Abstract
The construction and performance of a scroll coil double-resonance probe for solid-state NMR on stationary samples is described. The advantages of the scroll coil at the high resonance frequencies of 1H and 31P include: high efficiency, minimal perturbations of tuning by a wide range of samples, minimal RF sample heating of high dielectric samples of biopolymers in aqueous solution, and excellent RF homogeneity. The incorporation of a cable tie cinch for mechanical stability of the scroll coil is described. Experimental results obtained on a Hunter Killer Peptide 1 (HKP1) interacting with phospholipid bilayers of varying lipid composition demonstrate the capabilities of this probe on lossy aqueous samples.
Introduction
The combination of high resonance frequencies and lossy aqueous samples is problematic for probes that utilize conventional solenoid coils, especially those used to perform solid-state NMR experiments that require large B1 fields. High magnetic fields, and correspondingly high resonance frequencies are essential in order to obtain the requisite sensitivity and resolution for studies of proteins and other biopolymers. Magnetically aligned phospholipid bilayers (bicelles) have recently been shown to be highly effective at providing a fully-hydrated, planar bilayer environment for structural studies of membrane-associated peptides and proteins (1,2); the only drawback to these samples is that they are very lossy at high frequencies due to the presence of high concentrations of lipids, proteins, and salts in aqueous solution. Similar problems are encountered with hydrated samples of mechanically aligned phospholipid bilayers (3) as well as with the unoriented protein samples used in magic angle sample spinning experiments (4,5). The analysis of RF-induced sample heating has led to the development of alternative coil geometries, e.g. scroll and other specialized coils, which reduce RF sample heating by lowering the inductance of the coil and minimizing the electric field generated in the sample (4,6–8). Furthermore, many of these designs have the added benefit of high B1 homogeneity.
In this article, we describe the implementation of a scroll coil, which consists of a single sheet of copper wrapped concentrically with a sheet of a non-conducting material, in a 1H/31P double-resonance probe optimized for solid-state NMR experiments on stationary samples of magnetically aligned bilayers in aqueous solution. The previously reported applications of scroll coil NMR probes have been for 1H/13C/15N triple-resonance magic angle sample spinning experiments (4,5). The low inductance of a scroll coil limits its efficiency at the relatively low frequencies associated with 13C and especially 15N, limiting the impact of 1H/15N double-resonance probes for stationary samples; however, the low inductance is very well suited for the higher 1H and 31P resonance frequencies, even at magnetic field strengths as low as 11.7 T. Because of the relatively small electric field generated within the sample volume of a scroll coil, sample heating is minimized and probe tuning is not significantly perturbed by samples with quite different dielectric properties. As a result, the probe has sufficient flexibility to be used to test pulse sequences on single crystal samples, and then to be used to study aqueous protein samples without replacing fixed capacitors or other tuning elements.
The performance of the 1H/31P double-resonance probe is demonstrated with results obtained on a synthetic sample of a pro-apoptotic peptide that targets specific tissue for programmed cell death. These hunter-killer peptides (HKPs) bind to cell surface receptors, are subsequently internalized, and then initiate apoptosis by disrupting the mitochondrial membrane (9). They consist of a protein homing motif connected to a pro-apoptotic sequence containing D-amino acids to confer resistance to proteolytic degradation. Examples have been designed to target tumor vasculature (9), adipose tissue, specifically white fat vasculature (10), prostate tissue (11), and collagen-induced arthritis in mice (12). The specificity of HKPs toward mitochondrial membrane disruption is crucial for their application as therapeutics since plasma membrane disruption would lead to general toxicity. The conformation and interaction of two HKPs with micelles have been studied by solution-state NMR (13,14). Because the mitochondrial membrane contains a relative high percentage of anionic phospholipids and HKPs show a preference for binding to vesicles containing anionic phospholipids, it is of interest to examine their interactions with bilayers containing anionic phospholipids. These studies are technically demanding because they require a solid-state NMR probe whose performance is not degraded by the lossy nature of the biologically relevant samples.
Results and Discussion
Even with many years of experience building double- and triple-resonance probes with solenoid coils, our initial efforts to construct scroll coil probes were stymied by highly unstable tuning and self-resonance frequencies. The lack of stability was found to be mechanical rather than electrical in origin. There is a significant amount of capacitance in the scroll coil design and consequently, small changes in dimensions, such as those resulting from RF heating-induced expansion of components, give rise to significant changes in the observed self-resonance frequency. This necessitated the development of a construction protocol that produces mechanically stable scroll coils.
