNA decreases branching, p42 MAPK phosphorylation, and expression of FGFR1b, FGFR2b, and FGF1, and branching is rescued by FGF10
The function of laminin α5 was also investigated using siRNA to Lama5 in WT SMGs. Branching morphogenesis and Lama5 expression were decreased by ∼50% after 36 hours (Fig. 6A and 6B) and there was a 59% decrease p42 MAPK phosphorylation 24 hours after Lama5-siRNA treatment compared to control siRNA (Fig. 6C; Western blot). Lama5-siRNA also decreased laminin α5 protein in the basement membrane to 56% of control as measured by quantitative immunofluorescence, normalizing the amount of laminin to E-Cadherin, an epithelial cell marker (Fig. 6D and 6E). The first round of clefting occurred within 24 hours probably because endogenous laminin α5 was present and the siRNA knockdown was not measurable until ∼24 hours post transfection, but by 48 hours, subsequent rounds of branching were delayed. Therefore, siRNA knockdown did not allow investigation of the delay in the first round of branching. However, analysis of FGFR and FGF1 gene expression by real-time PCR shows similar findings to SMGs from Lama5-/- mice (Fig. 6F); FGFR1b, FGFR2b, and FGF1 were all decreased by greater than 50%, and Lama1 expression did not change. Importantly, the decrease in branching could be rescued by the addition of exogenous FGF10, which binds FGFR2b, but not by addition of FGF2, which binds FGFR1b (Fig. 6A and B). The exogenous FGF10 also increased gene expression of Lama5 and FGFR2b in the siRNA-treated SMGs to control levels (data not shown). The addition of FGF1, which binds both FGFRs partially rescued branching (data not shown). Western blot analysis indicated a 59% decrease of p42 MAPK phosphorylation 24 hours after Lama5-siRNA treatment compared to control siRNA. Thus, the reduced p42 MAPK signaling and FGFR expression resulting from less laminin α5 in the BM, likely provides negative feedback for epithelial morphogenesis.
Fig. 6.

siRNAi to Lama5 decreases branching morphogenesis, p42 MAPK signaling, and downregulates FGFR1b, FGFR2b, and FGF1 expression, but branching is rescued by exogenous FGF10, not FGF2. A. E12 SMGs were cultured in the presence of nonsilencing (control-siRNA) and silencing (Lama5-siRNA) siRNA. Light micrographs at 36 hours show the decrease of branching morphogenesis with Lama5-siRNA compared to control-siRNA and the rescue of branching by the addition of FGF10 (100 ng/ml) but not FGF2 (20 ng/ml). B. The graph shows quantitation of the number of end buds expressed as a ratio of the number at 36 hours/the number at 2 hours. C. Western blot analysis shows there is a 59% decrease in p42 MAPK phosphorylation 24 hours after Lama5-siRNA treatment compared to control-siRNA. D. Lama5-siRNA decreases laminin α5 protein in the basement membrane to 56% of control levels. After siRNA treatment, the SMGs were stained for laminin α5 (green), perlecan (red), and E-cadherin (blue). E. The immunofluorescence was measured using Metamorph image analysis software from confocal sections, and the amount of laminin staining was expressed as a ratio of the E-cadherin staining (an epithelial cell marker) F. Lama5-siRNA also downregulates FGFR1b, FGFR2b, and FGF1 expression but not Lama1and decreases Lama5 expression by 50% compared with control-siRNA. After 36 hours, gene expression was analyzed by real-time PCR normalizing to 29S and expressed as a relative fold-change compared to control-siRNA. P values are unpaired T-tests compared to 29S controls **P< 0.01, *P< 0.05.
