Abstract
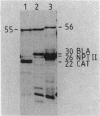
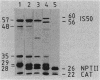
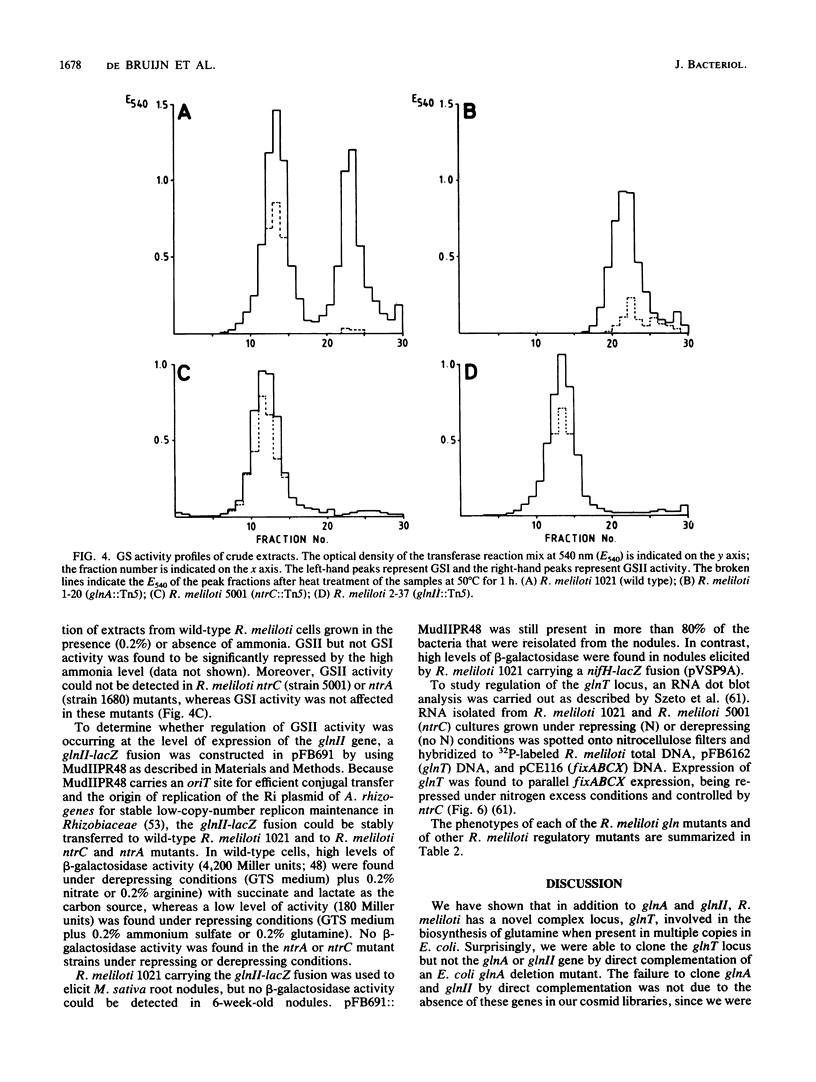
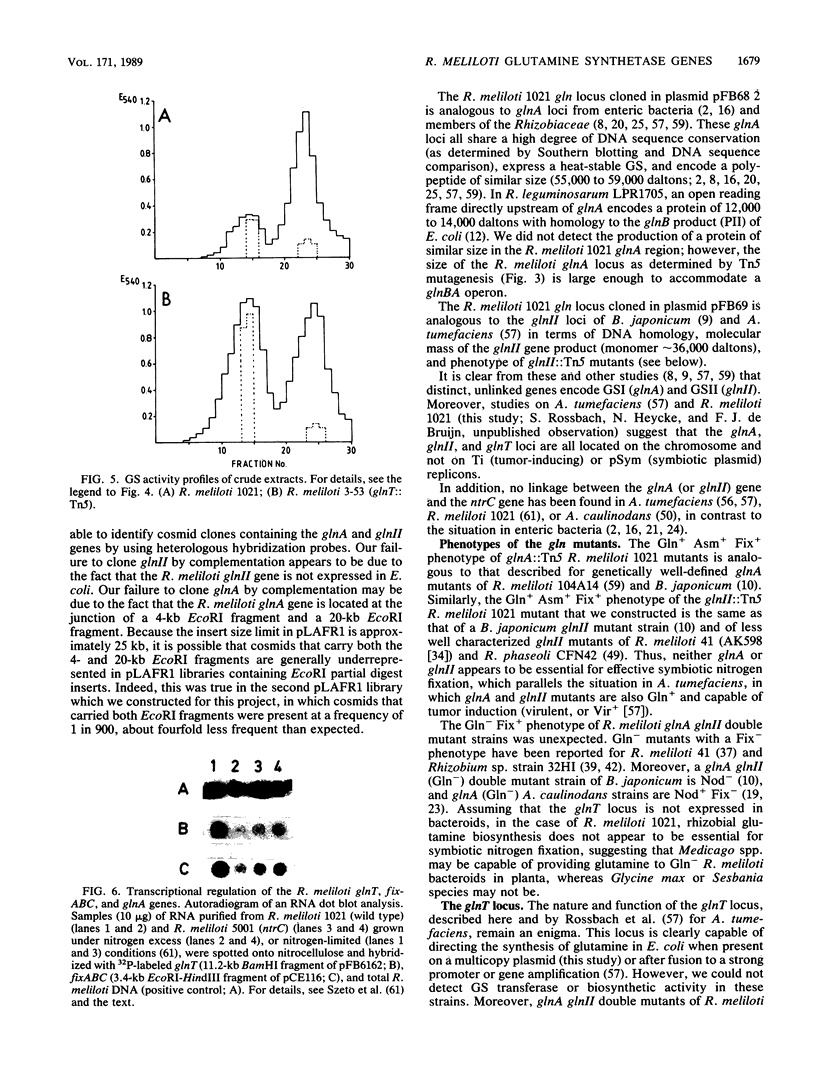
We have cloned and characterized three distinct Rhizobium meliloti loci involved in glutamine biosynthesis (glnA, glnII, and glnT). The glnA locus shares DNA homology with the glnA gene of Klebsiella pneumoniae, encodes a 55,000-dalton monomer subunit of the heat-stable glutamine synthetase (GS) protein (GSI), and complemented an Escherichia coli glnA mutation. The glnII locus shares DNA homology with the glnII gene of Bradyrhizobium japonicum and encodes a 36,000-dalton monomer subunit of the heat-labile GS protein (GSII). The glnT locus shares no DNA homology with either the glnA or glnII gene and complemented a glnA E. coli strain. The glnT locus codes for an operon encoding polypeptides of 57,000, 48,000, 35,000, 29,000, and 28,000 daltons. glnA and glnII insertion mutants were glutamine prototrophs, lacked the respective GS form (GSI or GSII), grew normally on different nitrogen sources (Asm+), and induced normal, nitrogen-fixing nodules on Medicago sativa plants (Nod+ Fix+). A glnA glnII double mutant was a glutamine auxotroph (Gln-), lacked both GSI and GSII forms, but nevertheless induced normal Fix+ nodules. glnT insertion mutants were prototrophs, contained both GSI and GSII forms, grew normally on different N sources, and induced normal Fix+ nodules. glnII and glnT, but not glnA, expression in R. meliloti was regulated by the nitrogen-regulatory genes ntrA and ntrC and was repressed by rich N sources such as ammonium and glutamine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Broughton W. J., Dilworth M. J. Control of leghaemoglobin synthesis in snake beans. Biochem J. 1971 Dec;125(4):1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Guerinot M. L., Chelm B. K. Characterization of the gene encoding glutamine synthetase I (glnA) from Bradyrhizobium japonicum. J Bacteriol. 1985 May;162(2):698–703. doi: 10.1128/jb.162.2.698-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T. A., Martin G. B., Chelm B. K. Differential transcription of the two glutamine synthetase genes of Bradyrhizobium japonicum. J Bacteriol. 1987 Dec;169(12):5861–5866. doi: 10.1128/jb.169.12.5861-5866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna-Romano S., Riccio A., Guida M., Defez R., Lamberti A., Iaccarino M., Arnold W., Priefer U., Pühler A. Tight linkage of glnA and a putative regulatory gene in Rhizobium leguminosarum. Nucleic Acids Res. 1987 Mar 11;15(5):1951–1964. doi: 10.1093/nar/15.5.