A scroll coil “blank” was cut from a 0.010 inch thick sheet of oxygen free copper (McMaster-Carr, www.mcmaster.com). The coil blank shown in Figure 1A is 0.8 cm wide by 6.0 cm long measured between the inside edges of the leads. The polytetraflouroethylene (PTFE) dielectric used was in the form of adhesive backed tape 0.0115 inch thick by 0.75 inch wide (Saint-Gobain Performance Plastics, www.plastics.saint-gobain.com). The PTFE tape was adhered to one side of the copper, and trimmed to a width approximately 2 mm larger than that of the coil blank to prevent electrical shorting at the edges. This assembly was then wrapped around a cylindrical former with a 5 mm outer diameter; the relatively stiff mechanical properties of the copper sheet means that this process must be performed carefully in order to ensure uniform curvature throughout each turn. The resulting scroll coil (Figure 1B) was then cinched tightly using a nylon 0.141 inch wide 40 lb tinsel strength cable tie (Thomas & Betts, www.tnb.com). The resulting scroll coil was mechanically stable with a self resonance frequency of 629 MHz measured on a Hewlett-Packard 8753A network analyzer (Agilent, www.agilent.com).
Figure 1.
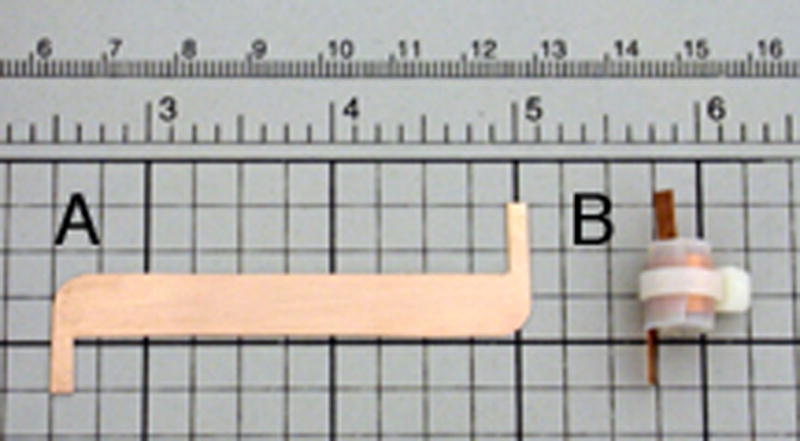
A. Copper blank for scroll coil. B. 5 mm inner diameter scroll coil including the cable tie cinch that provides mechanical stability.
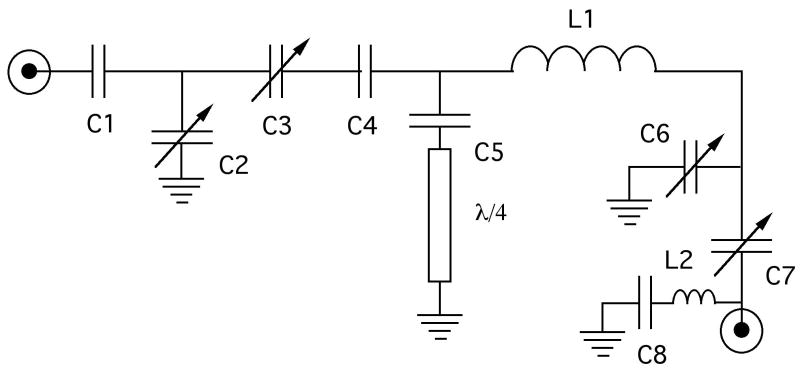
The scroll coil is incorporated into the circuit diagrammed in Figure 2, which is an updated version of the original Cross et al (15) circuit and is similar to those used in other applications (16–21) The circuit is double-tuned to 500 MHz (1H) and 202 MHz (31P). Two variable capacitors (Polyflon NRP/VC10-12-06A, www.polyflon.com) are used in both the 1H and 31P tuning networks, indicated as C2 and C3 for the 1H channel and C6 and C7 for the 31P channel. The ceramic chip capacitors are from American Technical Ceramics (www.atceramics.com) or Voltronics. (www.voltronicscorp.com) 31P to 1H isolation is accomplished with a grounded λ/4 line. The combination of the length of the λ/4 line and the C5 capacitor assembly are chosen to resonate the sample coil at the 1H frequency. 1H to 31P isolation is achieved with an LC trap tuned to the 1H frequency using the inductance of a curved lead approximately 1.5 cm in length (L2) in series with capacitor C8. The effectiveness of this trap was optimized by monitoring the transmission between the 1H and 31P ports at the 1H tuning frequency using a network analyzer while varying the value of C8, an isolation of −32 db was achieved. Capacitor C5 was located on the opposite side of the sample inductor (L1) in the initial versions, however the placement shown in Figure 2 results in superior isolation of the 31P and 1H channels. The 31P to 1H isolation was monitored using the same method as described above, while varying the value of capacitor C5 until a 31P to 1H isolation of −30 db was achieved. Temperature regulation of the sample is accomplished with a ceramic heating element (Doty Scientific, www.dotynmr.com) housed in a custom made glass dewar (New Era Enterprises, www.newera-spectro.com). A photograph of the completed probe is shown in Figure 3.
