Abstract
The role of cellular interactions in the mechanism of secondary cone photoreceptor degeneration in inherited retinal degenerations in which the mutation specifically affects rod photoreceptors was studied. We developed an organ culture model of whole retinas from 5-week-old mice carrying the retinal degeneration mutation, which at this age contain few remaining rods and numerous surviving cones cocultured with primary cultures of mixed cells from postnatal day 8 normal-sighted mice (C57BL/6) retinas or retinal explants from normal (C57BL/6) or dystrophic (C3H/He) 5-week-old mice. After 7 days, the numbers of residual cone photoreceptors were quantified after specific peanut lectin or anti-arrestin antibody labeling by using an unbiased stereological approach. Examination of organ cultured retinas revealed significantly greater numbers of surviving cones (15–20%) if cultured in the presence of retinas containing normal rods as compared with controls or cocultures with rod-deprived retinas. These data indicate the existence of a diffusible trophic factor released from retinas containing rod cells and acting on retinas in which only cones are present. Because cones are responsible for high acuity and color vision, such data could have important implications not only for eventual therapeutic approaches to human retinal degenerations but also to define interactions between retinal photoreceptor types.
Despite recent advances, our understanding of the mechanisms underlying visual malfunction and cell loss in retinitis pigmentosa (RP) has provided few clinically significant clues to improve retinal cell survival (1–3). RP forms a group of inherited photoreceptor dystrophies characterized largely by early features of rod photoreceptor impairment (1, 4). Most identified forms of RP are caused by defects in proteins restricted to rod photoreceptors (5–8). Yet, at various stages of the disease, a loss of the photopic function reflecting degeneration of cone photoreceptors is found consistently (1, 4). Of extreme importance for the patient, cone cell death and its functional consequences appear to be secondary events. No current data exist to account for cone cell death, but observations made on animal models of RP provide arguments for cone survival depending on the presence of rods. In several models with selective elimination of rods, secondary loss of cones is observed (9–11). The cause of rod cell death in these animals, either transgenic or spontaneous mutants, cannot account for direct cone cell loss (9–17). Putative mechanisms of cone cell death include the liberation of endotoxins by degenerating rods, environmental alteration, or deprivation of rod-derived trophic factor(s). Recent studies using chimeras of normal and transgenic mice carrying mutations in rods showed that photoreceptor cell death was diffuse rather than restricted to areas with mutations in rods, providing evidence for a role of cellular interactions between photoreceptors in survival and degeneration of these cells (18). Based on these data, the authors suggested that photoreceptors draw from a common pool of trophic factor necessary for photoreceptor survival.
Rod photoreceptor degeneration caused by a mutation in the β subunit of rod cGMP phosphodiesterase has been described in both the retinal degeneration (rd) mouse and in human RP (6, 9, 16). In the rd mouse, a differential effect of the mutation on rods and cones was demonstrated by Carter-Dawson et al. (10, 11, 19); that is, cones die after rods. This model appears appropriate to test the hypothesis of the dependence of cones on the viability of rods. Indeed, we found that transplantation of rod-rich photoreceptor transplants to these animals at an age when most rods have disappeared induced a significant increase in host cone survival (20).
To search for rod-derived trophic factors influencing cone survival, we used an in vitro model permitting a more controlled and reproducible assessment of photoreceptor cell interactions in the rd mouse retina. We demonstrate the existence of such a factor. In view of the importance of cones for high acuity vision and color perception (21), such a beneficial effect has important implications for both the understanding of retinal degeneration and the development of therapies.
MATERIALS AND METHODS
Experimental procedures adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research.
Animals and Tissue Preparation.
Both normal C57BL/6 and mutant C3H/He strains were obtained from both Janvier Animal Suppliers, Le Genest St. Isle, France (C3H/He/J) and Charles River France, Saint-Aubain-Lès-Elbeuf, France (C3H/He/N). All animals were reared in cyclic light (12 hr on/12 hr off, with a room illumination of ≈15 lux) at 25°C from birth. The animals were killed with anaesthetic overdose: i.e., 0.5 ml of ketamine (Imalgene 500, 5 mg/ml or 140 mg/kg body weight; Rhone Merieux, Lyon, France). After enucleation and removal of the anterior segment, the retina was separated from the posterior segment in DMEM. Retinas destined for organ culture experiments were isolated from 5-week-old C3H mice homozygous for the rd locus (C3H/He, rd/rd). This age was selected in view of earlier work (17, 18) showing that, at this age, almost no rods are left whereas >50% of cones are still present. Retinas from rd mice aged 5 and 6 weeks also were isolated to estimate the cone cell loss during the sixth 1-week period in vivo.
