Abstract
We previously reported that lithium stimulated extracellular glutamate accumulation in monkey and mouse cerebrocortical slices. We report here that this is caused by lithium-induced inhibition of glutamate uptake into the slice. Glutamate release was amplified 5-fold over inhibition of uptake. When the effects of lithium and the specific glutamate transporter inhibitors, l-trans-pyrrolidine-2,4-dicarboxylic acid and dihydrokainic acid, were plotted as glutamate accumulation vs. inhibition of glutamate uptake, the plots were superimposable. This finding strongly indicates that lithium-induced glutamate accumulation is caused entirely by inhibition of uptake. With cerebrocortical synaptosomes, inhibition of glutamate uptake was greater than in slices, suggesting that presynaptic nerve endings are the primary site of inhibition of uptake by lithium. Inhibition of uptake was caused by a progressive lowering of Vmax, as the lithium concentration was increased, whereas the Km remained constant, indicating that lithium inhibited the capacity of the transporter but not its affinity. Chronic treatment of mice with lithium, achieving a blood level of 0.7 mM, which is on the low side of therapeutic, up-regulated synaptosomal uptake of glutamate. This would be expected to exert an antimanic effect. Lithium is a mood stabilizer, dampening both the manic and depressive phases of bipolar disorder. Interestingly, although the uptake of glutamate varied widely in individual control mice, uptake in lithium-treated mice was stabilized over a narrow range (variance in controls, 0.423; in lithium treated, 0.184).
We previously observed that the antibipolar drug, lithium, stimulated the release of the excitatory neurotransmitter, glutamate, from cerebral cortex slices of rhesus monkey and mouse (1, 2). This release was accompanied by an increase in inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] accumulation. The increase in Ins(1,4,5)P3 accumulation was caused by the selective activation of the N-methyl-d-aspartate (NMDA) receptor/channel by glutamate. Activation of the NMDA receptor is known to cause increased Ins(1,4,5)P3 accumulation (3).
At that stage of the investigation, the mechanism of Li-induced glutamate release was not known. There were several possibilities. Lithium could increase the discharge of glutamate from presynaptic nerve endings and/or glial cells. Lithium could inhibit the reuptake of glutamate by the glutamate transporter(s) in presynaptic nerve endings and/or in glial cells.
In the present study, we have investigated the mechanism of lithium-induced glutamate release and the chronic consequences of this inhibition. Evidence is presented that shows that glutamate release is caused by lithium-inhibited uptake of glutamate in presynaptic nerve endings (synaptosomes).
The clinical benefit of lithium requires 1–2 weeks of therapy. We therefore have investigated the effect of chronic lithium ingestion in mice on synaptosomal uptake of glutamate. Synaptosomal uptake was up-regulated. Because this would remove glutamate from the synaptic cleft, it would tend to exert an antimanic effect. Lithium is a mood stabilizer. Interestingly, glutamate uptake in individual control mice varied rather widely, but with lithium-treated mice glutamate uptake in separate mice was stabilized over a narrow range.
EXPERIMENTAL PROCEDURES
Slice Preparation and Incubation.
White male adult ICR mice were sacrificed by cervical dislocation, and the brains were quickly removed and chilled. Cross-chopped slices (0.5 × 0.35 × 0.35 mm) were prepared and incubated in batch as previously described (2) with the following modifications. Stadie-Riggs cerebrocortical slices were prepared from two mice and quickly cross-chopped with a MacIlwain slicer. A set of cerebrocortical slices from two mice was submitted to the initial restorative incubation before sacrifice and dissection of the next set from two mice. This small-scale, rapid preparation significantly reduced, stabilized, and improved the reproducibility of subsequent glutamate levels in the media. At the conclusion of the batch restorative incubations (4), aliquots of slice suspension [1–2 mg/3.2 ml of Krebs-Henseleit bicarbonate saline (KHBS)] were taken, the media were immediately replaced with fresh KHBS, and the suspensions were incubated for 30 min at 37.5°C. The media were removed, drugs then were added in fresh medium, and the slices were preincubated for 30–45 min with and without lithium as indicated before determination of glutamate uptake or medium glutamate. Because LiCl additions were balanced by NaCl reductions, to maintain buffer isotonicity control buffers were prepared in the same manner, substituting an equivalent amount of choline chloride for LiCl.
Determination Glutamate Uptake in Slices.
