Abstract
Psychostimulants including amphetamine and cocaine induce locomotion and stereotypy and suppress eating. Although the capacity of cocaine to alter locomotion is usually viewed as related to dopamine neurotransmission, recent studies suggest that norepinephrine, acting through alpha1-adrenergic receptors (α1-ARs) can facilitate cocaine-stimulated locomotion. Of the three α1-AR subtypes (α1A, α1B, and α1D) identified to date, inactivation of the α1B-AR subtype diminishes cocaine-stimulated locomotion, whereas the impact of inactivation of the α1A-AR subtype on either eating or locomotion is unknown. In the present study, we assessed the relative impact of icv administration of the α1B-AR antagonist 5-methylurapidil (5-MU) on cocaine-stimulated hyperlocomotion and hypophagia, using a concurrent method (Wellman et al., 2005). Rats were infused icv with one of 3 doses of 5-MU (0, 3, or 30 nmol) and then injected (i.p.) with 0, 2.5, 5.0, 10.0, or 20.0 mg/kg cocaine HCl on each of five tests. Rats always received the same 5-MU dose, but a different cocaine dose on each trial. Feeding and locomotion were assessed concurrently during a 45 min postinjection period. Significant suppression of eating was noted at 2.5 mg/kg cocaine, a dose that does not alter forward locomotion in the rat. Administration of 5-MU did not alter locomotion in rats treated with saline, but did significantly increase baseline food intake. Neither cocaine-induced hypophagia nor hyperlocomotion were altered by icv administration of 5-MU. These results suggest that the capacity of α1-AR agonists (e.g. phenylpropanolamine) to suppress eating may be related to activation of the α1A-AR subtype, whereas cocaine does not act through the α1A-AR subtype to suppress eating nor does this subtype modulate cocaine-induced hyperlocomotion.
Keywords: Body Weight, Cumulative Food Intake, Adrenoceptors, Horizontal Activity, Cocaine
Introduction
The capacity of psychostimulants to enhance forward locomotion in rats (Berthold et al., 1992; Drouin et al., 2002a,b; Wellman et al., 2002) has been related to increases in synaptic dopamine levels within the forebrain (Bassareo and DiChiara, 1999; DiChiara et al., 2004) as well as to an action of norepinephrine on alpha1-adrenergic receptors (α1-ARs). Of the three (1A, 1B or 1D) α1-AR subtypes identified to date, the α1B-AR subtype is reported to modulate cocaine (or amphetamine)-stimulated locomotion (Darraq et al., 1998; Drouin et al., 2002a,b).
Psychostimulants also inhibit eating (Bellinger et al., 2003; Blavet and DeFeudis, 1982; Blosser et al., 1987; Cooper and van der Hoek, 1993; Wellman et al., 2003). Hypophagia has been attributed to dopamine receptor activation in the perifornical hypothalamus (Leibowitz, 1975; Leibowitz and Rossakis, 1979; Wellman, 2005) as well as to activation of α1-ARs in brain (Wellman et al., 1993). Pretreatment of rats with the non-selective α1-AR antagonist prazosin attenuates the hypophagia induced by amphetamine, phenylpropanolamine (PPA), phentermine,and sibutramine (Mitchell et al., 1998; Wellman, et al., 1993) whereas intracranial infusion of α1-AR agonists (e.g. phenylephrine or cirazoline) induces hypophagia in rats (Wellman et al., 1993). The general role of α1-ARs in feeding or locomotion is unknown, as is the specific role of these receptors in psychostimulant induced hyperlocomotion and hypophagia (Baldo et al., 2002; Bassareo and DiChiara, 1999).
Although the capacities of psychostimulants to alter locomotion and to inhibit eating are commonly assessed using separate paradigms, these capacities may interact in important ways. Hyperactivity induced by a psychostimulant may compete with other motor behaviors, including eating. Moreover, changes in hunger (and satiation) may alter the biobehavioral actions of psychostimulants (Bell et al., 1997; Carr, 2002; Carroll, 1985; Carrol and Lac, 1993; Specker et al., 1994). Short-term measures of feeding and locomotion are often conducted under conditions of food deprivation (and reduced body weight) to ensure adequate food consumption (Baldo et al., 2002; Wellman et al., 2005). The aforementioned observations suggest that it is important to concurrently assess changes in eating and locomotion after psychostimulant treatment in non food-deprived animals. We have developed a concurrent method in which rats are offered a palatable mash diet that is suspended via a leash from an electronic balance positioned on the ceiling of an activity chamber (Wellman et al., 2005). In this method, rats are maintained at normal body weights and are not subjected to food deprivation. The use of a palatable diet ensures that the rats will consume a reliable amount of food during a one hour test. The method generates minute-by-minute recordings of food consumption that are gathered in parallel with minute-by-minute measures of locomotion.