Tissue Culture.
After isolation, retinas were flattened gently at the bottom of culture wells, the photoreceptor layer facing up, in 0.5 ml DMEM. Control retinas were cultured in DMEM only for 7 days whereas treated retinas were cocultured for the same period under Falcon minicell inserts containing dissociated cell cultures prepared from retinas of postnatal day 8 normal or rd mice (22) or intact explanted retinas from 5-week-old normal or rd mice, respectively. This experimental system avoided direct contacts between the populations. The first experimental series using C3H/He/J mice comprised:
retinas cultured in DMEM alone (n = 12);
retinas co-cultured with dissociated cells from two 8-day normal mouse retinas (n = 12);
retinas co-cultured with dissociated retinal cells from two 8-day rd retinas (n = 12);
retinas co-cultured with 2 whole retinal explants from 5 week normal mouse retinas (n = 12);
retinas co-cultured with 2 whole retinal explants from 5 week rd mouse retinas (few remaining rod photoreceptors) (n = 12);
The same as for condition 5 but using four explanted rd retinas, to compensate for mass differences between 5 week C57BL/6 and rd retinas (n = 10).
All controls included the retina from the fellow eye. An equal number of right and left eyes were studied in each experimental setting, and all retinas in this series were processed for peanut agglutinin (PNA) labeling. A second independent series of cultures prepared from C3H/He/N mice examining only conditions 1 and 2 (n = 10 for each treatment) and using an antibody rather than lectin cytochemistry to visualize cones (see below) also was performed.
Morphological and Immunocytochemical Analysis.
After 7 days in culture, the retinas were fixed for 1 hr with 4% paraformaldehyde, rinsed in PBS, permeabilized in PBS containing 0.1% Triton X-100 (5 min), and immersed in PBS containing 0.1% BSA and 0.1% Tween 20 (solution A) for 30 min. The different probes used were screened initially on intact retinas isolated from 5-week normal and rd mice to ensure their specificity. Labeling of cones in cultured retinas was performed by using the following methods, with all incubations being performed in solution A: (i) Incubation in PNA lectin from Arachis hypogae (Sigma) (50 μg/ml for 1 hr) (23) followed by anti-PNA polyclonal antibody (Chemicon) (10 μg/ml for 1 hr) and finally with goat-anti rabbit IgG-Texas Red (Molecular Probes) (10 μg/ml for 1 hr). In some trials, cultured retinas were incubated in a mix of PNA-biotin (Sigma) (50 μg/ml) and anti-arrestin polyclonal antibody (generous gift of I. Gery, National Institutes of Health) (10 μg/ml) for 2 hr and were visualized with a mix of Streptavidin-Texas Red (5 μg/ml) and goat-anti rabbit IgG-BODIPY FL (both from Molecular) (5 μg/ml) for 1 hr; (ii) Incubation in anti-arrestin polyclonal antibody and rho-4D2 anti-opsin mAb (24) (10 μg/ml each) for 2 hr, then incubation in goat anti-rabbit IgG-Texas Red (10 μg/ml) and goat anti-mouse IgG-BODIPY FL (10 μg/ml for 1 hr). After extensive rinsing, the retinas were flat mounted in PBS/glycerol (1:1), with the photoreceptor layer facing up, and were examined by using a Nikon Optiphot 2 fluorescence photomicroscope equipped with the corresponding filter sets.
Cell Counting.