After preincubation of slices, 1 μCi of [3H]l-glutamate and various amounts of carrier l-glutamate (1–2,500 μM, final concentration) were added and the incubation continued for 3.5 min before removal of medium. At 4 min, the pack of wet slices was rinsed with 5 ml of KHBS (+ choline chloride or lithium chloride) at 37.5°C. The rinsed slices were taken up in a final volume of 2 ml of 3% perchloric acid, homogenized for 20 s with a polytron at a moderate speed to prevent foaming, and centrifuged at 15,000 × g for 10 min. The extracts were neutralized with KOH and centrifuged, and the supernatants were counted for radioactivity by liquid scintillation spectroscopy. The pellets were resuspended and analyzed for protein by the BCA assay (Pierce). “Uptake” at 0°C was used to control for nonspecific radioactivity.
Enzymatic Determination of Medium Glutamate.
After the slices were allowed to settle to the bottom of the flask, samples of medium were removed from slice suspensions with care to avoid taking slices with the medium. After centrifugation of the medium at 3,000 × g for 5 min to remove any slice or slice debris carried over, the media were boiled for 5 min to denature any soluble proteins. The media were stored at −20°C until assay. Glutamate was determined by measurement of fluorescence of the NADH formed after reaction of medium sample with glutamate dehydrogenase (EC 1.4.1.3) as previously described (2).
Preparation of Synaptosomes.
Mouse cerebrocortical synaptosomes were prepared by direct Percoll density gradient centrifugation of an S1 fraction essentially as described by Harrison et al. (5). Briefly, fresh 0.5-mm thick Stadie-Riggs slices from the cerebral cortices of two mice were homogenized by 10 strokes in 5 ml of chilled isotonic sucrose in a glass homogenizer at 4°C. After a 10-min centrifugation at 1,000 × g, the supernatant (S1) was directly layered on a gradient made up of 2-ml layers of 20%, 10%, and 6% Percoll in isotonic sucrose at pH 7.4 at 4°C. After a 5-min centrifugation at 32,500 × g, the synaptosomes were harvested at the interface between the 10% and 20% layers, diluted with 11 ml of isotonic sucrose, and centrifuged at 15,000 × g for 10 min. The loose pellet was gently resuspended in 100 μl of isotonic sucrose and stored briefly on ice before use.
Determination of Synaptosomal Glutamate Uptake.
A concentrated synaptosome suspension (approximately 5 mg/ml) was diluted with KHBS to 150 μg/ml. Aliquots (200 μl) were carefully measured into 25-ml Erlenmeyer flasks, immediately diluted with 3.2 ml of KHBS with lithium chloride or choline chloride, gassed with 5% CO2/95% O2, and incubated with shaking for 30 min at 37.5°C. At the end of the preincubation, determination of glutamate uptake was initiated by addition of 1 μCi of [3H]l-glutamate and various amounts of carrier l-glutamate (1–25 μM). Glutamate uptake was stopped by rapid filtration of the suspension on Whatman GFB glass fiber filters, followed immediately by rinsing with 10 ml of KHBS (+ lithium chloride or choline chloride) at 37.5°C. The wet filters were counted for radioactivity in 10 ml of hydrofluor (National Diagnostics). An aliquot of the initial concentrated suspension was analyzed for protein by the BCA method (Pierce). Uptake at 0°C was used as a control for nonspecific radioactivity and subtracted from total dpm on the filter. The initial glutamate concentration used in calculation of the rate of uptake included added carrier glutamate and endogenous medium glutamate for both slices and synaptosomes.
Chronic Treatment of Mice with Lithium.
Mouse feed containing 0.4% lithium carbonate was provided by Teklad, Madison, WI (diet no. 92271). Feeding with lithium was continued for 2 weeks. This treatment produced a lithium blood level of 0.7 mM (G. V. Los and L.E.H., unpublished observations), which is on the low side of the therapeutic range in humans (0.5–1.5 mM). In mice, higher doses of lithium were too toxic, leading to some fatalities.
Statistical Treatment.
Significance was determined by using the Student’s t test (unpaired), and where appropriate the P values are given in the figure legends. Variance, as used in the legend of Fig. 8, is the average squared deviation of a set of values from their mean. The average is determined by dividing the sum of the squared deviations by one less than the number of samples.
Figure 8.