FGFR expression influences Lama5 but not FGF1 and Lama1 expression
We also used siRNA to reduce FGFR expression in WT SMGs to determine the hierarchy of gene expression. Knockdown of either FGFR1 or FGFR2 expression significantly decreased branching morphogenesis by 48 hours (Fig. 7A), and knockdown of both FGFRs inhibited branching more than either alone (Fig. 7A). The knockdown of one epithelial FGFR also downregulated the expression of the other by 48 hours, suggesting reciprocal regulation of FGFR1b and FGFR2b expression. In addition, decreasing FGFR expression reduced Lama5 expression, did not affect Lama1 expression, and increased FGF1 expression. The increased FGF1 expression may be an attempt to compensate for decreased receptor levels (Fig. 7B) and could also suggest that FGF1 expression is downstream from laminin α5, independent of FGFR levels. We therefore suggest that there is reciprocal regulation of expression of the epithelial FGFRs and Lama5, that is independent of Lama1. Downstream signaling from epithelial FGFRs involves Erk1/2 and PI3K signaling (Steinberg et al., 2005); therefore, we investigated the receptor signaling downstream of laminin α5.
Fig. 7.
Decreasing FGFR1 or FGFR2 expression with siRNA decreases both branching and Lama5 expression but does not decrease Lama1 expression. A. Light micrographs of E12 SMGs cultured for 48 hours with either a scrambled control siRNA, siRNA to FGFR1 and FGFR2, or a combination of both FGFR siRNA. The decrease in branching is quantitated by counting the number of end buds after 48 hours of siRNA transfection. Knockdown of both FGFRs inhibits branching more than either alone. ANOVA ** P<0.01 compared to control. B. The knockdown of either FGFR1 or FGFR2 results in decreased Lama5 and increased FGF1 expression, but does not affect Lama1 expression. The knockdown of one epithelial FGFR also decreases the expression of the other by 48 hours, suggesting each receptor regulates the expression of the other. The gene expression was measured by real-time PCR, and the results are expressed in fold increase in gene expression compared to glands transfected with a nonsilencing control siRNA. P values are unpaired T-tests compared to 29S controls **P< 0.01, *P< 0.05.
Blocking β1 Integrin function decreases FGFR1b, FGFR2b, and FGF1 expression but also decreases Lama5 expression
Integrin α3β1 and α6β1 are epithelial cell surface receptors for laminin α5. Both were present in the epithelium at cell-matrix and cell-cell junctions, and their distribution was similar in WT and Lama5-/- SMGs (Fig. 8A). Some α6 integrin staining at the periphery of the SMG bud was not associated with β1 (data not shown) and was likely paired with β4. Function-blocking β1 integrin antibodies decrease branching morphogenesis of E13 SMGs in culture (Kadoya et al., 1995; Sakai et al., 2003). We saw similar effects on branching with anti-β1 integrin antibodies (Fig. 9B) and also found reduced expression of FGFR1b, FGFR2b, and FGF1 (Fig. 8C), which might be expected if β1 integrins function as receptors for laminin α5. Surprisingly, β1 integrin antibodies also reduced Lama5 expression but did not affect Lama1 expression. Therefore, β1 integrin signaling regulates epithelial FGFR and FGF1 expression as well as Lama5 expression.
Fig. 8.

Integrin localization is similar in E17 Lama5-/- and WT SMGs. Perturbing β1 integrin function results in decreased Lama5, FGFR1b, FGFR2b, and FGF1 expression but not Lama1, and the integrin Itga3-/-:Itga6-/- SMGs have a similar phenotype to Lama5-/- SMGs. A. Integrins α3 and α6 are localized in the epithelium at cell-matrix and cell-cell junctions and have similar expression in both Lama5-/- and WT SMGs. B. Branching is decreased by ∼50% with function-blocking β1 integrin antibodies compared to an IgG control (200 μg/ml). E13 SMGs were cultured with antibodies for 44 hours, and the number of buds was counted and expressed as a ratio (T44/T2 hours). C. Function-blocking β1 integrin antibodies decrease gene expression of FGFR1b, FGFR2b, FGF1, and Lama5 compared to IgG controls. Expression of Lama1 was not affected. Gene expression was measured after 44 hours by real-time PCR, normalized to 29S expression, and reported as a fold change in expression compared to IgG controls. P values are unpaired T-tests compared to IgG control **P < 0.01, *P < 0.05. D. Histology of SMGs from the Itga3-/-:Itga6-/- double integrin knockout mice shows a severe developmental phenotype that resembles the Lama5-/- glands. The glands are smaller, and branching is disrupted. The glands have a more dramatic phenotype than that reported for either the single Itga3-/- or Itga6-/- integrin knockout mice.
