Abstract
Using detrended fluctuation analysis (DFA), we studied the scaling properties of the time instances (occurrence) of the fetal breathing, gross-body, and extremity movements scored on a second by second basis from the recorded ultrasound measurements of 49 fetuses. The DFA exponent α of all the three movements of the fetuses varied between 0.63 and 1.1. We found an increase in α obtained for the movement due to breathing as a function of the gestational age while this trend was not observed for gross-body and extremity movements. This trend was argued as the indication of the maturation of lung and functional development of respiratory aspect of the fetal central nervous system. This result may be useful in discriminating normal fetuses from high-risk fetuses.
Keywords: Time series, Biological Signal Processing
1 Introduction
Before the mid 1970's maternal perception of fetal movement was the most common antepartum surveillance tool used for the assessment of fetal well-being. Studies such as done in Ref. [1] had demonstrated that daily recording and assessment of fetal movement helped prevent adverse health events in the fetus and newborn. However, a weakness of this technique was demonstrated by a study that reported the ability of women to accurately perceive fetal movements varied widely among individuals [2].
With the advent of modern technology such as ultrasonography, direct observation of fetal movement has become convenient [3-6]. Even so, after acquiring such data, expertise is needed to classify the different patterns of movements such as that of breathing, gross-body and extremity. In this work, we studied the scaling behavior exhibited by the instances of breathing, gross-body and extremity movements to understand their dynamics with fetal maturation.
There is increasing evidence that fetal behavior reflects functional development of the central nervous system [7]. It has been shown [8] that both normal and high risk fetuses show an increase in breathing activity and a decrease in other movements as a function of gestational age. It has been postulated that movements of the embryo and fetus are a fundamental expression of early neural activity [9]. In low risk fetuses (delivering at term as healthy infant), the onset of general movements of the head, trunk and extremity movements occurs at 7.5-8.5 weeks [10]. The frequency of generalized movements increases during the first trimester but then begins to decrease with increasing maturation during the second trimester (16 to 32 weeks) [11, 12]. This decrease in the number and frequency of movements has been observed to continue until delivery [13]. Observation of a normal fetal movement count of not fewer than 10 movements in 12 h is associated with a good outcome and is considered as a reassurance to the obstetricians to defer the delivery to gain further fetal intrauterine maturation [14].
Fetal breathing movements have been thought of as a complex phenomenon with a composite, progressive pattern of development during gestation [15]. The onset of fetal breathing occurs at about 10 weeks of gestation. It has been shown from the observations made over 24 hr that at 24-28 weeks of gestation, fetuses breathe about 14 % of the time [16] and by 34 to 40 weeks breathing increases to about 30% of the time [17]. From 25 to 32 weeks, episodes of shorter breaths decrease and those lasting for more than 30 s increase [18]. Episodic breathing is interspersed with apnoeic periods whose duration varies with the gestational age. Variation of breathing pattern as a function of gestational age can be thought of as morphological maturation of the fetal lung and the functional development of respiratory and sleep centers of the fetal central nervous system [19]. Fetal breathing has been shown to be influenced by various internal and external factors. For instance, an increase in the fetal breathing is noted with the maternal ingestion of coffee [20], and with a raise in maternal glucose level [21, 22]. Absence of fetal breathing has been shown to be a potential tool to predict preterm birth [23]. A decrease in the breathing activity is observed following premature rupture of membranes [24, 25], usually three days prior to onset of labor [26, 27] and following maternal alcohol intake, in normal pregnancies [28].
In a comparison study of breathing movements of three different age groups, 30-32 weeks, 33-36 weeks and 37-38 weeks, of normal fetuses, it has been shown that the increased breathing movements of a longer duration are observed for the fetuses in 33-36 weeks of gestation. Based on these observations, it has been argued that the biochemical transformations for the maturation of fetal lung are occurring during this period of fetal life [29].