Selective antagonists of the various α1-AR subtypes have been developed including the α1A-AR antagonist 5-methylurapidil (5-MU) (Camargo et al., 2000; Marek et al., 1996). In the present study, we assessed the relative impact of icv administration of 5-MU on cocaine-stimulated locomotion and eating, using a concurrent method. Rats were infused (icv) with one of 3 doses of 5-MU (0, 3, or 30 nmol) and then injected (i.p.) with 0, 2.5, 5.0, 10.0, or 20.0 mg/kg cocaine HCl on each of five tests.
Materials and Methods
Animals
This study was approved by the Texas A&M University Laboratory Animal Care Committee. Adult male Sprague-Dawley rats (Harlan Industries: Houston, TX) weighing approximately 250-275 grams at the beginning of the study were housed in plastic hanging cages in colony room maintained at 22.0 ± 1 °C under a 12 hr light/dark cycle (lights off at 1100hr). The rats were maintained on standard chow pellets (Teklad) and tap water in the home cage for 23 hours per day. A mash diet (see below) was offered for an hour per day in a separate test chamber. Rats were identified using permanent ink markings placed on the tail.
Drugs
Artificial CSF (aCSF) consisted of 128 mM NaCl, 3.9 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 25 mM NaHCO3, 0.3 mM NaH2PO4 (pH 7.3). Solutions of 5-MU were prepared by dissolving 5-MU (Sigma Chemical; St. Louis, MO) into aCSF at a concentration of either 0.3 or 3.0 mg/ml (a 2 ul infusion of these solutions would result in 1.5 or 15 nmol/infusion/side). A vehicle saline solution for systemic injection was prepared as 0.9% NaCl in distilled water. Solutions of cocaine hydrochloride (2.5, 5, 10 or 20 mg/ml) were prepared in saline (cocaine HCl was kindly provided by Dr. Kevin Gormley of the Basic Research Division of NIDA). Drug doses were calculated as the salt and all systemic injections were administered i.p. in a volume of 1 ml/kg.
Diet
A palatable mash diet (4.03 kcal/g) consisted of 470 g of Teklad ground rat chow, 10 g of maltodextrin (Product # 31410: Fluka BioChemica, St. Louis, MO), 440 g of sweetened condensed milk (Hill Country Fare: San Antonio TX), and 80 g of corn oil (Albertson's; Boise, ID). The mash diet contained 13.2% fat, 14.64% protein, and 49.8% carbohydrate. The mash diet was prepared fresh every 3 days, stored in a sealed plastic container in a refrigerator and allowed to reach room temperature prior to use in the activity-feeding cages. The texture of this diet is such that it is possible to press a ball of the diet into a suspended plastic cylinder for presentation within a locomotor chamber. The mash diet is palatable (Farley et al., 2003), easily consumed, and is not readily spilled by a rat.
Surgery
Rats were mildly food deprived for 1-4 hours before the surgery. Each rat was injected (i.p.) with 0.4 mg/kg atropine sulphate to reduce bronchial secretions and anesthetized with a combination of ketamine (80 mg/kg) and xylazine (20 mg/kg) given i.p. For lateral cerebroventricular infusions, a double guide shaft assembly was fashioned from two 17 mm lengths of 20-ga stainless steel tubing (Small Parts Inc; Miami Lakes, FL) spaced 2.4 mm apart. Each rat was mounted in a stereotaxic frame and the scalp incised using sterile technique. A 2% lidocaine jelly was applied to the incised edges of the scalp. The periosteum was mechanically retracted and skull bleeding was terminated using a styptic gel (Kwik-Stop, Gimborn Pet, Atlanta, GA). The tip of each guide shaft was positioned at +1.2 mm anterior to bregma and 1 mm above the lateral ventricles, using coordinates derived from Paxinos and Watson (2005). Each cannula pedestal was secured to the skull using 4 stainless steel screws, a layer of cyanoacrylate and then a layer of dental acrylic (Lang Dental; Wheeling, IL). The lateral edges of the scalp incision were coated with a 0.1% gentamicin sulfate ointment (E. Fougera; Melville, NY) and the ends of the incision were closed using cyanoacrylate. A metal obdurator cut flush to the end of each shaft was used to seal each cannula guide shaft. Sodium ampicillin (Reseach Products Intl; Mount Prospect, IL) was given (IM) at 55,000 ug/0.3 ml to prevent infection. Each rat was allowed a week to recover after surgery.