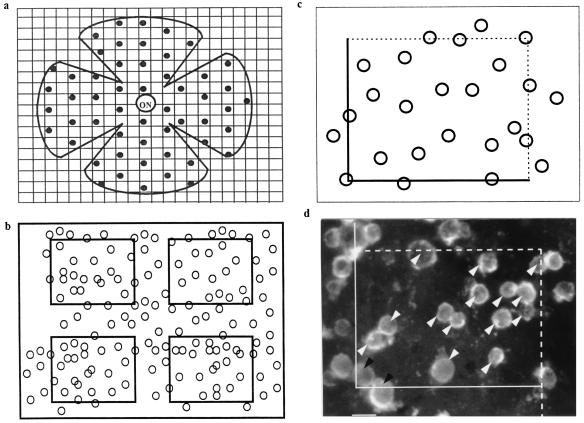
The total numbers of labeled cones were estimated in the flat-mounted retinas by using a stereological approach to obtain unbiased samples (25). Because the surviving cones form a monolayer, the optical dissector method aimed at avoiding oversampling errors could be restricted to a sharp focus on every single counted area. The cells were counted on 200 sampled, nonoverlapping 1,825-μm2 zones determined in a systematic random fashion to sample equally the whole retinal surface extending from the center of the optic nerve head over a radius of 2 mm (Fig. 1). The whole retinal surface that did not differ between the various experimental paradigms represented, on average, 12.8 mm2 and was divided into 1,600 fields of 8,000 μm2-areas. Among these, 50 fields distributed throughout the whole retinal surface were chosen with a stage encoder by using a systematic random sampling procedure: An initial random choice was made within the first measurement interval, and all subsequent sampling was performed at predetermined intervals throughout the entire retinal surface. Each field was observed by using a Nikon Plan 40× objective with a numerical aperture of 0.70 differential interference contrast microscopy using fluorescence optics on an Optiphot 2 Nikon photomicroscope equipped with a Sony trinitron color graphic display camera (Sony, Tokyo). Each image was focused on a plane allowing reliable counting of all cones present in the area under observation, was digitized by using a Multiscan 17se II (Iilyama, Croissy-Beaubourg, France) and a Matrox Millenium (2 M) graphic card (Matrox, Quebec, Canada), and was stored. By using the software automator for Windows provided by Biocom (Paris), the 50 fields were zoomed and divided into 4 fields of 1,825 μm2. Cell counts were performed on the 200 fields viewed on the computer screen by using an unbiased counting frame (25) (Fig. 1). This strategy for sampling was calibrated previously by comparison of the estimates with numbers obtained after counting of the entire retina with double and triple sample numbers.
Figure 1.
(a) Systematic random sampling of the retinal surface for stereological counting. ON, optic nerve. (b) Each sample was subdivided into four counting fields after focusing and digitization. (c) Counting was performed by using a stereological dissector. (d) Example of a counted sample from a 5-week-old rd mouse retina cocultured for 8 days with dissociated cells from 8-day-old C57 rod-containing retina. White arrow head, counted cells; black arrow head, excluded cells. (Bar = 5 μm.)
Statistical Analysis.
For comparisons of cocultured vs. control retinas, difference analysis was performed with the StatWorks Data student’s t statistic software (Cricket Software, Malvern, PA) by using the parametric method of Student’s t test for unpaired series (26) for one variable: i.e., the total cone number estimates per retina. Because the numbers found in control cultured C3H/He/J retinas did not vary significantly (mean ± SD = 102118 ± 6436), a series of 24 PNA-labeled controls taken from all experiments using this strain were used for comparison. The use of Student’s t test for paired series comparing the numbers in paired cocultured and control retinas did not change the significance of the data.
RESULTS
Cytochemical Labeling.
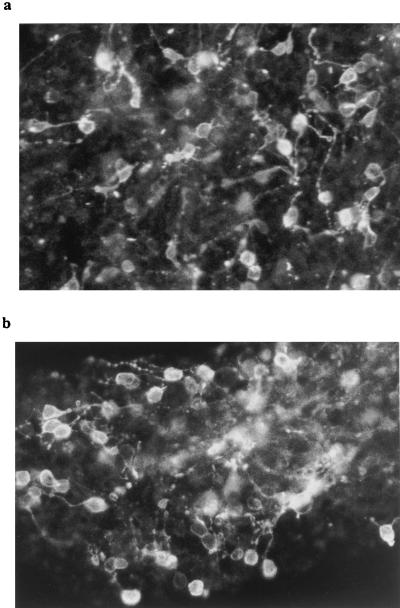
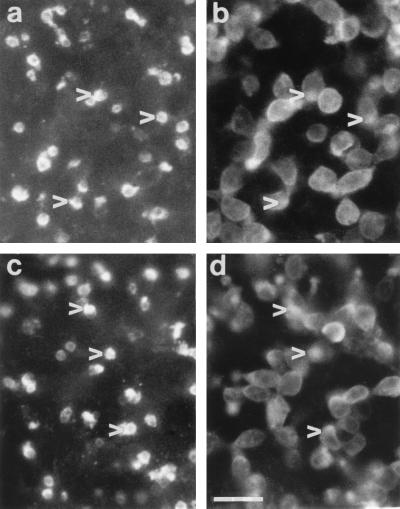
Rod photoreceptors in dissociated cell cultures of 8-day normal and rd retinas were identified by using rho-4D2 and represented in both cases ≈50% total neurons (Fig. 2). The specificity of labeling of the different lectin and antibody probes was verified by using in vivo and in vitro normal and rd retinal tissue. Double labeling of rd retina with PNA and anti-arrestin antibody showed that the former stained only the photoreceptor outer segment region whereas the latter stained the entire cell body, inner and outer segment (data not shown). Double labeling with anti-arrestin and anti-opsin antibodies showed that opsin-immunopositive rods formed a minor subset of the arrestin-immunopositive cells (both rods and cones). PNA labeling of cultured retinas revealed small rounded structures present exclusively at the outer surface (Fig. 3 a and c). Double immunolabeling of some retinas with arrestin antiserum revealed underlying cell bodies and small irregular outer segments overlapping with the PNA stain (Fig. 3 b and d). Correspondence between the two labels did not change between control and test retinas (Fig. 3a–d). In addition, cross sections taken from PNA-labeled cultured retinas that were reembedded confirmed that the labeling was restricted to superficially located cones (Fig. 4).