Figure 2.
Circuit diagram for the single-coil double-tuned probe. L1 represents the scroll coil. The values of the capacitors are: C1 = 4.7 pF, C4 = 1.5 pF, C5 = 10.0 pF and C8 = 8.2 pF.
Figure 3.
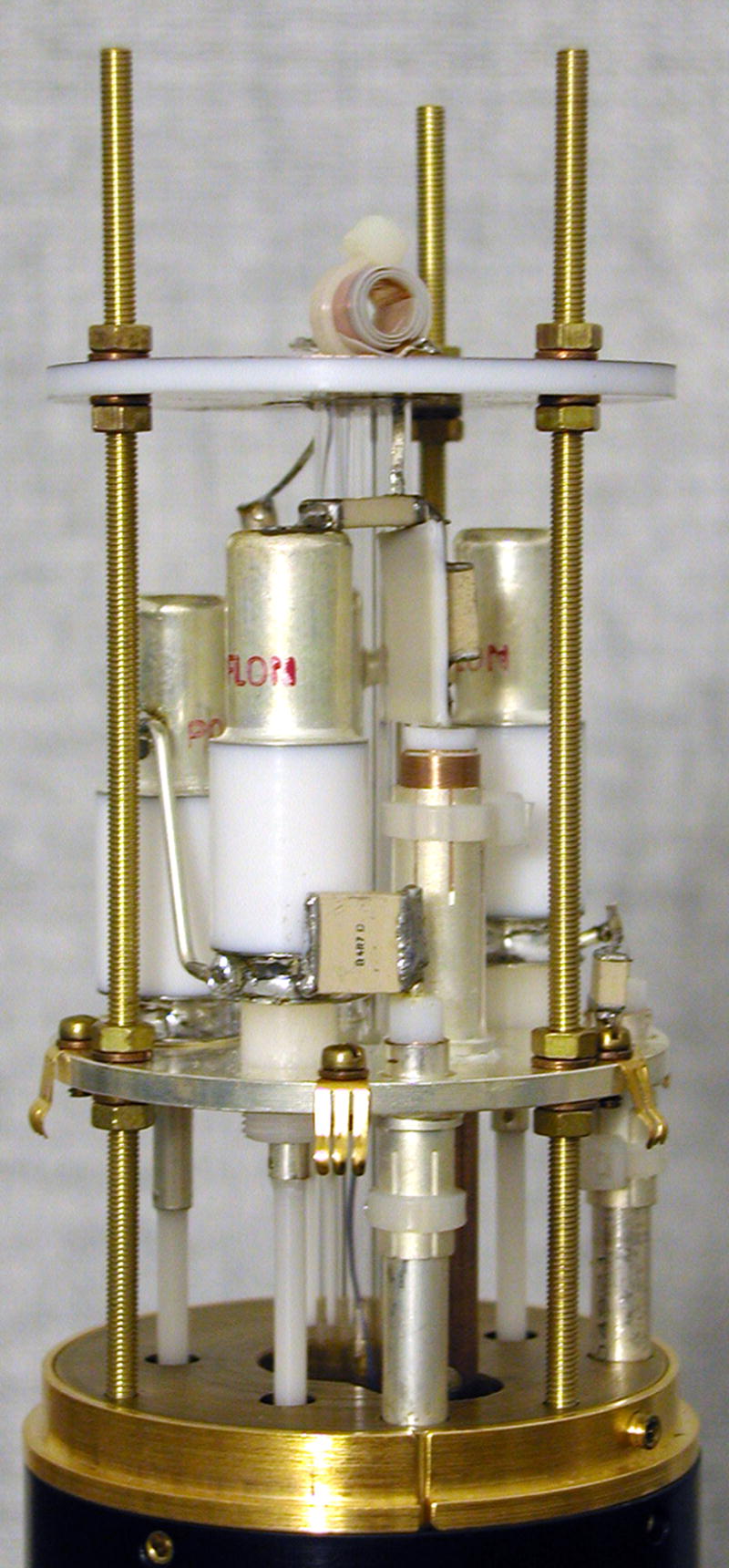
A photograph of the complete probe assembly.