Though the occurrences of fetal movements were analyzed by conventional statistical measures, we considered the scaling behavior of these activities in order to understand the correlation in the occurrences of these events. We then analyzed the instances of the fetal breathing, gross-body and extremity movements scored (by experts) on a second by second basis from ultrasound measurements. We performed detrended fluctuation analysis (DFA) [30] to understand the correlation aspects of these three patterns. We found that all three patterns exhibit long range correlations with DFA exponent α ranging from 0.63 to 1.1. There is an increasing trend in the exponents obtained for the movements corresponding to fetal breathing as a function of the gestational age, but we did not observe such a trend for other two movement activities.
2 Materials and Methods
In this work, a series of ultrasound imager-based fetal movement studies was conducted in which fetal movement was classified with a time resolution of one second [5, 6, 31, 32]. The study was approved by the local Institutional Review Board and each subject signed an informed consent. Patients were placed in the supine position and spontaneous fetal movement was observed by ultrasound imaging for 1400 seconds. The video output of the imager was recorded on a VHS video recording device and time stamped at one second intervals in a manner that could be read by computer upon playback. Expert sonographer later scored the tapes using a PC linked to the VHS playback device. Only visually clear ultrasound recordings with discernable fetal movements were scored. Also, the expert had trained on a series of fetal ultrasound recordings designated the “gold standard”. That is, an expert had to reach a certain level of proficiency in movement scoring to be considered expert. The gold standard was developed through group scoring sessions of the maternal-fetal medicine physician in charge of the research as well as others involved with the study design and implementation. After many practice sessions, the scoring of the expert in training was graded against the gold standard recordings.
The tapes were scored on a second by second basis for three types of fetal movements: breathing, gross-body and extremity. Instances of missing or poor images due to patient movement were also marked. On ultrasound exam, fetal breathing is seen as diaphragmatic and chest wall expansion and relaxation which can range from shallow to deep in amplitude and regular to irregular by rate. In early fetal life, breathing movements tend to be erratic but develop a more regular pattern with advancing gestational maturity. At times this activity is physically perceptible to the mother. Fetal breathing is thought to originate from the medullary respiratory centers as part of the process of respiatory development and results in primary diagphragmatic rather than chest wall activity.
Fetal gross body movements involve motility of the trunk and head and can be vigorous or weak. Typically on ultrasound they are seen as rolling over, arching of the back, jerking, etc. Extremity movements (flexion and extension of arms and legs) often accompanies gross movement but can also occur without trunk motility. Each study resulted in a single text file indicating the time and type of fetal movement on a second by second basis.
We acquired recordings from 49 fetuses whose mothers presented with healthy singleton pregnancies in the gestational age range between 28 and 39 weeks. The inclusion criteria were gravid women with no known disease or abnormality and no maternal medication during pregnancy. These subjects were recruited upon arrival for their clinical obstetrical appointment and the study was performed during the time before the physician exam or immediately afterwards. However, there was no experimental protocol for this study. To study the scaling properties of the occurrences of these activities, we converted the active and passive paces into binary sequences, 1 and 0, respectively. These binary sequences were then analyzed for long range correlations using DFA.
DFA is an elegant tool to quantify reliably the correlations in non-stationary data. It has been applied to wide variety of datasets ranging from heart-beat time series [33, 34], atmospheric temperature data [35], to DNA sequences [36]. Let xi, i = 1 to N be the observations (time series) made as a function of time (not necessarily as continuous function of time) and N be the length of the series. The methodology of DFA involves four steps [37]:
Construct profile function as follows: , where x̄ indicate the mean of xi. Subtraction of mean in this step is not mandatory as it will be eliminated in step 3.
Divide the profile function in to M = N/s disjoint time windows (indexed by ν) of size s. When the length of the window is not integer multiple of the length of the data, a short portion of the data at the end of the record will be left unanalyzed. In order not to discard this portion, we divide the profile starting from the other end [38]. Thus we have a total of (ν = 1 to) 2M disjoint segments of size s.