Fig.9.
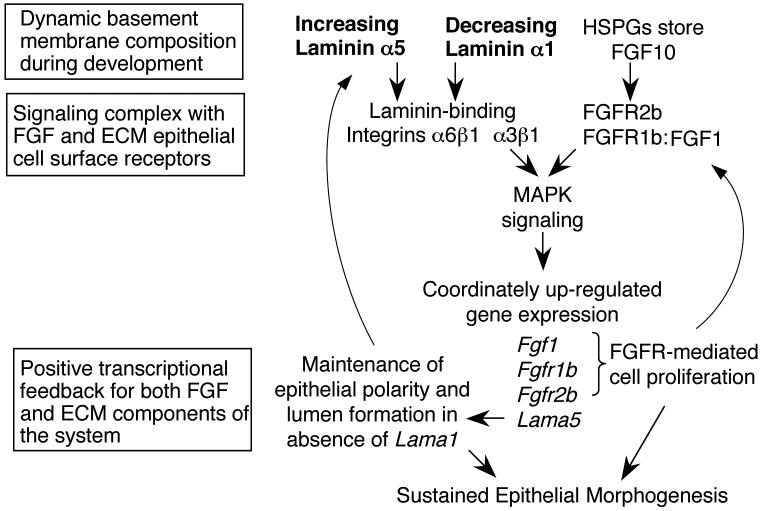
Model diagram showing the reciprocal control of laminin α5 and FGFR expression. β1 integrin and epithelial FGFR signaling provides positive feedback resulting in increased laminin and FGFR synthesis, growth factor signaling, and epithelial morphogenesis. Important research questions remain; defining the in vivo specificity of integrin interactions for laminin isoforms within a signaling complex, and defining the role of heparan sulfate containing proteoglycans, which bind both FGFs and laminin isoforms and may also provide specificity to the system.
Loss of integrins α3 and α6 results in a phenotype similar to Lama5-/- SMGs
Altered SMG phenotypes have been reported for both the α3 (Menko et al., 2001) and α6 (Georges-Labouesse et al., 1996) integrin-null mice, but neither is as severe as the Lama5-/- glands. Here, we analyzed E14.5 Itga3-/-:Itga6-/- SMGs (De Arcangelis et al., 1999), which had a more severe phenotype than that of Itga3-/- or Itga6-/- alone, and were strikingly similar in appearance to the Lama5-/- SMGs (Fig. 8D). There was a delay in epithelial branching with abnormal morphogenesis, and epithelial cell organization was disrupted at later stages. The phenotype of the double integrin knockout SMGs, while correlative, supports our data that the Lama5-/- SMG phenotype may be due to disruption of epithelial integrin function. From these data, we propose a model where signaling from laminin α5 through β1 integrin receptors regulates FGFR1b, FGFR2b, and FGF1 expression, as well as Lama5 expression (Fig. 9) independent of Lama1. These data link laminin α5 function in the BM with FGFR receptor signaling pathways, both of which are required for epithelial morphogenesis.
Discussion
Laminin α chains have both overlapping and unique functions as well as spatiotemporal expression patterns in epithelial basement membranes during development. BMs form with the polymerization of laminin-111 (α1, β1, γ1) acting as a scaffold for the recruitment of other components including laminin isoforms (Sasaki et al., 2004). Defining the function of specific α chains is required to understand the regulation of epithelial morphogenesis. Here we show that although laminin α5 is not required for gland initiation, it plays an important role in initial cleft formation and epithelial morphogenesis; is necessary for sublingual gland formation; and later in development is required for epithelial cell organization and lumen formation. Our data suggests that laminin α5 controls SMG epithelial morphogenesis through β1 integrin signaling by regulating FGFR expression, which also reciprocally regulates the expression of Lama5 and that this regulation is independent of Lama1.