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald R. G., Ludwig R. A. Rhizobium sp. strain ORS571 ammonium assimilation and nitrogen fixation. J Bacteriol. 1984 Jun;158(3):1144–1151. doi: 10.1128/jb.158.3.1144-1151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espin G., Alvarez-Morales A., Cannon F., Dixon R., Merrick M. Cloning of the glnA, ntrB and ntrC genes of Klebsiella pneumoniae and studies of their role in regulation of the nitrogen fixation (nif) gene cluster. Mol Gen Genet. 1982;186(4):518–524. doi: 10.1007/BF00337959. [DOI] [PubMed] [Google Scholar]
- Filser D. M., Moscatelli C., Lamberti A., Vincze E., Guida M., Salzano G., Iaccarino M. Characterization and cloning of two Rhizobium leguminosarum genes coding for glutamine synthetase activities. J Gen Microbiol. 1986 Sep;132(9):2561–2569. doi: 10.1099/00221287-132-9-2561. [DOI] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Comparative properties of glutamine synthetases I and II in Rhizobium and Agrobacterium spp. J Bacteriol. 1980 Nov;144(2):641–648. doi: 10.1128/jb.144.2.641-648.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. L., Keister D. L. Identification of two glutamine synthetases in Agrobacterium. J Bacteriol. 1980 Feb;141(2):996–998. doi: 10.1128/jb.141.2.996-998.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Ronson C. W., Ausubel F. M. Regulation of nitrogen fixation genes. Annu Rev Genet. 1986;20:567–591. doi: 10.1146/annurev.ge.20.120186.003031. [DOI] [PubMed] [Google Scholar]
- Hirschman J., Wong P. K., Sei K., Keener J., Kustu S. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7525–7529. doi: 10.1073/pnas.82.22.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Hunt T. P., Magasanik B. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8453–8457. doi: 10.1073/pnas.82.24.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koduri R. K., Gots J. S. A DNA-binding protein with specificity for pur genes in Escherichia coli. J Biol Chem. 1980 Oct 25;255(20):9594–9598. [PubMed] [Google Scholar]
- Ludwig R. A. Control of ammonium assimilation in Rhizobium 32H1. J Bacteriol. 1978 Jul;135(1):114–123. doi: 10.1128/jb.135.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Regulation of Rhizobium nitrogen fixation by the unadenylylated glutamine synthetase I system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5817–5821. doi: 10.1073/pnas.77.10.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A., Signer E. R. Glutamine synthetase and control of nitrogen fixation in Rhizobium. Nature. 1977 May 19;267(5608):245–248. doi: 10.1038/267245a0. [DOI] [PubMed] [Google Scholar]