The basic performance characteristics of the probe summarized in Table 1 demonstrate that it is very efficient on both channels. The results are reported for a 5 mm sample tube loaded with 160 μL of 85% phosphoric acid at room temperature. Both channels of the probe can be tuned and matched to samples with a wide range of dielectric constants, including air, crystalline solids, and aqueous samples containing significant salt concentrations, using only the variable capacitors. The RF fields generated at typical power levels are given in Table 1; the 1H and 31P channels were tested with continuous irradiation for 30 msec and 10 msec, respectively, and showed no evidence of arcing or instability. Table 1 also indicates that the RF homogeneity measured by comparing the amplitude of a signal following an 810° pulse to that of a 90° pulse and expressing this ratio as a percentage is >95% on both channels.
Table 1.
Summary of probe performance.
| Channel | Nutation Frequency, Power | Homogeneity (A810/A90) × 100 % |
|---|---|---|
| 1H | 89 kHz, 44 Watts | > 95 % |
| 31P | 68 kHz, 125 Watts | > 95 % |
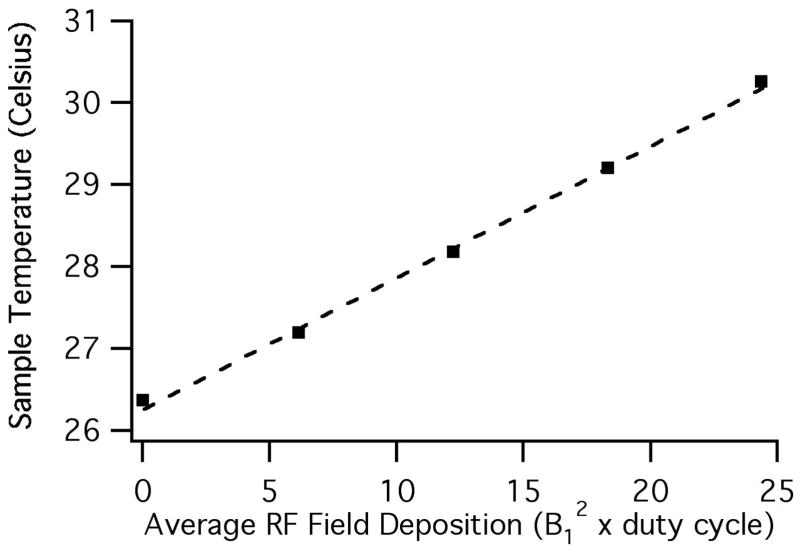
The RF heating effects of the scroll coil have been analyzed previously (4). We assessed sample heating by monitoring the 1H chemical shift of the H6 resonance of Na5[TmDOTP] (Macrocyclics, www.macrocyclics.com), the sodium salt of the complex between the thulium ion and the macrocyclic chelate 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetrakis(methylene phosphonate) (22, 23). The Na5[TmDOTP] sample included an additional 70 mM of NaCl so that its dielectric properties are comparable to a “worst-case” lossy aqueous sample, as judged by the power required to achieve a 50 kHz 1H B1 field in a solenoid coil probe. This allows for a meaningful comparison between the RF heating measurements made using the Na5[TmDOTP] sample and the RF heating in representative biological samples. The RF heating data are presented in Figure 4, where the sample temperature determined from the Na5[TmDOTP] H6 chemical shift is plotted as a function of the average RF field deposition, calculated as the product of B12 and a duty cycle factor. The duty cycle factor is the ratio of the time that the RF irradiation is on to the total duration of the pulse sequence. In this case, B1 is set to 50 kHz and four data points were obtained each using an 8 minute pulse train (to ensure thermal equilibrium) consisting of 1H pulses of 2.5, 5.0, 7.5 or 10.0 msec duration with a 1 sec delay between pulses, followed by a 90° pulse and a 16 msec acquisition time. As an example, an NMR experiment utilizing 50 ms of 1H irradiation and a 5 second recycle delay with a 50 kHz B1 would give rise to an average RF field deposition of 25; typical experiments on proteins have RF field deposition values between 4 and 10, and the value is 4.3 for the spectra displayed in Figure 5. Using the scroll coil, the temperature of the sample is increased by 3.9 °C at an average RF field deposition of 24.4. For comparison, a probe of similar construction, but utilizing a conventional 6 turn 5 mm solenoid coil resulted in a 25 °C temperature increase at the same average RF field deposition (data not shown).