Both laminin α5 and α1 are initially abundant in SMGs, and during epithelial morphogenesis rapid BM turnover, in concert with decreasing Lama1 synthesis, changes the BM composition so that laminin α5 increases during development. The laminin staining (Fig. 3A-D) clearly shows laminin-rich matrix present at the sites of cleft formation. Other ECM molecules, including perlecan (Fig. 7B), collagen III (Hayakawa et al., 1992), and fibronectin (Sakai et al., 2003), are present at the site of cleft formation, and fibronectin is required for clefting. Our data suggest laminin α5 plays an important role which begins during the first round of clefting independent of epithelial proliferation, supporting previous reports that cleft formation is independent of cell proliferation (Spooner et al., 1989). However, after 20 hours, the gland begins to bud but with abnormal morphology. We have treated SMGs with either SU5402 or soluble recombinant FGFR2b, both inhibitors of FGFR signaling and epithelial proliferation, and clefting occurs (Hoffman et al., 2002; Steinberg et al., 2005). We conclude that loss of Lama5 causes a specific patterning defect that begins during the first round of branching independent of proliferation, possibly because of decreased laminin α5 matrix in the clefts that influences the mechanical properties of the matrix during cleft formation.
Laminin α5 has important functions affecting epithelial cell organization and lumen formation. The E17.5 Lama5-/- SMGs are smaller than WT, have a defect in cytodifferentiation as the cells have decreased ZO-1 staining, and lack lumens. The appearance of cells in the end buds of E14.5 Lama5-/- SMGs resembles those in WT endbuds. This suggests laminin α5 is not required for organization of the outer epithelial layer of cells in contact with the BM. Once cytodifferentiation occurs, beginning after ∼E15, the epithelial cells of the terminal lobules of Lama5-/- SMGs do not form lumens. In the absence of laminin α5, with decreasing α1 expression, epithelial cytodifferentiation is disrupted. We conclude that laminin α5 is required for correct epithelial cell organization and lumen formation.
Laminin α5 also plays an important role in SLG initiation, as the gland is absent in ∼83% Lama5-/- mice. This novel finding may result from a critical timing defect, since the SLG initiates a day later than the SMG. Little is known about the mechanisms involved in SLG initiation; our data suggest they may be different from the SMG, but further analysis is required.
FGFR2b/FGF10 signaling is critical for SMG formation in both humans and mice in vivo (De Moerlooze et al., 2000; Entesarian et al., 2007; Entesarian et al., 2005; Ohuchi et al., 2000; Rohmann et al., 2006), and FGFRs are important for cell proliferation during ex vivo mouse SMG morphogenesis (Hoffman et al., 2002; Steinberg et al., 2005). We show that regulation of both epithelial FGFRs and Lama5 expression involves downstream signaling pathways from both FGFRs and laminin α5. This coordinate regulation likely involves cross talk between growth factor and integrin signaling pathways that have similar signaling components, particularly in this case through MAPK signaling (Danen and Sonnenberg, 2003; Yamada and Even-Ram, 2002). Our previous results showed that the MAPK pathway downstream of FGFR2b increases FGFR1b and FGFR2b expression as well as MMP2 production, which is involved in matrix remodeling (Steinberg et al., 2005). Thus, the initial problems with epithelial clefting and morphogenesis observed in the Lama5-/- SMGs may also involve disruption of matrix remodeling through MMPs, although the specific MMPs involved in SMG epithelial morphogenesis are not well defined. MT1-MMP-/- embryos have decreased SMG branching morphogenesis (Oblander et al., 2005), although the phenotype is quite different from that of Lama5-/- SMGs.