Effect of chronic lithium treatment of mice on glutamate uptake by cortical synaptosomes. White male adult mice (average weight 31 g) were fed rodent chow ± 0.4% Li2CO3 for 14 days. Food, water, and saline (0.9% NaCl) were available ad libitum. Water and saline were supplemented with 1% myo-inositol. After sacrifice, synaptosomes were prepared from cortical slices from individual mice, and the initial rate of uptake of 10 μM l-glutamate was determined as described in Experimental Procedures. Assays were performed in quintuplicate. n = 18 for both conditions. Average control uptake was 5.22 ± 0.15 nmol/mg per min. Average uptake from lithium-treated mice was 5.98 ± 0.1 nmol/mg per min. Lithium differed from control with a significance of P < 0.001. The variance (σ2) for uptake from control mice was 0.423; the variance for uptake from lithium-treated mice was 0.184.
RESULTS
Effect of Lithium Concentration on the Inhibition of Glutamate Uptake in Cerebrocortical Synaptosomes.
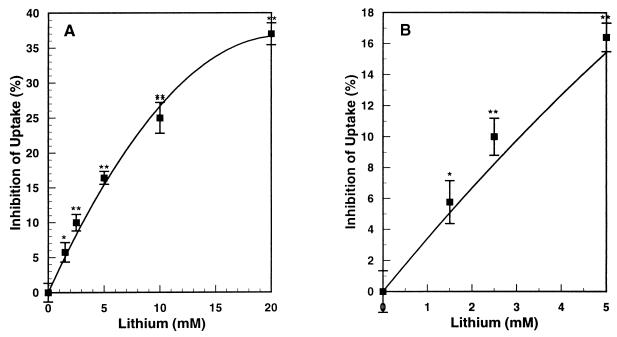
In routine experiments, cerebrocortical synaptosomes were preincubated without or with lithium for 30 min (appropriate concentrations of choline were substituted for lithium in the control synaptosomes, and sodium was adjusted accordingly to maintain constant osmolarity). This was followed by incubation for 4 min with various concentrations of [3H]l-glutamate. Glutamate uptake was linear over this period. Over the range of concentrations of lithium used here, the corresponding reductions in Na+ concentrations had no effect on glutamate uptake. Fig. 1 shows the effect of increasing concentrations of lithium on the inhibition of uptake of glutamate by cerebrocortical synaptosomes. At the therapeutic concentration of 1.5 mM lithium, the inhibition was statistically significant. Although this inhibition at 1.5 mM was not great, it should be pointed out that with cerebrocortical slices, when glutamate release was plotted against inhibition of glutamate uptake, release was amplified 5-fold over inhibition of uptake (see below). Thus, a 6% inhibition of uptake would be amplified to a 30% increase in release. Inhibition of uptake progressively increased from 6% at 1.5 mM to 35% at 20 mM lithium.
Figure 1.
Effect of lithium concentration on inhibition of mouse cortical synaptosomal glutamate uptake. Synaptosomes were prepared from fresh 0.5-mm slices of mouse cerebral cortex by direct Percoll density gradient centrifugation of an S1 fraction as described in Experimental Procedures. Aliquots of synaptosomes (30 μg) were preincubated for 30 min in 3.2 ml of KHBS with various concentrations of lithium or choline chloride. The suspensions then were adjusted to 5 μM [3H]l-glutamate, uptake stopped after 4 min by rapid filtration and rinsing with 10 ml of fresh buffer, and the radioactivity on the filter counted by liquid scintillation (see Experimental Procedures). Displayed are averages of three separate experiments, each in triplicate ± SE. Significance of differences relative to zero lithium: ∗, P = 0.007; ∗∗, P < 0.001. B is an enlargement of the low lithium portion of A.
Effect of Glutamate Concentration on the Velocity of Glutamate Uptake in Cerebrocortical Synaptosomes in the Presence and Absence of Lithium.