Figure 4.
Sample temperature measured by chemical shifts as a function of the average RF field deposition for a B1 field of 50 kHz. The dashed line is a linear fit to the experimental data with a slope of 0.16 (°C/kHz2).
Figure 5.
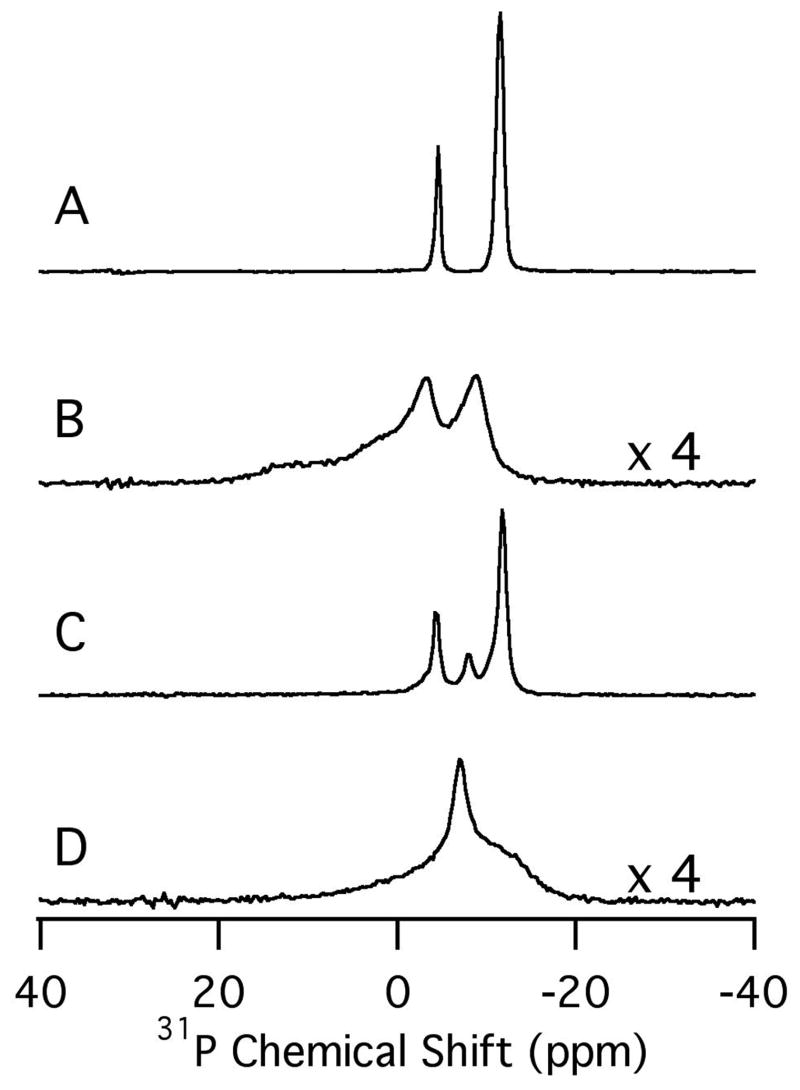
Experimental 31P NMR spectra of the magnetically-aligned phospholipid bilayer samples. A. DMPC/6-O-PC. B. DMPC/6-O-PC with HKP1. C. DMPC/DMPG/6-O-PC. D. DMPC/DMPG/6-O-PC with HKP1. The vertical scale of the spectra in B and D has been increased by a factor of 4 compared to that in A and C.