- Maas R. An improved colony hybridization method with significantly increased sensitivity for detection of single genes. Plasmid. 1983 Nov;10(3):296–298. doi: 10.1016/0147-619x(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Mason H. D., Sagle M., Polson D. W., Kiddy D., Dobriansky D., Adams J., Franks S. Reduced frequency of luteinizing hormone pulses in women with weight loss-related amenorrhoea and multifollicular ovaries. Clin Endocrinol (Oxf) 1988 Jun;28(6):611–618. doi: 10.1111/j.1365-2265.1988.tb03852.x. [DOI] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. R., Darrow R. A., Keister D. L. Effect of oxygen tension on nitrogenase and on glutamine synthetases I and II in Rhizobium jaonicum 61A76. Biochem Biophys Res Commun. 1978 Mar 15;81(1):224–231. doi: 10.1016/0006-291x(78)91653-4. [DOI] [PubMed] [Google Scholar]
- Ratet P., Schell J., de Bruijn F. J. Mini-Mulac transposons with broad-host-range origins of conjugal transfer and replication designed for gene regulation studies in Rhizobiaceae. Gene. 1988;63(1):41–52. doi: 10.1016/0378-1119(88)90544-6. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Albright L. M., Ausubel F. M. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J Bacteriol. 1987 Jun;169(6):2424–2431. doi: 10.1128/jb.169.6.2424-2431.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach S, Schell J, de Bruijn F J. The ntrC gene of Agrobacterium tumefaciens C58 controls glutamine synthetase (GSII) activity, growth on nitrate and chromosomal but not Ti-encoded arginine catabolism pathways. Mol Gen Genet. 1987 Oct;209(3):419–426. doi: 10.1007/BF00331144. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville J. E., Kahn M. L. Cloning of the glutamine synthetase I gene from Rhizobium meliloti. J Bacteriol. 1983 Oct;156(1):168–176. doi: 10.1128/jb.156.1.168-176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V., Jones J. D., Ow D. W., Ausubel F. M. Klebsiella pneumoniae nifA product activates the Rhizobium meliloti nitrogenase promoter. Nature. 1983 Feb 24;301(5902):728–732. doi: 10.1038/301728a0. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Nixon B. T., Ronson C. W., Ausubel F. M. Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J Bacteriol. 1987 Apr;169(4):1423–1432. doi: 10.1128/jb.169.4.1423-1432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Ausubel F. M. The cloning and transposon Tn5 mutagenesis of the glnA region of Klebsiella pneumoniae: identification of glnR, a gene involved in the regulation of the nif and hut operons. Mol Gen Genet. 1981;183(2):289–297. doi: 10.1007/BF00270631. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]
- de Bruijn F. J. Transposon Tn5 mutagenesis to map genes. Methods Enzymol. 1987;154:175–196. doi: 10.1016/0076-6879(87)54077-0. [DOI] [PubMed] [Google Scholar]