The data inside ν-th window of the profile are fitted by a m-th order polynomial pm. Depending on the order of the polynomial, DFA is designated as DFA(m). In general DFA of order m will remove a polynomial trend of order m from profile and hence a polynomial trend of order m − 1 from the original time series. Thus successive higher orders of DFA will systematically eliminate the trends present in the data and reliably quantify the correlations.
- Finally we compute the DFA fluctuation function F(s) as follows:
where is the best fit polynomial of order m for the ν-th window of the profile. In order to establish the relation between F(s) and s, steps (2) to (4) are repeated for different values of s. Usually the minimum value of s is chosen as m+2 (Note: m is the order of the DFA) [37] and maximum is chosen either as one quarter or one half of the length of record. Here we compute the fluctuations functions up to one half of the length of the record (i.e. N/2). For long range correlated data F(s) follows a power law: F(s) ∼ sα, where α is called DFA fluctuation exponent and is related to the exponent β obtained from power spectrum by α = (1+β)/2. For short range and uncorrelated data α takes a value of 0.5. For anti-correlated data α takes a value less than 0.5. For long range correlated data α takes a value greater than 0.5.
3 Results
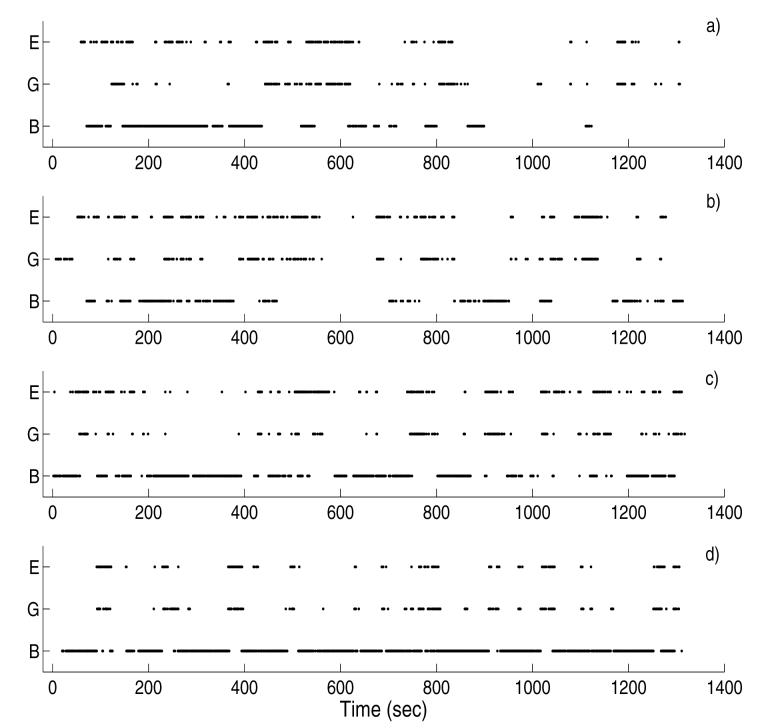
Figure 1 a)-d) shows the paces of breathing (B), gross-body (G) and extremity (E) movements of (randomly chosen) four fetuses at different gestational ages, 28 weeks, 31 weeks, 34 weeks and 37 weeks, respectively. The paces of breathing are interspersed by apnoeic periods. The duration and occurrence of the apnoeic pace is more pronounced in the earlier gestation compared to later gestational age. This observation is in agreement with the earlier studies [19, 26, 27] which reported that episodes of fetal breathing increased with gestational age. We also observed that episodes of extremity movements occur more frequently compared to the gross body movements and that the episodes of the gross-body and extremity movements decrease as a function of gestational age. This observation is also in agreement with the earlier report [8].
Fig. 1.
The occurrences of breathing (B), gross-body (G) and extremity (E) movements (scored on a second by second basis for 1400 seconds) from four randomly chosen fetuses at different gestational ages. The gestational ages of the fetuses are: a) 28 weeks, b) 31 weeks, c) 34 weeks, and d) 37 weeks. Regions of active paces are marked by dots while the passive paces are left as white space. In the foregoing analysis, the active and passive states were converted into binary symbols, 1 and 0, respectively.