Apparatus
The concurrent assessments of eating, drinking, and locomotion were made in a set of 8 automated optical beam activity monitors (Model RXYZCM-16; Accuscan Instruments, Columbus, OH, USA). Each monitor is housed within a 40 × 40 × 30.5 cm acrylic cage. These monitors were modified to include an electronic balance affixed to the ceiling of each chamber. A food ring, packed with the mash diet, was suspended from the weigh-below hook of each balance. Software programs were used to record forward locomotion and food intake for each minute of a 45 minute test session. A 100 ml calibrated drinking tube (Ancare Corp., Bellmore, NY) with a metal sipper spout was inserted through a hole drilled above the back left corner of the chamber and the end of the spout is positioned just above the vertical infrared beams. A metal ball bearing placed within each sipper tube serves to minimize water spillage. Details on the fabrication of this system are given in (Wellman et al., 2005).
Daily Testing Procedures
In these studies, rats have continuous access to a chow pellet diet and tap water in the home cage for 23 hours per day. Each rat was adapted to the mash test diet for a 3-4 day period. In this phase, a leash filled with food was suspended from the home cage wall for an hour per day.
After diet adaptation, the rats were exposed daily to activity-monitoring procedures. Rats were weighed to the nearest g at 1000 hr and transported to the locomotor testing room in separate plastic cages. At 1015 hr, under red light (15 Watt) and white noise, the rats were placed in their respective chambers for 15 min, without access to food or water. This period served to adapt the rats to the chambers and to reduce initial locomotor activity. After 15 min, each rat was injected (i.p.) with 1 ml/kg saline and replaced in the chamber. The balance lid was placed on the chamber, the balance was zeroed and the food leash was checked for clearance from the left and front walls of the chamber. The drinking tube start volume was recorded to the nearest ml. The activity monitor and the food monitor programs were then initiated for 45 min. At the end of the 45 min period, the final volumes of the water tubes were recorded, and the leash assembly was weighed to verify actual food consumption. Rats were then transported back to the colony room and again allowed access to chow pellets and tap water in the home cage until the next test day. Testing chambers were thoroughly cleaned after each test session with a 70% isopropyl alcohol solution and dried with paper towels.
Rats were randomly assigned to 5-MU injection conditions (0, 3 or 30 nmol). ICV drug infusions were given every third day (i.e., days 1, 4, 7, 10, and 13) and were separated by two days in which eating and locomotion are assessed without intracranial drug infusion/systemic drug injection. On a drug day, each rat was transported to the testing room and placed into a chamber, without food or water, for 15 min. The obdurators were then removed and a set of 25-ga microinjector needles were positioned within each guide shaft (1 mm beyond the guide shaft into the ventricle) and a 2.0 uL infusion made over 60 sec using an infusion pump (Model 780230, KD Scientific, Holliston, MA). After an additional 30 sec, the microinjector needles were removed. Each rat was then injected (i.p.) with the appropriate dose of cocaine and placed into the activity chamber with access to food and water for a 45 min test period. Each rat always received the same icv dose of 5-MU, but a different cocaine dose on each of the five drug trials.
Histology
Rats were euthanized with a fatal overdose of sodium pentobarbital (100 mg/kg, i.p.) and then infused with a black ink using microinjector needles that extended 1.0 mm beyond the tip of the guide shaft (using 0.2 ul per side for 30 sec). Each rat brain was removed from the skull and the presence of black ink within the ventricles served as the criterion for a successful guide shaft placement within the lateral ventricles.