Figure 2.
Five-day in vitro cultures of normal (a) and rd (b) mice retinal cells from postnatal day 8 animals, stained with rho-4D2 anti-rod opsin antibody. In both cases, numerous immunoreactive cells are visible. (Bar = 10 μm.)
Figure 3.
Explanted rd mice retinas cultured for 1 week in DMEM alone (a and b) or in the presence of dissociated young retinal cells (c and d), then double immunolabeled with PNA lectin (a and c) and arrestin antiserum (b and d). The same fields are shown in a and b and in c and d and reveal that PNA lectin labels rounded matrix-associated structures protruding from the retinal surface whereas arrestin is present throughout the photoreceptor cell bodies and outer segments. The differing focal plane of the two labels makes superimposition difficult, but the overlapping nature of the staining pattern can be observed (corresponding arrowheads in paired fields a and b and in paired fields c and d). (Bar = 10 μm.)
Figure 4.
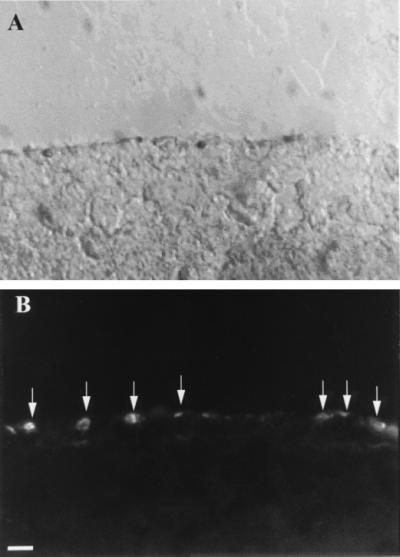
Cross section through a PNA-labeled flat-mounted retina, showing that labeling is restricted to cones. (a) Nomarski image of outer 50 μm of retina; (b) The same field viewed by fluorescence microscopy, showing lectin staining present only at the surface (arrows). (Bar = 10 μm.)
Cone Losses During the Sixth Postnatal Week in Vivo.
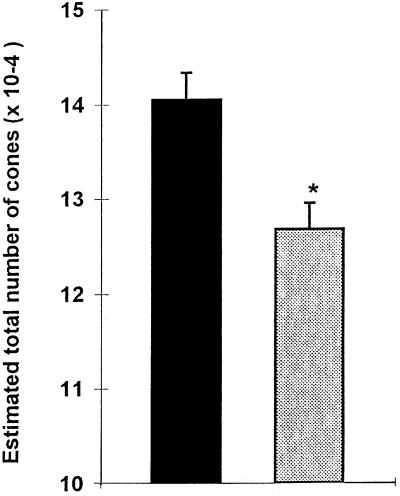
The stereological method used as described in Materials and Methods for estimating the total number of PNA-labeled cones allowed us to detect changes in cone numbers over a short period of time. The average number of PNA-labeled cones in 5-week-old C3H/He/J mice in vivo was 140508 ± 7008 (mean ± SD), which decreased by 10% to 126682 ± 6816 at 6 weeks (Fig. 5). This difference was statistically significant (P < 0.01). The number of PNA-labeled cones in C3H/He/N retinas was ≈25% less than in C3H/He/J mice (data not shown).
Figure 5.
Estimated total number of cones (mean ± SD) in retinas from 5-week and 6-week rd mice in vivo. *P < 0.01.
Cone Changes in the Coculture Models.