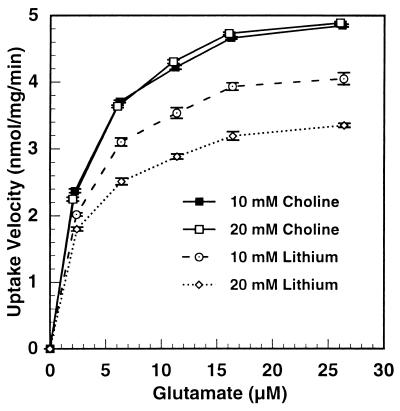
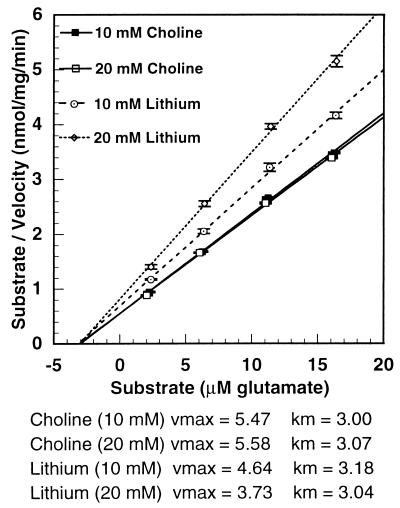
Fig. 2 shows the velocity of uptake of glutamate over a concentration range of 2.5 to 26 μM glutamate in cerebrocortical synaptosomes and at different lithium concentrations. The dose-response curves show typical Michaelis-Menten kinetics. The uptake of glutamate was progressively inhibited by increasing concentrations of lithium, but the shapes of the curves were similar. Fig. 3 is a Hanes-Wilkinson transformation of the dose-response curve from the data. The Vmax of glutamate uptake was progressively reduced by increasing lithium concentrations, dropping from 5.5 nmol/mg per min in the control to 3.7 nmol/mg per min at 20 mM lithium. On the other hand, there was no effect of lithium on the Km, averaging 3.07 μM glutamate. Thus, lithium inhibited the capacity of the transporter without affecting its affinity for glutamate. The Hanes-Wilkinson plot indicates a single transporter in synaptosomes, unlike the situation in cortical slices (6). A single transporter in synaptosomes has been reported previously (7).
Figure 2.
Glutamate concentration dependence of [3H]l-glutamate uptake by mouse cortical synaptosomes and effect of two concentrations of lithium. Mouse cortical synaptosomes were prepared as described in Experimental Procedures. Aliquots (45 μg) were preincubated in 3.2 ml KHBS for 30 min with lithium or choline chloride as indicated. The initial rate of glutamate uptake then was determined at various glutamate concentrations. Samples of incubation medium were taken before assay for determination of endogenous medium glutamate. Substrate levels reflect the sum of endogenous and added glutamate at the start of the uptake determination. Determination of uptake and enzymatic determination of glutamate are described in Experimental Procedures. All lithium points differed from their respective choline controls with a significance of P ≤ 0.001.
Figure 3.
Linear transformation of the kinetic data describing the effect of lithium on glutamate uptake by mouse cortical synaptosomes. The plots are a Hanes-Wilkinson transformation of the data in Fig. 6; i.e., S/v = (1/Vmax)S + (Km/Vmax). Ordinate intersected at Km/Vmax. Abscissa intersected at −Km. All lithium points differed from their respective choline controls with a significance of P ≤ 0.001.
Effect of Lithium Concentration on the Inhibition of the Initial Rate of Glutamate Uptake by Cerebrocortical Slices.
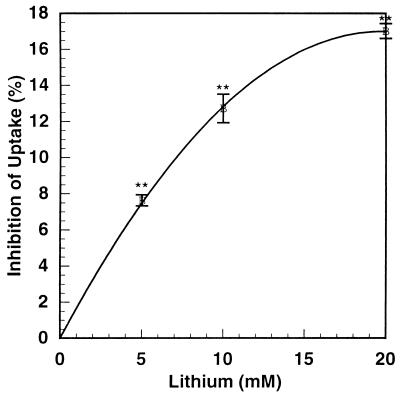
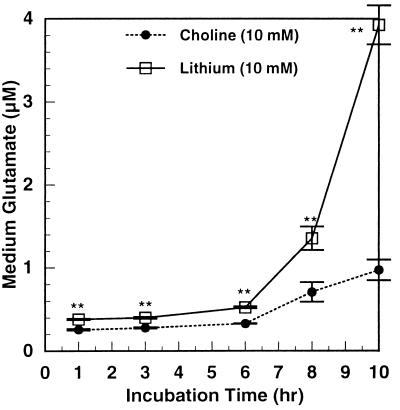
Fig. 4 shows the inhibition of initial glutamate uptake in cerebrocortical slices by concentrations of lithium ranging from 5 to 20 mM. Inhibition increased linearly up to 10 mM, tending to reach a plateau at 20 mM. Over this concentration range, the inhibition ranged from 7% to 17%. At 5 mM lithium, this inhibition is half as great as in cerebrocortical synaptosomes. But, as shown below, the small inhibition of glutamate uptake in slices leads to considerable amplification of extracellular glutamate accumulation (see Fig. 7). Also, if incubations of slices were continued for 10 hr in the presence of antibiotics, 10 mM lithium increased extracellular glutamate 4-fold (Fig. 5). Beyond 8 hr, there was a rapid upshoot in the lithium effect. Although this may be caused in part by lithium toxicity with 10 mM lithium at 10 hr, it suggests that with chronic therapy with therapeutic concentrations (0.5–1.5 mM) the effects of lithium on glutamate release may be greater than that seen with short-term incubations presented here. When the effect of glutamate concentration on the uptake of glutamate in cerebrocortical slices was studied, there were clearly two components of uptake: a high affinity component up to 0.05 mM glutamate and a low affinity component from 0.05 to 2.5 mM (data not shown). Uptake mediated by both transporters was inhibited by lithium.