The percentage of occurrences of these three movements is shown in Fig. 2 as a function of gestational age. Unlike adults, the fetus does not breathe continuously. For example, two of the fetuses showed no breathing behavior during the entire duration of the study and one of these showed no extremity movements either. All three movements showed a significant trend as a function of gestational age. The frequency of breathing movements showed an increasing trend as a function of gestational age (ρ=0.29;p=0.042), while the frequency of gross-body movements (ρ=−0.29;p =0.042) and extremity movements (ρ=−0.38;p =0.007) showed a decreasing trend as a function of gestational age. ρ is the correlation coefficient and p is the probability computed under the null hypothesis that there is no correlation between the % of occurrences and gestational age at 95 % confidence level. Compared to the gross-body movements the frequency of the extremity movements decreases at the faster rate with the gestational age.
Fig. 2.
Percentage of occurrences of (a) breathing movements, (b) gross-body movements, and (c) extremity movements and their variation as a function of gestational age. Breathing movements showed an increasing trend as a function of gestation while gross-body and extremity movements showed a decreasing trend as a function of gestation. The degree of association expressed by correlation coefficient ρ and the probability obtained under the hypothesis that there is no association between the two variables (% of occurrence and gestational age) are given in the inset. For the sake of clarity, the ordinate axes in b) and c) are plotted in different scales. Each point in the plot corresponds to each fetus. The (solid) trend lines shown in each plot indicate the nature of the trend.
The profile functions of the breathing movements, gross-body movements and extremity movements for a fetus in 35 weeks of gestation are shown in Fig. 3(a-c). The parabolic nature of profile for breathing movement and gross-body movements indicate the presence of linear trends in the data. This mandates the use of DFA(2) [38] to quantify the correlations correctly and is employed in this study. Using DFA(2) we studied the correlations in the occurrence of the movements. If the successive events (movements) are (negatively) correlated, the dataset will exhibit long range (anti) correlations with α > 0.5 (< 0.5) and the extent of correlation dictated by the magnitude of α. If there is no correlation between the successive events or the correlation exhibits only up to certain time scale (short range correlations), then the dataset will display an exponent of 0.5.
Fig. 3.
The profile functions of (a) breathing movements (b) gross-body movements and (c) extremity movements of a fetus in 35 weeks of gestational age. The parabolic nature of the profile (see a) and b)) is an indication of linear trends in the data.
For a fetus in 32 weeks of gestational age, the DFA(2) fluctuation functions for the paces of breathing (Fig. 4a), gross-body (Fig. 4b) and extremity (Fig. 4c) movements are shown in Log-Log representation. The estimated exponents for each type of movements are given in the inset. All the three movements exhibit long range correlations with the DFA exponent greater than 0.5. In order to confirm the long range correlations exhibited by the three different movements, we randomly shuffled the data and performed the scaling analysis. The shuffled data should not contain any correlations and should yield an exponent of 0.5. This is indeed obtained for the shuffled data in our case. The results are shown (’x’) in Fig. 4(a-c). The results of the scaling analysis for all the three types of movements are given in Fig. 4(e-f) for another different fetus in 35 weeks of gestational age. Also in this case, all the three movements display exponents very different from their shuffled counter parts indicating the presence of long range correlations in the dynamics.
Fig. 4.
Log-Log plot of DFA(2) fluctuation functions (shown in ○) obtained for (a) breathing, (b) gross-body, and (c) extremity movements for a fetus in 32 weeks of gestational age. The data shown in (d), (e) and (f) are same as in (a), (b) and (c) respectively, except for another different fetus in 35 weeks of gestational age. The fluctuation functions obtained for the shuffled data are shown in ’x’. The solid lines are obtained by fitting a straight line to F(s) and s (between 7-700 sec) in Log-Log representation. The estimated exponents (slope) α for the original data and αs for the shuffled data are given in the inset. Unit of s is sec. For the sake of clarity, F(s) of original data are shifted by a constant factor.