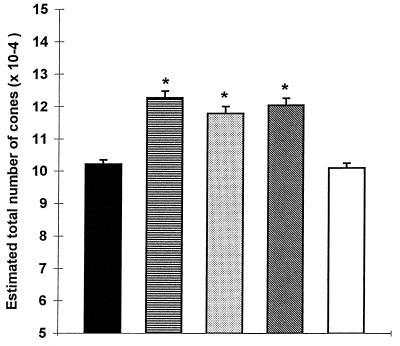
In the C3H/He/J strain, PNA labeling of 5-week-old rd mouse retinas cultured alone (control) for 8 days revealed 102118 ± 6436 (mean ± SD) cones (Fig. 6). In contrast, in retinas cocultured with cells from 8-day-old rd or normal retinas, the average number of PNA-labeled cones was 117805 ± 7581 (15% increase) and 122692 ± 7384 (20% increase), respectively. Statistical analysis with the Student’s t test provided P value <0.0001 (t statistics −6.498 and −8.611, respectively, with 34° of freedom). Rd retinas cocultured with 5-week-old normal retinal explants also showed significantly higher numbers of PNA-labeled cones, 120359 ± 7462 (t-statistic −7.603, 34° of freedom, P < 0.0001). No such difference was found when the mutant retina was cocultured with 5-week-old rd mice retinal explants (100875 ± 5420 in controls vs.102118 ± 6436) (t statistic 0.574, 34° of freedom, P > 0.5). When the number of 5-week rd retinas in the cocultures was increased to four (approximately the same total tissue mass as two 5-week normal retinas), we observed a significant reduction in PNA-labeled cone numbers in the target rd retina (77589 ± 2852) (Fig. 6).
Figure 6.
Estimated total number of cones (mean ± SD) in 5-week-old rd (C3H/He/J) mice retinas cultured in DMEM alone and from 5-week-old rd mice retinas cocultured with cells from 8-day-old normal mice retinas with cells from 8-day-old rd mice retinas, with retinal explants from 5-week-old normal mice, and with retinal explants from 5-week-old rd mice. *P < 0.0001.
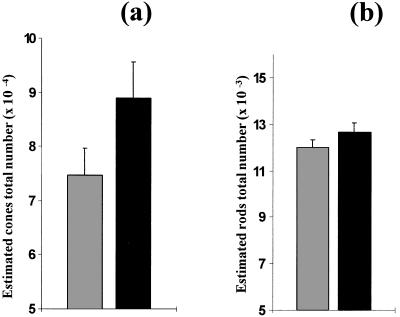
In the second series using cocultures of C3H/He/N retinas in which both rod and cone numbers were estimated through double immunolabeling using anti-rod opsin and -arrestin antibodies (rods being immunolabeled by both antibodies whereas cones were stained uniquely with anti-arrestin antibody), lower overall cone numbers were scored, but the ratio of the difference between controls and those cocultured in the presence of dissociated cells from 8-day normal mice retinas was similar (74818 ± 4901 vs. 88911 ± 6796, or an increase of 19%) (Fig. 7a). Rods represented ≈10% total photoreceptor numbers, and, in contrast to cones, their numbers were not altered significantly by coculture (11988 ± 341 vs. 12652 ± 429) (Fig. 7b).
Figure 7.
Estimated total number (mean ± SD) of cones (a) and rods (b) in retinas from 5-week-old rd mice (C3H/He/N) cultured in DMEM alone and in retinas from 5-week-old rd mice cocultured with 8-day-old normal C57BL/6 mice. *P < 0.0001. There was no significant difference between rod numbers.
DISCUSSION
The stereological approach used in this study allows a more precise estimation of cone cell numbers in the rd mouse retina compared with other studies (9, 10, 27). Previous reports stated that counting of morphologically identified cones in representative transverse sections did not reveal any changes between 36 and 47 postnatal days (10). Our stereological sampling of the entire retinal surface demonstrated a statistically significant decrease of 10% between 35 and 42 postnatal days. Stereology is accepted as the most unbiased and reproducible method for enumerating neurons in the central nervous system (25, 28). This approach compensated the differences between the superior and inferior hemispheres because the total surface of the retina was sampled. It reduced greatly the large variation observed in other studies relying on the sampling of relatively few tissue sections (9, 27). We calculated SD values of 5–8% of the mean value, permitting reliable statistical treatment of the data. Cones were identified by using two independent probes, PNA lectin and anti-arrestin antibody, which concorded in their staining patterns. PNA binds specifically to the glycocalyx surrounding cone outer segments in both normal (23) and rd mice (29) in vivo whereas arrestin was detected throughout the cell body and outer segments of both cones and rods (30). These markers identified faithfully photoreceptors in normal and rd mice retinas in vivo and in the different culture paradigms. Although cone loss was slightly greater in vitro than during the corresponding period in vivo (presumably because of manipulation of the retina and maintenance in chemically defined medium for one week), this loss was reduced significantly by coculture with dissociated cells from young postnatal retina or intact adult normal retina. Because such effects were not observed in the presence of rod-less retina (indeed an equivalent tissue mass of rd retina actually exacerbated cone death, possibly because of competition for trophic factors or liberation of toxic substances), we hypothesize that retinas containing rods release a diffusible survival-promoting factor for cones.