Figure 4.
Inhibition by lithium of the initial rate of glutamate uptake by mouse cortical slices. Mouse cortical slices were prepared, restored, and divided into aliquots (average protein = 1.42 mg) as described in Experimental Procedures. After an initial 30-min incubation, the media were replaced with KHBS containing the indicated concentrations of lithium chloride (or choline chloride in controls) and preincubated for 45 min. Initial uptake of [3H]l-glutamate (25 μM) then was determined as described in Experimental Procedures. Shown is the average of the results of three separate experiments, each in triplicate ± SE. ∗∗, P < 0.001.
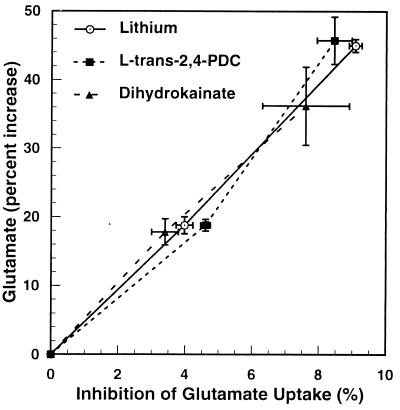
Figure 7.
Effects of lithium, PDC, or dihydrokainate on glutamate uptake and extracellular glutamate. Mouse cerebrocortical slices were prepared and restored as described in Experimental Procedures and then preincubated (average protein = 1.53 mg) in 3.2 ml of KHBS for 30 min with 5 or 10 mM LiCl, 1.25 or 2.5 μM PDC, or 15 or 30 μM dihydrokainate. Samples then were taken for enzymatic determination of medium glutamate, and the tissue was assayed for initial uptake of glutamate (5 μM) as described in Experimental Procedures. Shown is the average of three experiments with quadruplicate determinations in each experiment.
Figure 5.
Extracellular glutamate after lengthy incubation of mouse cortical slices with lithium. Slices of mouse cerebral cortex were prepared and incubated as described in Experimental Procedures. All buffers were filtered with 0.2 μm “Uniflo” syringe filters. Aliquots (average protein = 1.37 mg) were incubated in 3.2 ml of KHBS with 10 mM choline chloride or 10 mM lithium chloride. The final incubation buffer was supplemented with penicillin (100 units/ml) and streptomycin (0.1 mg/ml). At the times indicated, samples of incubation medium were taken for glutamate determination. The slices were treated with 3% perchloric acid and the acid-insoluble solid was analyzed for protein. Glutamate and protein analyses were as described in Experimental Procedures. ∗∗, P ≤ 0.001.
We hypothesized that the lesser inhibition of glutamate uptake by lithium in cerebrocortical slices as compared with cerebrocortical synaptosomes might be caused by dampening by other structures in the slice that show no inhibition of uptake. The most likely candidates would be glial cells. In fact, we found no inhibition of uptake in human astrocytes by lithium (G.V. Los and L.E.H., unpublished observations).
Inhibitory Effects of Lithium and the Glutamate Transporter Inhibitors, l-Trans-Pyrrolidine-2,4-Dicarboxylic Acid (PDC) and Dihydrokainic Acid, on Glutamate Uptake and Extracellular Glutamate Accumulation in Cerebrocortical Slices.