Data Analyses
Data were retained for subsequent analyses for those rats in groups 0, 3 and 30 nmol 5-MU for whom the inection cannula terminated within the lateral ventricles (n's=11, 11, and 13, respectively). Separate analyses of variance (ANOVA) were computed for each dependent measure (food intake; forward locomotion) using a split-plot (mixed model) design with a between-group factor of 5-MU dose (0, 3, or 30 nmol) and within-group factors of cocaine dose (0, 2.5, 5, 10 and 20 mg/kg) and time (15 min blocks). Additional comparisons between drugs at any specific time point were computed using Bonferroni contrasts. Differences that were p < 0.05 were deemed to be statistically significant.
Results
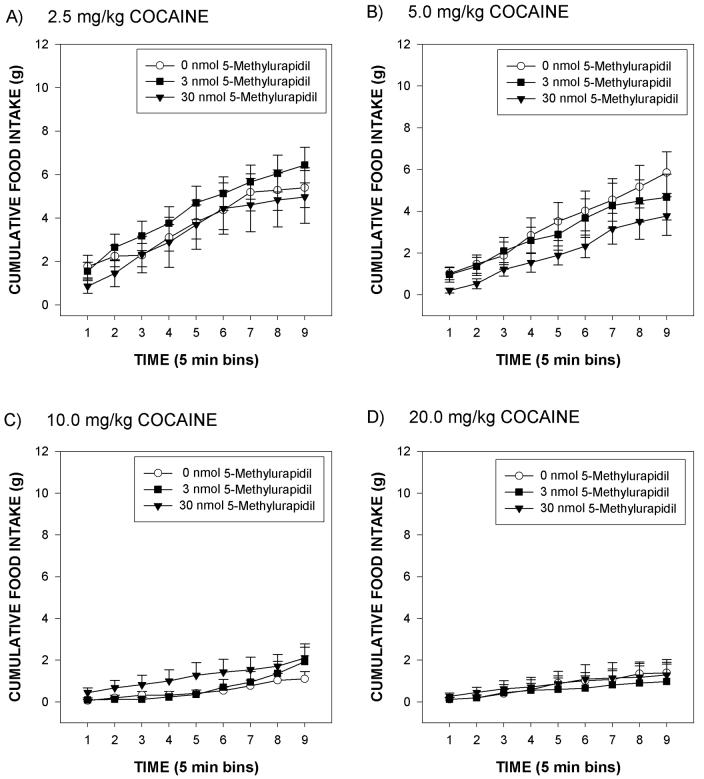
In the present study, rats readily consumed the mash diet after injection of vehicle with cumulative food intake values reaching an asymptote of approximately 8 g at postinjection 45 min (Figure 1A). ICV infusion of 5-MU increased cumulative food intake values in rats treated systemically with 0 mg/kg cocaine (Figure 1A). Systemic injection of cocaine produced a dose-dependent suppression of eating in rats treated with 0 nmol 5-MU (Figure 1B). Although the main effect of 5-MU [p = .226] was not significant in the ANOVA of the overall data, there was a significant 5-MU by time interaction [F(4,62) = 4.59, p < .003]. The capacity of 5-MU to increase food intake is unlikely to be a non-specific effect insasmuch as infusion of 5-MU did not significantly alter 45 min water intakes (mean group water intakes = 3.9, 2.9, and 3.5 mls, respectively). Additionally, the analyses indicated a significant effect of cocaine dose [F(4,124) = 57.5, p < .0001], of time [F(2,62) = 214.9, p < .0001] and a significant interaction between cocaine dose and time [F(8,248) = 19.0, p < .0001]. Further analyses indicated that 2.5 mg/kg cocaine significantly suppressed food intake relative to 0 mg/kg cocaine, that 5.0 mg/kg cocaine significantly suppressed food intake relative to 2.5 mg/kg cocaine, and that cocaine doses of 10 or 20 mg/kg induced near-total (and similar) suppressions of eating that were greater than that induced by 5 mg/kg cocaine [p < 0.05 each]. Finally, there were no significant interactions between 5-MU dose and cocaine dose (data depicted in Figure 2).
Figure 1.