The 1H/31P double-resonance probe was used to study the interaction of the hunter killer peptide HKP1 with magnetically aligned phospholipid bilayers in two different preparations. The first sample contains the zwitterionic lipids 1, 2-di-O-hexyl-sn-glycero-3-phosphocholine (6-O-PC) and 1, 2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC). The second sample includes about 20% anionic long-chain lipids prepared with a 20:80 molar ratio of 1, 2-dimyristoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] sodium salt (DMPG) and DMPC (Avanti Polar Lipids, www.avantilipids.com). Figure 5 A contains the one-dimensional 31P NMR spectrum obtained for the DMPC/6-O-PC sample at its optimal alignment temperature of 40°C. Figure 5B contains the spectrum obtained following the addition of approximately 2 mg of HKP1 to the sample. Similarly, Figure 5C and D compare the DMPC/DMPG/6-O-PC samples in the absence and presence of 2 mg of HKP1, respectively, at a temperature of 34°C, the experimentally determined optimal alignment temperature for this mixture of lipids. These initial results demonstrate that HKP1 disrupts bilayer formation for both lipid compositions. The differences in the 31P NMR spectra (Figure 5B and D) obtained in the presence of HKP1 indicate that the resulting samples may be significantly different from one another. Included here to demonstrate the probe performance on actual samples, further studies are being pursued to characterize the interactions of these peptides with phospholipid bilayers.
Conclusion
A 1H - 31P double tuned probe was built based on a scroll coil sample inductor. A protocol was established to construct a stable scroll coil using a teflon dielectric and a cable tie tensioning device, or cinch, to stress the structure thus improving dimensional and mechanical stability. A well-established tuning circuit was used and optimized for the constructed coil resulting in a highly efficient, and thus highly sensitive (24), probe. The scroll coil offers the significant advantage of reduced sample heating because of the low electric field component developed within the coil. A useful side effect of this is that the 1H resonance frequency shift and Q factor reduction upon the introduction of a high dielectric sample, such as a bicelle sample, are minimized. This allows the probe to easily tune to a variety of samples without a substantial reduction in probe performance. Furthermore, the scroll coil allows for a substantial reduction in sample heating by RF power deposition. The coil presented in this work is expected to elevate the sample temperature by less than 3.9 °C even for high duty cycle solid-state NMR experiments with samples containing a significant salt background.
Two minor drawbacks were identified while using this scroll coil probe for experiments. The first of which is that magnet shimming is somewhat more difficult with a scroll coil probe compared to an equivalent solenoid coil probe. However, we found that acceptable shimming (0.1 ppm – 0.2 ppm H2O line width using a standard room temperature shim coils) could always be achieved for the types of samples presented here. Secondly, the tuning frequency of the scroll coil probe shifts slightly more as a function of temperature than for a solenoid coil, the likely result of a capacitance change in the coil as thermally induced dimensional changes take place. This results in the necessity to retune the probe when the sample (and thus coil) temperature is changed by as few as 2 °C – 3 °C. Long-term stability of probe tuning was easily accomplished with the use of a standard variable temperature control system.
Preliminary results were presented for the interaction of HKP1 with bicelles consisting of both zwitterionic lipids and a mixture of zwitterionic lipids and lipids with negatively charged head groups. Regardless of bicelle composition, the HKP1 (in concentrations suitable for solid-state NMR studies of the peptide) disrupted bicelle formation. Although the nature of the resulting peptide lipid complexes that are formed can not be determined by 31P NMR analysis alone, the data indicate that the two different bicelle compositions gave rise to different interactions, as judged by the significant differences in the obtained 31P spectra. Additional studies are planned to further characterize these interactions.
Experimental
The 31P NMR experiments were performed on a spectrometer that consisted of a Chemagnetics console interfaced to a Magnex 500/89 wide-bore magnet. The 1H frequency is 499.9 MHz. All samples were equilibrated in the magnetic field at constant temperature for at least 30 minutes prior to the NMR measurements. One-dimensional 31P NMR chemical shift spectra were obtaining by direct excitation with a single pulse with radio-frequency field of 28 kHz. All 31P NMR spectra were obtained with a 6 sec recycle delay, 128 scans with a 10.24 ms acquisition time. Continuous wave 1H decoupling was accomplished using a B1 field strength of 50 kHz during the acquisition time. Temperatures of 40 °C and 34 °C corresponded to the optimal alignment of the liquid crystalline phase in the absence of peptide for the DMPC/6-O-PC and DMPC/DMPG/6-O-PC bicelles (25,26), respectively. All chemical shifts are referenced to the 31P resonance of external 85% phosphoric acid, which is assigned to 0 ppm at room temperature. NMR spectra were processed using nmrPipe (27) and visualized using IGOR pro (WaveMetrics, www.wavemetrics.com).