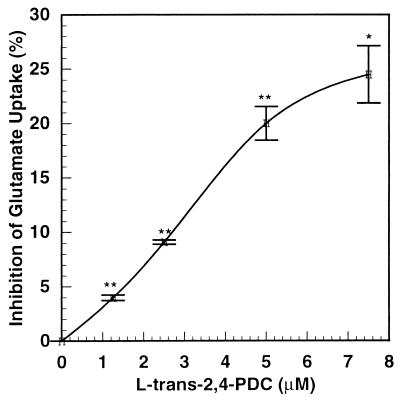
PDC selectively inhibits the glutamate transporter competitively (8). Fig. 6 shows the effect of increasing concentrations of the glutamate transporter inhibitor, PDC, on inhibition of glutamate uptake in cerebrocortical slices. The inhibition curve was slightly sigmoidal. Inhibition of glutamate uptake increased from 4% to 25% when PDC was increased from 1 μM to 8 μM. Fig. 7 shows the relationship between accumulation of extracellular glutamate and the inhibition of the glutamate transporter in the presence of lithium or each of the two glutamate transporter inhibitors, PDC and dihydrokainic acid. Extracellular glutamate was amplified approximately 5-fold over inhibition of glutamate uptake by all three agents. The plots of extracellular glutamate accumulation vs. inhibition of glutamate uptake for lithium, PDC, and dihydrokainic acid are superimposable. This suggests strongly that inhibition of glutamate uptake by lithium is responsible for the drug’s effect on extracellular glutamate accumulation. PDC is taken up by synaptosomes, and it has been reported (9) that it can cause release of glutamate by an exchange diffusion mechanism. In those experiments, the concentrations of PDC were very high (10–100 μM). Dihydrokainic acid is not transported into the synaptosome (10). Yet, it showed the same relationship between glutamate accumulation and inhibition of uptake as with lithium or PDC, ruling out a significant release of glutamate by exchange diffusion induced by PDC at low concentrations (1.25–2.5 μM).
Figure 6.
Effect of PDC on glutamate uptake in mouse cortical slices. Mouse cortical slices were prepared and restored as described in Experimental Procedures. Aliquots of slices (average protein = 1.40 mg) were incubated in 3.2 ml of KHBS for 30 min ± PDC (0, 1.25, 2.5, 5.0, and 7.5 μM). The initial rate of glutamate uptake (5 μM) then was determined as described in Experimental Procedures. PDC is transportable by the glutamate carrier, but levels of inhibition remained constant for 30 min, indicating that uptake did not instrumentally affect the inhibition. ∗, P = 0.002; ∗∗, P ≤ 0.001.
Chronic Treatment of Mice with Lithium Up-Regulates and Stabilizes Glutamate Reuptake by Cerebrocortical Synaptosomes.
Lithium is a mood stabilizer; i.e., it will normalize mood from the direction of either depression or mania. This requires chronic treatment for 1–2 weeks. The inhibition of synaptosomal uptake of glutamate by lithium occurs immediately. We therefore studied the effects of chronic treatment of mice with a relatively low dose of lithium (0.4% lithium carbonate in mouse chow), achieving a lithium blood level of 0.7 mM, which is only slightly above the minimum therapeutic blood level in humans. If we increased the lithium carbonate in the chow to 0.6% to achieve higher blood concentrations, there were fatalities by 2 weeks.
Even at this relatively low blood level, synaptosomes from the cerebral cortices of lithium-treated mice showed a small, but highly significant, up-regulation of glutamate uptake (Fig. 8). This would be expected to exert an antimanic effect. What is perhaps even more interesting is that lithium stabilized glutamate uptake. There was a wide range in glutamate uptake from individual control mice, perhaps representing differing levels of excitability, but uptake in synaptosomes from separate lithium-treated mice was clamped in a narrower range. To express this quantitatively, the variance (σ2) of uptake in control mice was = 0.423, whereas in lithium-treated mice it was 0.184. This mechanism offers a possible explanation for the fact that clinically lithium stabilizes mood from the direction of either mania or depression.