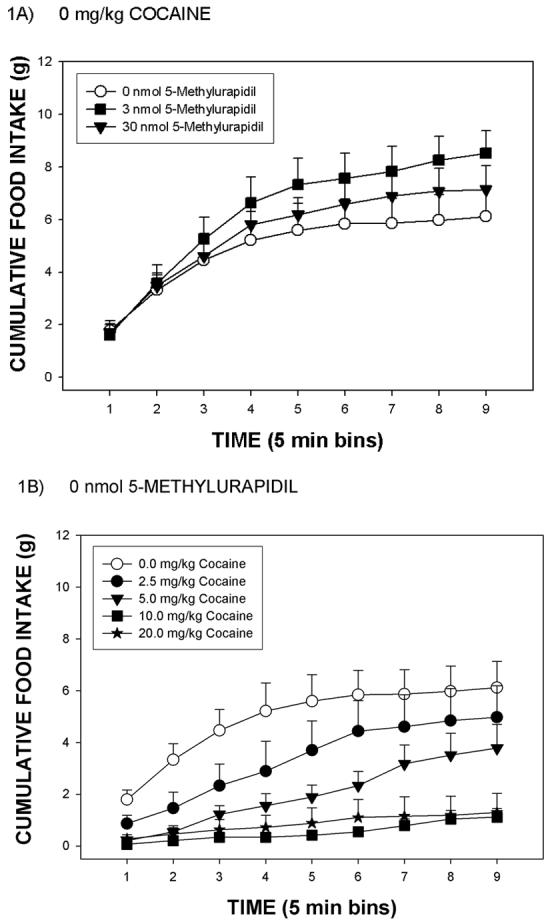
Panel A: Mean group cumulative changes in food intake (g) over a 45 min test period for rats infused icv with either 0, 3 or 30 nmol 5-methylurapidil and injected with vehicle (0 mg/kg cocaine). Panel B: Mean group cumulative changes in food intake (g) for rats infused icv with 0 nmol 5-methylurapidil and injected with 0, 2.5, 5.0, 10 and 20 mg/kg cocaine. The vertical line above each symbol represents the SEM.
Figure 2.
Mean group cumulative changes in food intake (g) over a 45 min test period for rats infused icv with either 0, 3 or 30 nmol 5-methylurapidil and then injected with 2.5 mg/kg cocaine (Panel A), 5.0 mg/kg cocaine (Panel B), 10 mg/kg cocaine (Panel C) and 20 mg/kg cocaine (Panel D). The vertical line above each symbol represents the SEM.
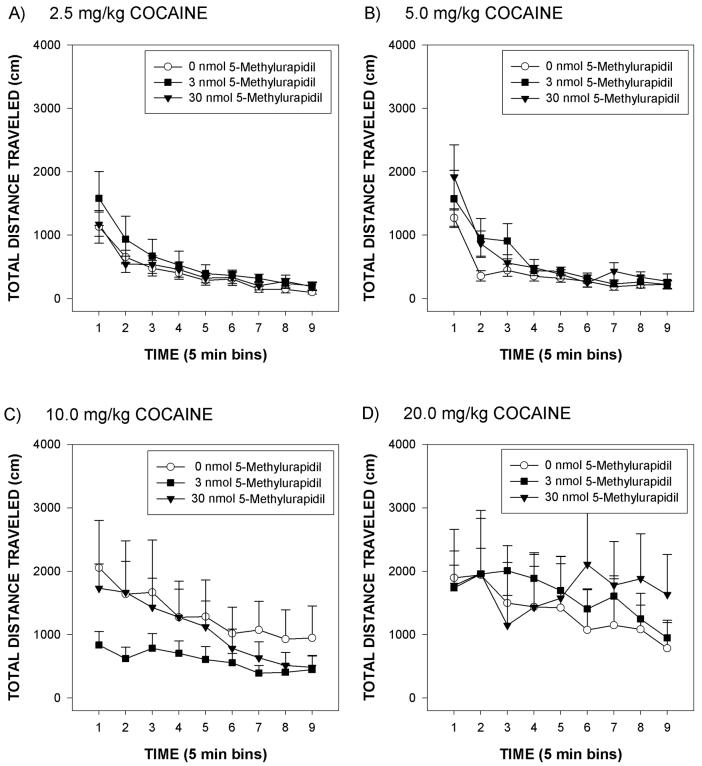
Changes in total distance traveled after cocaine or 5-MU are depicted in Figure 3. Rats infused icv with 0 nmol 5-MU and treated with 0 mg/kg cocaine traveled less than a 1000 cm in the first 5 min period, and decreased thereafter (Figure 3A). Whereas rats treated with 30 nmol 5-MU and 0 mg/kg cocaine were hyperphagic, these rats did not show significant changes in total distance traveled (Figure 3A). Rats treated with 0 nmol 5-MU and injected with various cocaine doses showed increased locomotion at cocaine doses of 10 or 20 mg/kg, but not at 2.5 or 5 mg/kg cocaine (Figure 3B). ANOVA of these data revealed a significant effect of cocaine dose [F(4,120) = 10.9, p < .0001], time [F(2,60) = 46.0, p < .0001] and cocaine by time [F(38,190) = 5.3, p < .001]. There was, however, no significant effect of 5-MU dose [p = 0.456] nor was there a significant interaction of the 5-MU dose condition with cocaine dose (data depicted in Figure 4).