In order to see the trend, if any, in the DFA exponents obtained for the movements, we present the fluctuation exponents of different fetuses as a function of gestational age in the form of a scatter diagram. The DFA(2) fluctuation exponents α calculated from the slope of the Log-Log plot of F (s) and s are shown, in Fig. 5a for the instances of breathing movements, in Fig. 5b for the instances of gross-body movements and in Fig. 5c for the extremity movements, as a function of gestational age. For all three movements, the exponents range between 0.63 and 1.15. These exponents are obtained by fitting a straight line to F (s) and s in Log-Log representation between 7 and 700 seconds. The paces of breathing movements showed a significant increasing trend (ρ=0.30;p =0.039) as a function of gestational age while the exponents obtained for the paces of gross-body (ρ=−0.15;p=0.3) and extremity movements (ρ=−0.04;p =0.8) did not show this trend.
Fig. 5.
DFA(2) fluctuation exponent α estimated from the slope of the Log-Log plot of F(s) and s for the paces of a) breathing, b) gross-body, and c) extremity movements as a function of gestational age. There is a positive trend in the exponents obtained for the instances of breathing movements as a function of gestational age which is not observed for the instances of the gross-body and extremity movements. The correlation (coefficient ρ) between α and gestational age with the probability computed based on the assumption that there is no correlation between the two variables are given in inset. In all the plots each data point corresponds to each fetus. In a) the solid line shows the nature of the trend. In b) and c) there is no significant trend between α and gestation age and hence the trend lines are not shown.
4 Discussion
The increase in the instances of fetal breathing as a function of gestational age indicates the functional development of respiratory muscles and lungs [20]. The decrease in the extremity and gross-body movements as a function of gestational age indicates the development of the fetal organs. As the fetus matures and increases in size, it may not be able to comfortably perform the body movements within the uterus and hence the instances of movements decrease as a function of gestational age [13, 14, 39, 40]. Though the percentage of occurrences of each movement gives a general idea about the nature of the dynamics pertaining to that movement, it is not possible to understand the nature of the correlation between the events of these movements. This is addressed by the scaling analysis and the scaling exponent α represents the extent of correlations between the events.
The scaling exponent α can be considered as the roughness exponent. If the events are not correlated as in the case of white noise (α=0.5), then they are considered as rough while the Brownian noise (α=1.5) (integrated version of zero mean white noise process) is considered as a smooth data. The case of α=1 forms the transition between the two extremes. All three types of movements displayed long range correlated behavior which indicates that an event is more likely to be followed by another event. For instance, long range correlations in the movements due to breathing indicate a long episodic breathing is more likely to be followed by another long episode of breathing. In the case of gross-body and extremity movements, the correlation between the instances of the movements remains almost constant for the entire period of gestational age. This indicates that the instances of movements occur with almost similar pattern throughout the pregnancy though there is a decrease in their occurrence with fetal maturation. For the case of movements due to breathing, in addition to the increase in the frequency of breathing with gestational age, the correlation between the episodic breathing events increases with gestational age. This is reflected as the increase in the scaling exponents as a function of gestational age. The increase in the movements due to fetal breathing in the last 10 weeks of gestation, especially during 33-36 weeks, has been related to the events taking place in the lungs such as thinning of the respiratory epithelium, transformation of type II alveolar epithelial cells into type I cells, the beginning of septation, and maximum collagen production [29]. Based on these earlier observations, the positive trend in the scaling exponent can be related to the complexity associated with the maturation of fetal lungs and respiratory systems.