To our knowledge, this study represents the first report of any objective rescue effect on cones in genetic retinal degenerations affecting primarily rods. The existence of diffusible trophic factors influencing photoreceptor survival initially was suggested from studies of chimeric normal and dystrophic rat retina in which regions of rod survival overgrew normal retinal pigmented epithelium (31). This hypothesis is supported by our recent findings (20) that transplantation of isolated outer nuclear layer fragments from C57BL/6 mice (and hence 97% pure in rods) promote survival of cones in the rd host retina, including at distance from the transplantation site (20). Taken together, these studies support a role for cellular interactions in cone survival within dystrophic and possibly normal retina. Our experimental paradigm, avoiding direct contact between the source of the putative factor and the target tissue, suggests that trophic interactions between photoreceptor populations are mediated by diffusible signal(s).
Our study is in agreement with previous findings (12–17) in several animal models and in numerous patients with RP corresponding to diffuse rod dystrophies demonstrating that cone photoreceptor death lags behind that of rods (12–17). These studies included spontaneous mutants such as the rd mouse in which this sequence of events was demonstrated well by Carter-Dawson et al. (10) as well as transgenic models carrying a mutation in the rhodopsin gene or constructs aimed at selectively destroying rod cells (12–17). All demonstrated a similar pattern of photoreceptor loss. In chimeric retinas composed of patches of normal and transgenic photoreceptors expressing a mutant rhodopsin gene, uniform retinal degeneration independent of the genotype was observed (18). The authors proposed that each photoreceptor contributes to and draws from a common pool of trophic factor. Apoptosis, often observed in the central nervous system as a consequence of growth factor deprivation, has been demonstrated to be involved in rod death (3). The mechanism of cone death has not as yet been reported.
Experiments using retinal cell transplantation also support a role for trophic interactions in photoreceptor cell survival. In the Royal College of Surgeons rat, an animal model of RP in which defective phagocytosis of rod photoreceptor outer segments by retinal pigmented epithelium leads to retinal degeneration (32), several investigators have demonstrated a rescue effect of retinal pigmented epithelium transplants on host rod photoreceptors (33, 34). Of importance, rescue also could be observed at some distance from the grafted cells, suggesting the existence of diffusible trophic factors released by the transplant. Furthermore, this salvaging effect also was observed after intraocular injection of growth factors (35) and transiently following sham surgery (36).
Data on the presence and effects of soluble polypeptide growth factors such as fibroblast growth factors and neurotrophins in photoreceptors and other retinal cells are numerous (33, 38). The therapeutic potential of these and other neurotrophic factors in neurodegenerative disease is considerable (39) because the beneficial effects of growth factors have been demonstrated in a variety of in vivo and in vitro retinal models (37–41). Trophic effects have been demonstrated recently intravitreal injection of ciliary neurotrophic factor in some mutants in which the primary gene defect is located within the photoreceptors (42). Rods were protected partially by this growth factor whereas no significant protection of cones could be demonstrated. It is interesting to note that rods were not protected under the experimental conditions used in our study, presumably because, by this stage, their apoptotic cycle already has been activated. Such data indicate that timing of treatments will be of paramount importance in protecting one or the other photoreceptor population.
Research aimed at alleviating human retinal degenerations currently is exploring three main avenues: gene therapy, pharmacology, and transplantation. The first includes attempts at replacing defective genes (43–46), the second includes attempts at slowing photoreceptor cell loss by using growth factors (37, 42) or protecting putatively remaining cones with vitamin supplements (47), and the third includes attempts at slowing such cell loss by grafting different retinal cell populations (48, 49). Recently, evidence has been provided using the same rd mouse model that early in vivo gene transfer produces a significant delay in photoreceptor cell death (46). This therapeutic approach is of major interest but carries some clinically significant limitations such as the diversity of genes mutated in human RP and the fact that replacement therapy is not applicable for dominant forms of RP (20% of all human RP). Moreover, Bennett et al. (46) emphasize that this effect was limited to the area of injection and suggest that the whole retina should be treated. In the vast majority of previous animal studies (30, 35, 40, 46), data have been presented on the rod cells and not on the more clinically important cones. Our data show that cones can be protected even after loss of almost all rods.
The present study sits astride pharmacological and transplantation approaches to retinal degeneration. The rescue of cones could be attempted either by transplantation of photoreceptor cells or by administration of the trophic factor. Identification of a diffusible substance accounting for improved cone survival would not only provide a significant incentive for possible surgical or pharmacological therapeutic approaches to treatment of RP. It also would provide a better understanding of cone survival and death pathways in retinal health and disease.