All five laminin α chain isoforms have been detected with antibodies in the mouse SMG during development (Kadoya et al., 1995; Kadoya et al., 1998; Kadoya and Yamashina, 1999; Kadoya and Yamashina, 2005; Miner et al., 1997). Our data are consistent with previous in situ data showing Lama1 expression at E13 in the tips of the epithelial buds and in the mesenchyme around the ducts but no expression by E17 (Kadoya et al., 1995). The detection of some laminin α1 expression by real-time PCR at E17 reflects the increased sensitivity of PCR compared with in situ analysis. Laminin α1 also plays an important role in SMG branching morphogenesis ex vivo as antibodies to laminin-1 inhibit branching (Kadoya et al., 1995). The Lama5-/- epithelium has increased intracellular β1 and γ1 chain cytoplasmic staining, suggesting laminin heterotrimer assembly and secretion may be disrupted by the absence of laminin α5. It has been reported that secretion of the β and γ chains requires simultaneous expression of the α chain (Yurchenco et al., 1997) and that α chain secretion may regulate laminin export and basement membrane network formation (De Arcangelis et al., 1996). It remains to be determined if the intracellular accumulation of laminin β1 and γ1 has a functional consequence.
The culture of Lama5+/- SMGs reduced budding by ∼25% after 20 hours, but by 42 hours there was no difference in the number of end buds, and the phenotype resembled the wildtype (Fig. 4A and B). Whereas, a 50% knockdown of laminin α5 by siRNA in a wildtype SMG resulted in a ∼45% decrease in bud number with ∼50% decrease in gene and protein expression after 36 hours (Fig. 6). Although we are cautious about comparing the phenotype of heterozygous SMGs with wildtype SMGs treated with siRNA, we could speculate that there may be developmental compensation in a heterozygote that does not occur during short-term perturbation in explant culture. The E12 SMGs treated with Lama5-siRNA still underwent normal branching for the first 24 hours in culture, likely due to endogenous laminin α5 protein and time for the siRNA transfection to take effect, and the decrease in budding was evident by 36 hours (Fig. 6A). Short-term perturbation experiments in SMG explant culture may show a different or more severe phenotype than a heterozygous SMG because developmental compensation might not occur. Alternatively, the actual gene expression level of individual cells in a specific region of the gland may have greater than 50% knockdown in gene expression, more similar to a knockout, whereas other cells within the same gland may have less than a 50% decrease and resemble wildtype cells. In addition, the organ culture conditions, i.e., culture on a flat filter in serum-free conditions, may make the morphogenesis of the gland more susceptible to perturbation.
In our experimental manipulation of SMGs the expression level of Lama1 was not affected by decreasing Lama5, FGFR1b, or FGFR2b expression. These data suggest Lama1 expression is independent of Lama5 and may explain why there is no compensatory increase in Lama1 expression. The mechanisms regulating laminin α1 expression, and in particular what downregulates its expression during development, remain important but unanswered questions. Mouse embryos with no Lama1 expression die before organogenesis begins, and conditional knockouts of Lama1 in the salivary glands have not been reported. Our own attempts to knock down Lama1 expression with siRNA have been unsuccessful, possibly because the expression of Lama1 is decreasing in the gland during the experiment (Fig. 1A). An unresolved issue is whether there are factors influencing the in vivo specificity of integrin interactions with laminin α1 and α5 isoforms or whether other specific receptors for laminin α5 exist. There is evidence that the G-domains of laminins, through their heparan sulfate-binding domains, may interact with sulfated carbohydrates in the BM or on the cell surface to mediate interactions with specific laminin isoforms (Timpl et al., 2000; Yurchenco and Wadsworth, 2004). Additionally, the interaction between laminins in the epithelial BM and FGFR/FGF complexes are likely mediated by glycosaminoglycans through heparan-binding regions of both components (Lonai, 2005). Integrins α3 and α6 are cell surface receptors for laminin α5, and mice deficient in integrin α3 (Itga3-/-) die at birth from epithelial and nervous system defects (DiPersio et al., 1997; Iyer et al., 2005; Manohar et al., 2004) and have a defect in salivary gland branching morphogenesis (Menko et al., 2001). In the absence of integrin α6 (Itga6-/-) embryos die at birth with epithelial detachment and CNS defects; however, embryonic development occurs normally (Georges-Labouesse et al., 1996), suggesting that functional redundancy or compensation by other integrins or nonintegrin laminin receptors occurs during organogenesis. A SMG phenotype in the Itga6-/- mouse was not described. The double knockout of integrins α3 and α6 reveals novel phenotypes, absent in each of the single null mutants, including a severe bilateral lung hypoplasia and limb malformations due to altered morphogenesis and a dramatic decrease in cell proliferation of the apical ectodermal ridge (De Arcangelis et al., 1999). Here, we describe a more severe phenotype in the SMGs of Itga3-/-:Itga6-/- double-knockout mice that resembles the Lama5-/- SMG and suggests that both α3β1 and α6β1 integrins may be the major receptors for laminin α5 in the developing SMG. Further analysis of the SMG basement membrane in Itga3-/-:Itga6-/- double-knockout mice may reveal if these integrins alter the composition or play a role in basement membrane assembly, although in the severely affected lungs of these mice there were no obvious modifications of the staining patterns of basement membrane components (De Arcangelis et al., 1999). We speculate that crossing the Itga3+/-:Itga6+/- with the Lama5+/- mice and analyzing the SMGs of the double and triple heterozygous embryos might uncover potential genetic interactions between Lama5, Itga3, and Itga6.