Figure 3.
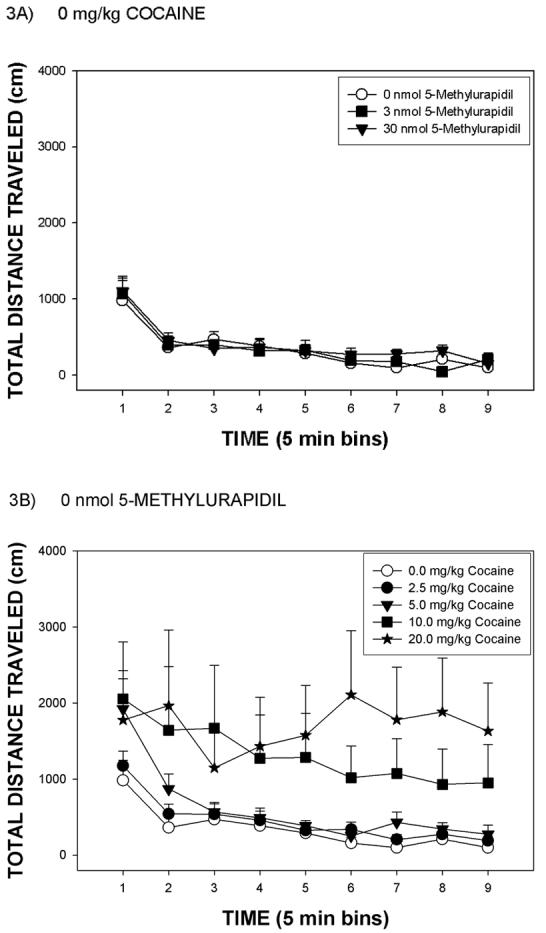
Panel A: Mean group total distance traveled (cm) over a 45 min test period for rats infused icv with either 0, 3 or 30 nmol 5-methylurapidil and injected with vehicle (0 mg/kg cocaine). Panel B: Mean group total distance traveled (cm) for rats infused icv with 0 nmol 5-methylurapidil and injected with 0, 2.5, 5.0, 10 and 20 mg/kg cocaine. The vertical line above each symbol represents the SEM.
Figure 4.
Mean group total distance traveled (cm) over a 45 min test period for rats infused icv with either 0, 3 or 30 nmol 5-methylurapidil and then injected with 2.5 mg/kg cocaine (Panel A), 5.0 mg/kg cocaine (Panel B), 10 mg/kg cocaine (Panel C) and 20 mg/kg cocaine (Panel D). The vertical line above each symbol represents the SEM.
Discussion
The major finding of the present study is that icv infusion of 5-methylurapidil, an antagonist at the α1A-AR subtype, significantly increased baseline food intake without an effect on forward locomotion. This pattern, along with the observation that icv 5-MU did not increase water intake, suggests a specific action of this α1A-AR antagonist on eating. Camargo et al., (2000) infused 5-MU into the lateral hypothalamus, at doses of 20-80 nmol, and did not find a change in water intake over a 4 hr post-infusion period. The observation that antagonism of α1A-ARs by 5-MU increased food intake is consistent with the proposition that α1-AR agonist drugs (e.g. cirazoline, phenylephrine, and phenylpropanolamine) generally function to suppress food intake (Wellman et al., 1993; Wellman, 2000). The fact that antagonism of α1A-ARs by 5-MU increased food intake suggests, but does not prove, that the α1A-AR subtype may mediate the hypophagia induced by α1-AR agonist drugs. A final conclusion will require studies in which 5-MU (and other α1A-AR antagonists) is used centrally to block the systemic effects of an α1-AR agonist such as cirazoline or phenylephrine on feeding.