The HKP1 peptide was synthesized as described previously (13). The samples used in the NMR experiments without peptide and samples containing HKP1 were prepared for a total lipid concentration cL = 28% (w/v) and lipid molar ratios: q=[DMPC]/[6-O-PC]= 3.2; and q = [DMPC]+[DMPG]/[6-O-PC]= 3.2, [DMPG]:[DMPC]=20:80. The DMPC/6-O-PC and DMPC/DMPG/6-O-PC bicelles were prepared as previously described (1,2) by preparing an aqueous solution containing the short-chain lipid (6-O-PC) and then adding this solution either to a dispersion of the long-chain lipid (DMPC), or to a dispersion of the long-chain lipids (DMPC) and (DMPG). The resulting solution was vortexed and freeze/heated (0 °C/40 °C) a few times and then allowed to equilibrate to room temperature. Samples of HKP1 in bicelles were prepared by adding a 160 μl volume of the bicelles directly to about 2 mg of the purified, lyophilized polypeptide. Sodium hydroxide was used to adjust to pH = 6. In all cases, the bicelles were non-viscous between 0 °C and 10 °C. A 1.5 cm long, flat-bottomed NMR tube with 5 mm outer diameter (New Era Enterprises, www.newera-spectro.com) was filled with the sample solution, using a pre-cooled glass pipette, at 4 °C. The NMR tube was sealed with a tight-fitting rubber cap, pierced with a thin syringe to remove residual air from the sample and create a tight seal.
Acknowledgments
We thank Dr. Francesca Marassi for the use of her spectrometer at the Burnham Institute for RF heating measurements. The National Institutes of Health supported the research described in this article. The spectroscopic developments were supported by grants RO1EB001966 and RO1GM075877, and utilized the Resource for NMR Molecular Imaging of Proteins supported by grant P41EB002031. The biochemistry of Hunter Killer Peptides was supported by grant R15GM068431. A.A.D. was supported by postdoctoral fellowship F32GM65833.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Angelis AA, Nevzorov AA, Park SH, Howell SC, Mrse AA, Opella SJ. High-resolution NMR spectroscopy of membrane proteins in aligned bicelles. J Am Chem Soc. 2004;126:15340–15341. doi: 10.1021/ja045631y. [DOI] [PubMed] [Google Scholar]
- 2.De Angelis AA, Jones DH, Grant CV, Park SH, Mesleh MF, Opella SJ. NMR experiments on aligned samples of membrane proteins. Methods Enzymol. 2005;394:350–382. doi: 10.1016/S0076-6879(05)94014-7. [DOI] [PubMed] [Google Scholar]
- 3.Li CG, Mo YM, Hu J, Chekmenev E, Tian CL, Gao FP, Fu RQ, Gor’kov P, Brey W, Cross TA. Analysis of RF heating and sample stability in aligned static solid-state NMR spectroscopy. J Magn Reson. 2006;180:51–57. doi: 10.1016/j.jmr.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Stringer JA, Bronnimann CE, Mullen CG, Zhou DH, Stellfox SA, Li Y, Williams EH, Rienstra CM. Reduction of RF-induced sample heating with a scroll coil resonator structure for solid-state NMR probes. J Magn Reson. 2005;173:40–48. doi: 10.1016/j.jmr.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Frericks HL, Zhou DH, Yap LL, Gennis RB, Rienstra CM. Magic-angle spinning solid-state NMR of a 144 kDa membrane protein complex: E-coli cytochrome bo(3) oxidase. J Biomolecular NMR. 2006;36:55–71. doi: 10.1007/s10858-006-9070-5. [DOI] [PubMed] [Google Scholar]
- 6.Gor’kov PL, Chekmenev EY, Li C, Cotten M, Buffy JJ, Traaseth NJ, Veglia G, Brey WW. Using low-E resonators to reduce RF heating in biological samples for static solid-state NMR up to 900 MHz. J Magn Reson. 2007;185:77–93. doi: 10.1016/j.jmr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Mullen C, Stringer J, Mehr K. Multiple tuned scroll coil, Varian, Inc. #7,081,753. United States Patent. 2006 July 25;