Acknowledgments
We thank Dr N. Sadeg for assistance with the statistical analysis, F. Stoeckel for photographic work, and Brad Hyman (Harvard University Medical School) for his expert advice in the use of stereology, and we thank the following organizations for their generous financial support of this work: Fédération Nationale des Aveugles et Handicapés Visuels, La Fondation de l’Avenir, the A. and M. Suchert Foundation, Caisse Nationale d’Assurances Maladies des Travailleurs Salariés–Institut National de la Santé et de la Recherche Médicale, RETINA-France, Mutuelle Générale de l’Education Nationale–Institut National de la Santé et de la Recherche Médicale, Direction des Recherches Etudes et Techniques/Direction Générale pour l’Armement, Association pour le Developpement et la Recherche sur la Régéneration de la Rétine et sa Transplantation, Institut Electricité Santé, L’Etablissement Français des Greffes, ESSILOR International and IPSEN Pharmaceuticals.
ABBREVIATIONS
- rd
retinal degeneration
- RP
retinitis pigmentosa
- PNA peanut agglutinin.
References
- 1.Berson E L. Invest Ophthalmol Visual Sci. 1993;34:1659–1676. [PubMed] [Google Scholar]
- 2.Adler R. Arch Ophthalmol. 1996;114:79–83. doi: 10.1001/archopht.1996.01100130075012. [DOI] [PubMed] [Google Scholar]
- 3.Chang G Q, Hao Y, Wong F. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 4.Gregory C Y, Bird A C. Br J Ophthalmol. 1995;79:186–190. doi: 10.1136/bjo.79.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Maghtheh M, Gregory C, Inglehearn C, Hardcastle A, Bhattacharya S. Hum Mutat. 1993;2:249–255. doi: 10.1002/humu.1380020403. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin M-E, Ehrhart T L, Berson E L, Dryja T P. Proc Natl Acad Sci USA. 1995;92:3249–3253. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajiwara K, Berson E-L, Dryja T P. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld P J, Dryja T P. In: Molecular Genetic Ocular Disorders. Wiggs J, editor. New York: Wiley; 1995. pp. 99–126. [Google Scholar]
- 9.Farber D B. Invest Ophthalmol Visual Sci. 1995;36:261–275. [PubMed] [Google Scholar]
- 10.Carter-Dawson L D, Lavail M M, Sidman R L. Invest Ophthalmol Visual Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- 11.Ogilvie J M, Tenkova T, Lett J M, Speck J, Landgraf M, Silverman S M. Curr Eye Res. 1997;16:244–251. doi: 10.1076/ceyr.16.3.244.15411. [DOI] [PubMed] [Google Scholar]
- 12.Lem J, Applebury M L, Falk J D, Flannery J G, Simon M I. Neuron. 1991;6:201–210. doi: 10.1016/0896-6273(91)90356-5. [DOI] [PubMed] [Google Scholar]
- 13.Usukura J, Khoo W, Abe T, Breitman M L, Shinohara T. Cell Tissue Res. 1994;275:79–90. doi: 10.1007/BF00305377. [DOI] [PubMed] [Google Scholar]
- 14.Olsson J E, Gordon J W, Pawlyk B S. Neuron. 1992;9:815–830. doi: 10.1016/0896-6273(92)90236-7. [DOI] [PubMed] [Google Scholar]
- 15.Al-Ubaidi M R, Hollyfield J G, Overbeek P A, Baehr W. Proc Natl Acad Sci USA. 1992;89:1194–1198. doi: 10.1073/pnas.89.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowes C, Li T, Danciger M, Baxter L C, Farber D-B. Nature (London) 1990;347:667–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 17.McCall M A, Gregg R-G, Merriman K, Goto Y, Peachey N S, Stanford L R. Exp Eye Res. 1996;63:35–50. doi: 10.1006/exer.1996.0089. [DOI] [PubMed] [Google Scholar]
- 18.Huang P C, Gaitan A E, Hao Y, Petters R M, Wong F. Proc Natl Acad Sci USA. 1993;90:8484–8488. doi: 10.1073/pnas.90.18.8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiminez A J, Garcia-Fernandez J M, Gonzalez B, Foster R G. Cell Tissue Res. 1996;284:193–202. doi: 10.1007/s004410050579. [DOI] [PubMed] [Google Scholar]
- 20.Mohand-Said S, Hicks D, Simonutti M, Tran-Minh D, Deudon-Combe A, Dreyfus D, Silverman M S, Ogilvie J M, Tenkova T, Sahel J. Ophthalmic Res. 1997;29:290–297. doi: 10.1159/000268027. [DOI] [PubMed] [Google Scholar]