5 Conclusion
We performed scaling analysis of breathing, gross-body, and extremity movements of 49 fetuses in the gestational age between 28 to 39 weeks. All three fetal movements of breathing, gross-body and extremity exhibited long range correlations characterized by the exponents in the range between 0.63 and 1.15. A positive trend was observed for breathing as a function of gestational age. The positive trend in the DFA exponent for the paces of breathing with gestational age indicates that the correlation between paces of the breathing events increases as a function of gestational age. The steady value of the exponents of the paces of the movements around 0.9 indicates that the correlation between the paces of the movements did not vary as a function of gestational age (though a slight decrease was observed for the later gestational ages).
Low-risk fetuses show higher number of (body) movements compared to high-risk fetuses [8]. Further, breathing episodes increase with gestation age for both low-risk and high-risk fetuses but with an earlier onset of these patterns for the high-risk fetuses. This has been argued as the early maturation of the high-risk fetuses while the low-risk fetuses show progessive maturation [8]. In future study, the scaling exponents of the three different movement patterns of the high-risk fetuses will be compared with the low-risk fetuses to understand the differences in their maturation.
6 Acknowledgment
This work is supported by NIH grant 5R01-NS-3627705A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadovsky E, Yaffe H. Daily fetal movement recording and fetal prognosis. Obstet. Gynecol. 1973;41:845–850. [PubMed] [Google Scholar]
- 2.Hertogs K, Roberts A, Campbell S. Do fetal movements reflect fetal well-being. Br. Med. J. 1981;282:1153–1154. [Google Scholar]
- 3.Besinger RE, Johnson TR. Doppler recording of fetal movements:Clinical correlation with real-time ultrasound. Obstet. Gynecol. 1989;74:277–280. [PubMed] [Google Scholar]
- 4.Maeda K. Computerized analysis of cardiograms and fetal movements. Baillieres Clin. Obstet. Gynaecol. 1990;4:797–813. doi: 10.1016/s0950-3552(05)80345-1. [DOI] [PubMed] [Google Scholar]
- 5.Lowery CL, Russel WA, Wilson JD, Walls RC, Murphy P. Time quantified fetal movement detection with two-transducer data fusion. Am. J. Obstet. Gynecol. 1995;172:1756–1764. doi: 10.1016/0002-9378(95)91408-0. [DOI] [PubMed] [Google Scholar]
- 6.Lowery CL, Russel WA, Wilson JD, Walls RC, Murphy P. Doppler movement detection as a potential screening tool for maternal sensitivity to fetal movement. J. Matern. Fetal Invest. 1997;7:7–11. [Google Scholar]
- 7.Hepper PG. The behavior of the fetus as an indicator of neural functioning. In: Lecanuet JP, Fifer W, Krasnegor N, Smotherman W, editors. Fetal Development: A Psychobiological Perspective. Lawrence Erlbaum; Hillsdale, NJ: 1995. pp. 405–417. [Google Scholar]
- 8.Kisilevsky BS, Hains SMJ, Low JA. Maturation of body and breathing movements in 24-33 week-old fetuses threatening to deliver prematurely. Early Hum. Dev. 1999;55:25–38. doi: 10.1016/s0378-3782(99)00007-9. [DOI] [PubMed] [Google Scholar]
- 9.de Vries JIP. The first trimester. In: Nijhuis JG, editor. Fetal behavior:Development and perinatal aspects. Oxford University Press; NY: 1992. pp. 3–16. [Google Scholar]