Laminin α5 plays a critical role during SMG development and during initiation of the SLG. In the Lama5-/- SMGs, alteration of the BM composition may also affect growth factor/ECM receptor signaling. Our previous studies using isolated submandibular gland (SMG) epithelium cultured in a laminin-111 gel with FGF7 or FGF10 showed that FGFR2b signaling is linked with increased epithelial expression of FGFR1b and FGF1 and that the epithelial FGF1 was required for FGF10-mediated epithelial morphogenesis (Steinberg et al., 2005). In addition, a positive feedback exists where FGFR signaling increases Lama5 expression in the epithelium (Fig. 9). Our findings suggest that laminin α5 controls SMG epithelial morphogenesis through β1 integrin signaling by regulating FGFR expression, which also reciprocally regulates the expression of Lama5 independent of Lama1. These data link the changing basement membrane composition during branching morphogenesis with FGFR expression and signaling.
Acknowledgements
The authors would like to thank Harry Grant and Melinda Larsen for critical reading of the manuscript. This work was supported by the Intramural Research Program of the National Institute for Dental and Craniofacial Research at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ivan T. Rebustini, Matrix and Morphogenesis Unit, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, MSC 4370, Bethesda, MD 20892-4370, USA.
Vaishali N. Patel, Matrix and Morphogenesis Unit, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, MSC 4370, Bethesda, MD 20892-4370, USA.
Julian S. Stewart, Matrix and Morphogenesis Unit, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, MSC 4370, Bethesda, MD 20892-4370, USA.
Ann Layvey, Matrix and Morphogenesis Unit, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, MSC 4370, Bethesda, MD 20892-4370, USA..
Elisabeth Georges-Labouesse, Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS, INSERM, ULP Illkirch, France.
Jeffrey H. Miner, Renal Division, Washington University School of Medicine, St. Louis, MO
Matthew P. Hoffman, Matrix and Morphogenesis Unit, Laboratory of Cell and Developmental Biology, National Institute of Dental and Craniofacial Research, National Institutes of Health, 30 Convent Dr, MSC 4370, Bethesda, MD 20892-4370, USA..