- 8.Doty DJ, Kulkarni J, Turner C, Entzminge G, Bielecki A. Using a cross-coil to reduce RF heating by an order of magnitude in triple-resonance multinuclear MAS at high fields. J Magn Reson. 2006;182:239–253. doi: 10.1016/j.jmr.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, Krajewski S, Lombardo CR, Rao R, Ruoslahti E, Bredesen DE, Pasqualini R. Anti-cancer activity of targeted pro-apoptotic peptides. Nature Medicine. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 10.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nature Medicine. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 11.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen D, Pasqualini R, Ruoslahti E. Targeting the prostate for destruction through a vascular address. PNAS. 2002;99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlag DM, Borges E, Tak PP, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E, Firestein GS. Suppression of murine collagen-induced arthritis by targeted apoptosis of synovial neovasculature. Arthritis Research. 2001;3:357–361. doi: 10.1186/ar327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plesniak LA, Parducho JI, Ziebart A, Geierstanger BH, Whiles JA, Melacini G, Jennings PA. Orientation and helical conformation of a tissue-specific hunter-killer peptide in micelles. Protein Sci. 2004;13:1988–1996. doi: 10.1110/ps.04853204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandoval CM, Geierstanger BH, Fujimura S, Balatbat C, Williams T, de Unamuno J, Whiles-Lillig JA, Ellerby LM, Ellerby HM, Jennings P, Plesniak LA. Structural evaluation of a novel pro-apoptotic peptide coupled to CNGRC tumor homing sequence by NMR. Chem Biol Drug Des. 2006;67:417–424. doi: 10.1111/j.1747-0285.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 15.Cross VR, Hester RK, Waugh JS. Single coil probe with transmission-line tuning for nuclear magnetic double resonance. Rev Sci Instrum. 1976;47:1486–1488. [Google Scholar]
- 16.Doty FD, Inners RR, Ellis PD. A multinuclear double-tuned probe for applications with solids or liquids utilizing lumped tuning elements. J Magn Reson. 1981;43:399–416. [Google Scholar]
- 17.Martin RW, Paulson EK, Zilm KW. Design of a triple resonance magic angle sample spinning probe for high field solid state nuclear magnetic resonance. Rev Sci Instrum. 2003;74:3045–3061. [Google Scholar]
- 18.Park SH, De Angelis AA, Nevzorov AA, Wu CH, Opella SJ. Three-dimensional structure of the transmembrane domain of Vpu from HIV-1 in aligned phospholipid bicelles. Biophysical Journal. 2006;91:3032–3042. doi: 10.1529/biophysj.106.087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gor’kov PL, Chekmenev EY, Fu R, Hu J, Cross TA, Cotten M, Brey WW. A large volume flat coil probe for oriented membrane proteins. J Magn Reson. 2006;181:9–20. doi: 10.1016/j.jmr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Jiang YJ, Pugmire RJ, Grant DM. An efficient double-tuned C-13/H-1 probe circuit for CP/MAS NMR and its importance in linewidths. J Magn Reson. 1987;71:485–494. [Google Scholar]
- 21.Zhang QW, Zhang H, Lakshmi KV, Lee DK, Bradley CH, Wittebort RJ. Double and triple resonance circuits for high-frequency probes. J Magn Reson. 1998;132:167–171. [Google Scholar]
- 22.Zuo CS, Bowers JL, Metz KR, Nosaka T, Sherry AD, Clause ME. TmDOTP5-: A substance for NMR temperature measurements in vivo. Magn Reson Med. 1996;36:955–959. doi: 10.1002/mrm.1910360619. [DOI] [PubMed] [Google Scholar]
- 23.Zuo CS, Metz KR, Sun Y, Sherry AD. NMR temperature measurements using a paramagnetic lanthanide complex. J Magn Reson. 1998;133:53–60. doi: 10.1006/jmre.1998.1429. [DOI] [PubMed] [Google Scholar]
- 24.Hoult DI, Richards RE. The signal-to-noise ratio of the nuclear magnetic resonance experiment. J Magn Reson. 1976;24:71–85. doi: 10.1016/j.jmr.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Sanders CR, Hare BJ, Howard KP, Prestegard JH. Magnetically-oriented phospholipids micelles as a tool for the study of membrane-associated molecules. Prog NMR Spectrosc. 1994;26:421–444. [Google Scholar]
- 26.Crowell KJ, Macdonald PM. Surface charge response of the phophatidylcholine head group in bilayered micelles from phosphorus and deuterium nuclear magnetic resonance. Biochem Biophys Acta. 1999;1416:21–30. doi: 10.1016/s0005-2736(98)00206-5. [DOI] [PubMed] [Google Scholar]
- 27.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. Nmrpipe—a multidimensional spectral processing system based on unix pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]