- 21.Dowling J E. The Retina: An Approachable Part of the Brain. Cambridge, MA: Harvard Univ. Press; 1987. pp. 1–271. [Google Scholar]
- 22.Sparrow J R, Hicks D, Barnstable C J. Dev Brain Res. 1990;51:69–84. doi: 10.1016/0165-3806(90)90259-2. [DOI] [PubMed] [Google Scholar]
- 23.Blanks J C, Johnson L V. Invest Ophthalmol Visual Sci. 1984;25:546–557. [PubMed] [Google Scholar]
- 24.Hicks D, Molday R S. Exp Eye Res. 1986;42:55–71. doi: 10.1016/0014-4835(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 25.Gundersen H J G, Bagger P, Bendtsen T F, Evans S M, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, et al. Acta Pathol Microbiol Immunol Scand. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 26.Dixon W J, Massey Jr F J. Introduction To Statistical Analysis. New York: McGraw–Hill; 1969. [Google Scholar]
- 27.LaVail M M, Matthes M T, Yasumura D, Steinberg R H. Exp Eye Res. 1997;65:45–50. doi: 10.1006/exer.1997.0308. [DOI] [PubMed] [Google Scholar]
- 28.Coggeshall R E, Lekan H A. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Blanks J C, Johnson L V, Hageman G S. Exp Eye Res. 1993;57:265–273. doi: 10.1006/exer.1993.1124. [DOI] [PubMed] [Google Scholar]
- 30.Nir I, Ransom N. Exp Eye Res. 1993;57:307–318. doi: 10.1006/exer.1993.1129. [DOI] [PubMed] [Google Scholar]
- 31.Mullen R J, LaVail M M. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- 32.Bok D, Hall M. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Turner J E. Exp Eye Res. 1988;47:911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 34.Lopez R, Gouras P, Kjeldbye H, Sullivan B, Reppucci V, Brittis M, Wapner F, Goluboff F. Invest Ophthalmol Visual Sci. 1989;30:586–588. [PubMed] [Google Scholar]
- 35.Faktorovich E G, Steinberg R H, Yasumura D, Matthes M T, Lavail M M. Nature (London) 1990;347:83–86. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 36.Silverman M S, Hughes S E. Curr Eye Res. 1989;9:183–191. doi: 10.3109/02713689008995205. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg R H. Curr Opin Neurobiol. 1994;4:515–524. doi: 10.1016/0959-4388(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 38.Hicks D, Bugra K, Faucheux B, Jeanny J, Laurent M, Malecase F, Mascarelli F, Raulais D, Cohen Y, Courtois Y. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Vol. 11. Oxford: Pergamon; 1991. pp. 333–374. [Google Scholar]
- 39.Lindsay R M, Wiegand S J, Altar C A, Distefano P S. Trends Neurosci. 1994;161:246–256. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 40.LaVail M M, Li L, Turner J E, Yasumura D. Exp Eye Res. 1992;55:555–562. doi: 10.1016/s0014-4835(05)80168-x. [DOI] [PubMed] [Google Scholar]
- 41.Adler R, Hewitt A T. In: Retinal Degenerations. Anderson R E, Hollyfield J G, Lavail M M, editors. Boca Raton, FL: CRC; 1991. pp. 79–86. [Google Scholar]
- 42.LaVail M M, Yasumura D, Matthes M T, Lau-Villacorta C, Unoki K, Sung C-H, Steinberg R H. Invest Ophthalmol Visual Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 43.Lem J, Flannery J G, Li T, Appelebury M L, Farber D B, Simon M I. Proc Natl Acad Sci USA. 1992;89:4422–4426. doi: 10.1073/pnas.89.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Travis G H, Bok D. Methods Neurosci. 1993;15:343–357. [Google Scholar]
- 45.Li T, Adamian M, Roof D J, Berson E L, Dryja T P, Roeisler B J, Davidson B L. Invest Ophthalmol Visual Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 46.Bennett J, Tanabe T, Sun D, Zeng Y, Kjeldbye H, Gouras P, Maguire A M. Nat Med. 1996;2:649–654. doi: 10.1038/nm0696-649. [DOI] [PubMed] [Google Scholar]
- 47.Berson E, Rosner B, Sandberg M A, Hayes K C, Nicholson B W, Weigel-DiFranco C, Willett W. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- 48.Del Cerro M. In: Progress in Retinal Research. Osborne N N, Chader G J, editors. Vol. 9. Oxford: Pergamon; 1989. pp. 229–272. [Google Scholar]
- 49.Litchfield T M, Whitely S J O, Lund D. Exp Eye Res. 1997;64:655–666. doi: 10.1006/exer.1996.0253. [DOI] [PubMed] [Google Scholar]