References
- Abrahamson DR, Irwin MH, St John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–91. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J Pathol. 2003;201:632–41. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–95. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–68. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–30. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest J, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–92. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. alpha3beta1 Integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–42. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M, Falk M, Salmivirta K, Durbeej M, Ekblom P. Laminin isoforms and epithelial development. Ann N Y Acad Sci. 1998;857:194–211. doi: 10.1111/j.1749-6632.1998.tb10117.x. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Receptors for laminins during epithelial morphogenesis. Curr Opin Cell Biol. 1996;8:700–6. doi: 10.1016/s0955-0674(96)80112-8. [DOI] [PubMed] [Google Scholar]
- Entesarian M, Dahlqvist J, Shashi V, Stanley CS, Falahat B, Reardon W, Dahl N. FGF10 missense mutations in aplasia of lacrimal and salivary glands (ALSG) Eur J Hum Genet. 2007 doi: 10.1038/sj.ejhg.5201762. [DOI] [PubMed] [Google Scholar]
- Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, Jonsson R, Wahren-Herlenius M, Dahl N. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125–7. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Jeffrey H Miner, Hiroko Ida, Emiko Fukumoto, Kenji Yuasa, Hiroshi Miyazaki, Matthew P Hoffman, Yoshihiko Yamada. Laminin 5 is required for dental epithelium growth and polarity and the development of tooth bud and shape. J. Biol. Chem. 2006 doi: 10.1074/jbc.M509295200. In press. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–3. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Hasenson S, Maatta M, Rousselle P, Kikkawa Y, Miner JH, Tervo T, Virtanen I. The immortalized human corneal epithelial cells adhere to laminin-10 by using Lutheran glycoproteins and integrin alpha3beta1. Exp Eye Res. 2005;81:415–21. doi: 10.1016/j.exer.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Kishi J, Nakanishi Y. Salivary gland morphogenesis: possible involvement of collagenase. Matrix Suppl. 1992;1:344–51. [PubMed] [Google Scholar]
- Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, Larsen M. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–78. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Iyer V, Pumiglia K, DiPersio CM. Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci. 2005;118:1185–95. doi: 10.1242/jcs.01708. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Abichaker G, Witcher D, Sala FG, Bellusci S, Hajihosseini MK, Melnick M. FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev Biol. 2005;5:11. doi: 10.1186/1471-213X-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–34. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya Y, Nomizu M, Sorokin LM, Yamashina S, Yamada Y. Laminin alpha1 chain G domain peptide, RKRLQVQLSIRT, inhibits epithelial branching morphogenesis of cultured embryonic mouse submandibular gland. Dev Dyn. 1998;212:394–402. doi: 10.1002/(SICI)1097-0177(199807)212:3<394::AID-AJA7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Yamashina S. Localization of laminin-5, HD1/plectin, and BP230 in the submandibular glands of developing and adult mice. Histochem Cell Biol. 1999;112:417–25. doi: 10.1007/s004180050423. [DOI] [PubMed] [Google Scholar]
- Kadoya Y, Yamashina S. Salivary gland morphogenesis and basement membranes. Anat Sci Int. 2005;80:71–9. doi: 10.1111/j.1447-073x.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- Kashimata M, Gresik EW. Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the alpha6-integrin subunit. Dev Dyn. 1997;208:149–61. doi: 10.1002/(SICI)1097-0177(199702)208:2<149::AID-AJA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Fujiwara H, Sonnenberg A, Sekiguchi K. Integrin binding specificity of laminin-10/11: laminin-10/11 are recognized by alpha 3 beta 1, alpha 6 beta 1 and alpha 6 beta 4 integrins. J Cell Sci. 2000;113(Pt 5):869–76. doi: 10.1242/jcs.113.5.869. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273:15854–9. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- Larsen M, Hoffman MP, Sakai T, Neibaur JC, Mitchell JM, Yamada KM. Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Dev Biol. 2003;255:178–91. doi: 10.1016/S0012-1606(02)00047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O, Sorokin L, Kedinger M, Simon-Assmann P. Developmental expression and cellular origin of the laminin alpha2, alpha4, and alpha5 chains in the intestine. Dev Biol. 1999;210:135–50. doi: 10.1006/dbio.1999.9270. [DOI] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, Bradley M, Keene DR, Oro AE, Miner JH, Marinkovich MP. Laminin-10 is crucial for hair morphogenesis. Embo J. 2003a;22:2400–10. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell. 2003b;4:613–24. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 2001;153:811–22. doi: 10.1083/jcb.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Kurpakus-Wheater M. Laminin alpha5 chain adhesion and signaling in conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:2615–21. [PubMed] [Google Scholar]