An advantage of the concurrent eating/locomotion procedure is the potential to dissociate changes in eating from changes in forward locomotion (Wellman et al., 2005). Such a dissociation was evident in the present study in that no change in locomotion was noted in rats for which icv administration of the α1A-AR antagonist 5-MU increased baseline eating behavior. It is thus unlikely that forward locomotion interfered with eating in this study. Suppression of locomotion is induced by infusion of α1B-AR subtype antagonist drugs (Stone et al., 2001, 2003). This pattern suggests that the α1A-AR subtype modulates eating but not locomotion, which is modulated by the α1B-AR subtype (Drouin et al., 2002a,b).
In the present study, cocaine produced dose-dependent increases in forward locomotion. It should be noted that cocaine significantly suppressed eating at doses of 2.5 mg/kg and higher, whereas forward locomotion was significantly increased only for cocaine doses at or above 5 mg/kg. The failure to note an effect of icv 5-MU on locomotion is unlikely to represent a floor effect in that in another study, we were able to detect a suppressive effect of nicotine on locomotion using this procedure (Wellman et al., 2005). A ceiling effect is also unlikely given that the rats were adapted to the chambers prior to drug testing, which functions to lower baseline forward locomotion (Miller et al., 1998) and because cocaine alone increased forward locomotion.
Cocaine is a mixed acting monoamine agonist that exerts effects on dopamine, norepinephrine, and serotonin pathways (Rothman et al., 2001). Norepinephrine, acting through α1B-ARs, has been reported to modulate the locomotor and reinforcing effects of cocaine and of amphetamine (Drouin et al., 2002a,b). In the present study, cocaine was shown to exert potent effects across a range of doses on concurrent measures of eating and on locomotion. There was, however, no evidence generated in the present study that supported a role for α1A-ARs for the hypophagic or hyperlocomotor effects of cocaine. This negative outcome is tentative given that a limited range of doses for a single α1A-AR antagonist was used in the present study.
Acknowledgements
The present study was supported by NIDA R21 DA017230 to PJW. Portions of the study were presented at the annual meeting of the Society for Neuroscience, San Diego, 2007.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balapole DC, Hansult CD, Dorph D. Effect of cocaine on food intake in rats. Psychopharmacology. 1979;64:121–122. doi: 10.1007/BF00427356. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behavioral Brain Research. 2002;137(12):165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Bassareo V, DiChiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology. 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Cepeda-Benito A, Wellman PJ. Meal patterns in male rats during and after intermittent nicotine administration. Pharmacology Biochemistry and Behavior. 2003;74:495–504. doi: 10.1016/s0091-3057(02)01033-x. [DOI] [PubMed] [Google Scholar]
- Berthold CW, 3rd., Gonzales RA, Moerschbaecher JM. Prazosin attenuates the effects of cocaine on motor activity but not on schedule-controlled behavior in the rat. Pharmacology Biochemistry and Behavior. 1992;43:111–115. doi: 10.1016/0091-3057(92)90646-w. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Herve D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical D-amphetamine injection. European Journal of Neuroscience. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Blavet N, DeFeudis FV. Inhibition of food intake in the rat. Neurochemical Research. 1982;7:339–348. doi: 10.1007/BF00965645. [DOI] [PubMed] [Google Scholar]
- Blosser JC, Barrantes M, Parker RB. Correlation between anorectic potency and affinity for hypothalamic (+)-amphetamine binding sites of phenylethylamines. European Journal of Pharmacology. 1987;134:97–103. doi: 10.1016/0014-2999(87)90136-1. [DOI] [PubMed] [Google Scholar]
- Burridge SB, Blundell JE. Amphetamine anorexia: antagonism by typical, but not atypical neuroleptics. Neuropsychopharmacology. 1979;18:453–457. doi: 10.1016/0028-3908(79)90069-8. [DOI] [PubMed] [Google Scholar]
- Camargo LA, Saad W,A, Camargo GP. Effects of subtypes alpha- and beta-adrenoceptors of the lateral hypothalamus on the water and sodium intake induced by angiotensin II injected into the subfornical organ. Brain Research. 2000;881(2):176–181. doi: 10.1016/s0006-8993(00)02840-7. [DOI] [PubMed] [Google Scholar]
- Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiology and Behavior. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine-seeking behavior in rats. Drug and Alcohol Dependence. 1985;6(2):95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110(12):5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, van der Hoek GA. Cocaine: a microstructural analysis of its effects on feeding and associated behaviour in the rat. Brain Research. 1993;608:45–51. doi: 10.1016/0006-8993(93)90772-f. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenalinedopamine coupling in the locomotor activating effects of D-amphetamine. Journal of Neuroscience. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002a;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. Alpha1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. Journal of Neuroscience. 2002b;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley C, Cook JA, Spar BD, Austin TM, Kowalski TJ. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obesity Research. 2003;11:845–851. doi: 10.1038/oby.2003.116. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Hernandez L, Schwartz DH, Mark GP, Hunter GA. Microdialysis studies of brain norepinephrine, serotonin, and dopamine release during ingestive behavior. Theoretical and clinical implications. Annals of the New York Academy of Science. 1989;575:171. doi: 10.1111/j.1749-6632.1989.tb53242.x. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. British Journal of Pharmocology. 1997;121:1613–1618. doi: 10.1038/sj.bjp.0701311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF. Amphetamine: possible site and mode of action for producing anorexia in the rat. Brain Research. 1975;84:160–167. doi: 10.1016/0006-8993(75)90811-2. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Rossakis C. Pharmacological characterization of perifornical hypothalamic dopamine receptors mediating feeding inhibition in the rat. Brain Research. 1979;172:115–130. doi: 10.1016/0006-8993(79)90899-0. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. Alpha 1B-adrenoceptor-mediated excitation of piriform cortical interneurons. European Journal of Pharmacology. 1996;305(13):95–100. doi: 10.1016/0014-2999(96)00158-6. [DOI] [PubMed] [Google Scholar]
- Miller DK, McMahon LR, Green TA, Nation JR, Wellman PJ. Repeated administration of ephedrine induces behavioral sensitization in rats. Psychopharmacology. 1998;140(1):52–56. doi: 10.1007/s002130050738. [DOI] [PubMed] [Google Scholar]
- Mitchell JC, Jackson HC, Heal DJ. Effects of monoamine antagonists on aminorex, phentermine and d-amphetamine hypophagia. Journal of Psychopharmocology. 1998;12:A38. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. fifth ed. Academic Press; New York: 2005. [Google Scholar]
- Rapoza D, Woolverton WL. Attenuation of the effects of cocaine on milk consumption in rats by dopamine antagonists. Pharmacology Biochemistry and Behavior. 1991;40:133–137. doi: 10.1016/0091-3057(91)90334-x. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Specker SM, Lac ST, Carroll ME. Food deprivation history and cocaine self-administration: an animal model of binge eating. Pharmacology Biochemistry and Behavior. 1994;48(4):1025–1029. doi: 10.1016/0091-3057(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Itteera A, Quartermain D. Pharmacological evidence for the role of central alpha 1B-adrenoceptors in the motor activity and spontaneous movement of mice. Neuropharmacology. 2001;40:54–61. doi: 10.1016/s0028-3908(00)00151-9. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Rosengarten H, Kramer HK, Quartermain D. Emerging evidence for a central epinephrine-innervated alpha 1-adrenergic system that regulates behavioral activation and is impaired in depression. Neuropsychopharmacology. 2003;28:1387–1399. doi: 10.1038/sj.npp.1300222. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Current Drug Targets. 2005;6(2):191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davies BT, Morien A, McMahon L. Modulation of feeding by hypothalamic paraventricular nucleus α1- and α2-adrenergic receptors. Life Sciences. 1993;53:669–679. doi: 10.1016/0024-3205(93)90243-v. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Ho DH, Cepeda-Benito A, Bellinger LL, Nation JR. Cocaine-induced hypophagia and hyperlocomotion in rats attenuated by prazosin. European Journal of Pharmacology. 2002;455:117–126. doi: 10.1016/s0014-2999(02)02616-x. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Ho DH, Davis KW. Concurrent measures of feeding and locomotion in rats. Physiology of Behavior. 2005;84(5):769–774. doi: 10.1016/j.physbeh.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Miller DK, Ho DH. Noradrenergic modulation of ephedrine-induced hypophagia. Synapse. 2003;48(1):18–24. doi: 10.1002/syn.10182. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94(4):469–492. [PubMed] [Google Scholar]