- 10.de Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behavior II. Quantitative aspects. Early Hum. Dev. 1985;12:99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- 11.Natale R, Nasello-Paterson C, Turlink R. Longitudinal measurements of fetal breathing, body movements, and hear rate accelerations, and decelerations at 24 and 32 weeks of gestation. Am. J. Obstet. Gynecol. 1985;151:256–263. doi: 10.1016/0002-9378(85)90022-5. [DOI] [PubMed] [Google Scholar]
- 12.Roodenburg PJ, Wladimiroff JW, van Es A, Prechtl HFR. Classification and quantitative aspects of fetal movements during second half of normal pregnancy. Early Hum. Dev. 1991;25:19–36. doi: 10.1016/0378-3782(91)90203-f. [DOI] [PubMed] [Google Scholar]
- 13.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of gross body fetal body movements over 24-hour observation intervals during the last 10 weeks of pregnancy. Am. J. Obstet. Gynecol. 1982;142:363–371. doi: 10.1016/s0002-9378(16)32375-4. [DOI] [PubMed] [Google Scholar]
- 14.Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. Br. Med. J. 1976;1:1305–1308. doi: 10.1136/bmj.1.6021.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosmi EV, Anceschi MM, Cosmi E, Piazze JJ, La R. Torre, Ultrasonographic, patterns of fetal breathing movements in normal pregnancy. Int. J. Gynecol and Obstet. 2003;80:285–290. doi: 10.1016/s0020-7292(02)00384-3. [DOI] [PubMed] [Google Scholar]
- 16.Natale R, Nasello-Paterson C, Connors G. Patterns of fetal breathing in the human fetal breathing during the last 10 weeks of gestation. Am. J. Obstet. Gynecol. 1980;56:24–30. doi: 10.1016/0002-9378(88)90146-9. [DOI] [PubMed] [Google Scholar]
- 17.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of human fetal breathing during the last 10 weeks of pregnancy. Obstet. Gynecol. 1980;56:24–30. [PubMed] [Google Scholar]
- 18.Higuchi M, Hirano H, Gotoh K, Otomo K, Maki M. Relationship between the duration of fetal breathing movements and gestational age and the development of the central nervous system at 25-32 weeks of gestation in normal pregnancy. Gynecol. Obstet. Inves. 1991;31:136–140. doi: 10.1159/000293131. [DOI] [PubMed] [Google Scholar]
- 19.Kozuma S, Nemoto A, Okal T, Mizuno M. Maturational sequence of fetal breathing movements. Biol. Neonat. 1991;60:36–40. doi: 10.1159/000251015. [DOI] [PubMed] [Google Scholar]
- 20.Salvador HS, Koos BJ. Effects of regular and decaffeinated on fetal breathing and heart rate. Am. J. Obstet. Gynecol. 1988;158:322–327. doi: 10.1016/0002-9378(89)90157-9. [DOI] [PubMed] [Google Scholar]
- 21.Adamson SL, Bocking A, Cousin AJ, Rapport I, Patrick JE. Ultrasonic measurement of rate and depth of human fetal breathing:Effect of glucose. Am. J. Gynecol. 1983;147:288–295. doi: 10.1016/0002-9378(83)91113-4. [DOI] [PubMed] [Google Scholar]
- 22.Harper MA, Meis PJ, Rose JC, Swain M, Burns J, Kardon B. Human fetal breathing response to intravenous glucose is directly related to gestational age. Am. J. Obstet. Gynecol. 1981;140:289–294. doi: 10.1016/s0002-9378(87)80232-6. [DOI] [PubMed] [Google Scholar]
- 23.Honest H, Bachmann LM, Sengupta R, Gupta JK, Kleijnen J. Accuracy of absence of fetal breathing movements in predicting preterm birth: a systematic review. Ultrasound Obstet. Gynecol. 2004;24:94–100. doi: 10.1002/uog.1062. [DOI] [PubMed] [Google Scholar]
- 24.Roberts AB, Goldstein I, Romero R, Hobbins JC. Fetal breathing movements after preterm rupture of membranes. Am. J. Obstet. Gynecol. 1991;164:821–825. doi: 10.1016/0002-9378(91)90523-t. [DOI] [PubMed] [Google Scholar]