- Lonai P. Fibroblast growth factor signaling and the function and assembly of basement membranes. Curr Top Dev Biol. 2005;66:37–64. doi: 10.1016/S0070-2153(05)66002-4. [DOI] [PubMed] [Google Scholar]
- Manohar A, Shome SG, Lamar J, Stirling L, Iyer V, Pumiglia K, DiPersio CM. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci. 2004;117:4043–54. doi: 10.1242/jcs.01277. [DOI] [PubMed] [Google Scholar]
- Menko AS, Kreidberg JA, Ryan TT, Van Bockstaele E, Kukuruzinska MA. Loss of alpha3beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev Dyn. 2001;220:337–49. doi: 10.1002/dvdy.1114. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C. Defective glomerulogenesis in the absence of laminin alpha5 demonstrates a developmental role for the kidney glomerular basement membrane. Dev Biol. 2000;217:278–89. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–56. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- Moulson CL, Li C, Miner JH. Localization of Lutheran, a novel laminin receptor, in normal, knockout, and transgenic mice suggests an interaction with laminin alpha5 in vivo. Dev Dyn. 2001;222:101–14. doi: 10.1002/dvdy.1169. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Nielsen PK, Yamada Y. Identification of cell-binding sites on the Laminin alpha 5 N-terminal domain by site-directed mutagenesis. J Biol Chem. 2001;276:10906–12. doi: 10.1074/jbc.M008743200. [DOI] [PubMed] [Google Scholar]
- Oblander SA, Zhou Z, Galvez BG, Starcher B, Shannon JM, Durbeej M, Arroyo AG, Tryggvason K, Apte SS. Distinctive functions of membrane type 1 matrix-metalloprotease (MT1-MMP or MMP-14) in lung and submandibular gland development are independent of its role in pro-MMP-2 activation. Dev Biol. 2005;277:255–69. doi: 10.1016/j.ydbio.2004.09.033. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Hori Y, Yamasaki M, Harada H, Sekine K, Kato S, Itoh N. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem Biophys Res Commun. 2000;277:643–9. doi: 10.1006/bbrc.2000.3721. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–64. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Rohmann E, Brunner HG, Kayserili H, Uyguner O, Nurnberg G, Lew ED, Dobbie A, Eswarakumar VP, Uzumcu A, Ulubil-Emeroglu M, Leroy JG, Li Y, Becker C, Lehnerdt K, Cremers CW, Yuksel-Apak M, Nurnberg P, Kubisch C, Schlessinger J, van Bokhoven H, Wollnik B. Mutations in different components of FGF signaling in LADD syndrome. Nat Genet. 2006;38:414–7. doi: 10.1038/ng1757. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol. 2004;164:959–63. doi: 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Mann K, Miner JH, Miosge N, Timpl R. Domain IV of mouse laminin beta1 and beta2 chains. Eur J Biochem. 2002;269:431–42. doi: 10.1046/j.0014-2956.2001.02663.x. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–41. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Hosokawa H, Ninomiya H, Miner JH, Masaki T. Adhesion of cultured bovine aortic endothelial cells to laminin-1 mediated by dystroglycan. J Biol Chem. 1999;274:11995–2000. doi: 10.1074/jbc.274.17.11995. [DOI] [PubMed] [Google Scholar]
- Spooner BS, Bassett KE, Spooner BS., Jr. Embryonic salivary gland epithelial branching activity is experimentally independent of epithelial expansion activity. Dev Biol. 1989;133:569–75. doi: 10.1016/0012-1606(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–34. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–17. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Vainionpaa N, Kikkawa Y, Lounatmaa K, Miner JH, Rousselle P, Virtanen I. Laminin-10 and Lutheran blood group glycoproteins in the adhesion of human endothelial cells. Am J Physiol Cell Physiol. 2005 doi: 10.1152/ajpcell.00285.2005. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–6. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- Yin Y, Kikkawa Y, Mudd JL, Skarnes WC, Sanes JR, Miner JH. Expression of laminin chains by central neurons: analysis with gene and protein trapping techniques. Genesis. 2003;36:114–27. doi: 10.1002/gene.10206. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Quan Y, Colognato H, Mathus T, Harrison D, Yamada Y, O’Rear JJ. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci U S A. 1997;94:10189–94. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–9. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]