- 25.Kivikoski A, Amon E, Vaalamo PO, Pirhonen J, Kopta MM. Effect of third-semester premature rupture of membranes on fetal breathing movements: A prospective case-control study. Am. J. Obstet. Gynecol. 1988;159:1474–1447. doi: 10.1016/0002-9378(88)90577-7. [DOI] [PubMed] [Google Scholar]
- 26.Richardson B, Natale R, Patrick J. Human fetal breathing activity during induced labor at term. Am. J. Obstet. Gynecol. 1979;133:247–255. doi: 10.1016/0002-9378(79)90674-4. [DOI] [PubMed] [Google Scholar]
- 27.Besinger RE, Compton AA, Hayashi RH. The presence or absence of fetal breathing movements as a predictor of outcome in preterm labor. Am. J. Obstet. Gynecol. 1987;157:753–757. doi: 10.1016/s0002-9378(87)80044-3. [DOI] [PubMed] [Google Scholar]
- 28.McLeod W, Brien J, Loomis C, Carmichael L, Probert C, Patrick J. Effect of methanol ingestion on fetal breathing movements, gross body movements and heart rate at 37 to 40 weeks gestation age. Am. J. Obstet. Gynecol. 1983;145:251–257. doi: 10.1016/0002-9378(83)90501-x. [DOI] [PubMed] [Google Scholar]
- 29.Florido J, Cortés E, Gutiérrez M, Soto VM, Miranda MT, Navarrete L. Analysis of fetal breathing movements at 30-38 weeks of gestation. J. Perinat. Med. 2005;33:38–41. doi: 10.1515/JPM.2005.006. [DOI] [PubMed] [Google Scholar]
- 30.Peng C-K, Buldyrev SV, Goldberger AL, Havlin S, Sciortino F, Simon M, Stanley HE. Nature. 1992;356:168–170. doi: 10.1038/356168a0. [DOI] [PubMed] [Google Scholar]
- 31.Lowery CL, Russell WA, Baggot PJ, Wilson JD, Walls RC, Bentz LS. Time quantified detection of fetal movements using a new fetal movement algorithm. American Journal Perinatology. 1997;1:7–12. doi: 10.1055/s-2007-994088. [DOI] [PubMed] [Google Scholar]
- 32.Lowery CL, Russell WA, Wilson JD, Walls RC, Murphy P. Electronic fetal breathing movement detection using fetal heart rate doppler transducers. Prenatal and Neonatal Medicine. 1996;1:241–246. [Google Scholar]
- 33.Peng C-K, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and cross over phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 34.Bunde A, Havlin S, Kantelhardt JW, Penzel T, Peter J-H, Voigt K. Correlated and uncorrelated regions in heart-rate fluctuations during sleep. Phys. Rev. Lett. 2000;85:3736–3739. doi: 10.1103/PhysRevLett.85.3736. [DOI] [PubMed] [Google Scholar]
- 35.Koscielny Bunde E, Bunde A, Havlin S, Roman HE, Goldreich Y, Schellenhuber H-J. Indication of a universal persistence law governing atmospheric variability. Phys. Rev. Lett. 1998;81:729–732. [Google Scholar]
- 36.Peng CK, Buldyrev SV, Havlin S, Simons M, Stanley HE, Goldberger AL. Mosaic organization of DNA nucleotides. Phys. Rev. E. 1994;49:1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 37.Kantelhardt JW, Ashkenazy Y, Ivanov P. Ch., Bunde A, Havlin S, Penzel T, Peter J-H, Stanley HE. Characterization of sleep stages by correlations in the magnitude and sign of heartbeat increments. Phys. Rev. E. 2002;65:051908-1–6. doi: 10.1103/PhysRevE.65.051908. [DOI] [PubMed] [Google Scholar]
- 38.Kantelhardt JW, Koscielny-Bunde E, Rego HA, Havlin S, Bunde A. Detecting long-range correlations with detrended fluctuation analysis. Physica A. 2001;295:441–454. [Google Scholar]
- 39.Davis L. Daily fetal movement counting. A valuable assessment tool. J. Nurse Midwifery. 1987;32:11–19. doi: 10.1016/0091-2182(87)90051-6. [DOI] [PubMed] [Google Scholar]
- 40.Mathews DD. Fetal well-being in gravidas with diminished fetal activity at term. Obstet. Gynecol. 1978;51:281–283. doi: 10.1097/00006250-197803000-00005. [DOI] [PubMed] [Google Scholar]