DISCUSSION
We previously reported that lithium released glutamate from cerebrocortical slices from monkeys and mice (1, 2). We show here that this release is caused by inhibition of uptake by cerebrocortical synaptosomes. The fact that the relationship between glutamate release and inhibition of glutamate uptake by lithium and by the specific glutamate transporter inhibitors, PDC and dihydrokainic acid, are quantitatively the same strongly suggests that inhibition of glutamate uptake by lithium fully accounts for lithium-induced release of glutamate. The magnitude of inhibition at comparable lithium concentrations was at least two times greater in cortical synaptosomes than in cortical slices. The lesser inhibition of glutamate uptake by lithium in cerebrocortical slices as compared with synaptosomes is apparently caused by no inhibition by lithium in astrocytes (G. V. Los and L.E.H., unpublished observations), which would blunt the overall effect in slices. It should be pointed out that it was difficult to measure glutamate release in synaptosomes because of the small amount of material.
Lithium treatment requires 1–2 weeks to exert its antibipolar effect. We therefore treated mice with lithium for 2 weeks and measured synaptosomal uptake in the absence of added lithium in the incubation medium. Compared with control mice, lithium up-regulated synaptosomal uptake of glutamate with high statistical significance (P < 0.001), even though uptake was increased only 15%. This would exert an antimanic effect. It is likely that this up-regulation would have been greater if the lithium blood levels could have been increased above the minimally effective concentration seen in humans, but in mice higher blood levels were too toxic. Lithium often is referred to as a mood stabilizer because it shifts mood toward normal from the direction of either depression or mania. In addition to up-regulating glutamate uptake, lithium stabilized glutamate uptake. There was a wide range of glutamate uptake in synaptosomes from individual control mice, but in lithium-treated mice uptake in synaptosomes from individual mice clustered closer together. In these studies, it is not possible to measure the effect of chronic lithium on extracellular glutamate in vivo. This will require microdialysis or push-pull perfusion in intact animals.
Elevation of extracellular glutamate by noxious stimuli is highly excitotoxic and can lead to neuronal death, primarily via activation of the NMDA receptor, which permits increased influx of calcium into the postsynaptic cell body. Lithium, also being excitotoxic in humans at supratherapeutic concentrations, may exert its excitotoxicity by elevating synaptic glutamate.
Since this paper was submitted, Nonaka et al. (11) treated embryonic neuronal cells with 100 μM glutamate (approximately 100 times the resting synaptic level), producing partial neuronal death by apoptosis. Chronic, but not acute, lithium protected against cell death. This may be caused at least in part by up-regulation of the glutamate transporter, which would lower extracellular glutamate. Other mechanisms also may be involved, such as down-regulation of the NMDA receptor and/or the Ins(1,4,5)P3 receptor. Following our earlier observation that lithium raises postsynaptic Ins(1,4,5)P3 via selective activation of the NMDA receptor by glutamate (1, 2), we have observed a small, but highly significant, down-regulation of the Ins(1,4,5)P3 receptor in mice chronically treated with lithium (G.V. Los and L.E.H., unpublished observations). This would exert an antimanic effect that would add to the antimanic effect exerted by up-regulation of the glutamate transporter.
Acknowledgments
We thank Dr. Georgyi Los for valuable discussions, Connie Bowes for technical assistance, and Karen Wipperfurth for assistance in preparation of the manuscript. This work was supported by National Institutes of Health Grant HL16318.
ABBREVIATIONS
- Ins(1
4,5)P3, inositol 1,4,5-trisphosphate
- NMDA
N-methyl-d-aspartate
- KHBS
Krebs-Henseleit bicarbonate saline
- PDC
l-trans-pyrrolidine-2,4-dicarboxylic acid
References
- 1.Dixon J F, Los G V, Hokin L E. Proc Natl Acad Sci USA. 1994;91:8358–8362. doi: 10.1073/pnas.91.18.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon J F, Hokin L E. Proc Natl Acad Sci USA. 1997;94:4757–4760. doi: 10.1073/pnas.94.9.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer M L, Miller R J. Trends Pharmacol Sci. 1990;11:254–260. doi: 10.1016/0165-6147(90)90254-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee C H, Dixon J F, Reichman M, Moummi C, Los G V, Hokin L E. Biochem J. 1992;282:377–385. doi: 10.1042/bj2820377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison S M, Jarvie P E, Dunkley P R. Brain Res. 1988;441:72–80. doi: 10.1016/0006-8993(88)91384-4. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin A M, Quastel J H. J Neurochem. 1976;26:431–441. doi: 10.1111/j.1471-4159.1976.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler D D. Neurochem Res. 1987;12:667–680. doi: 10.1007/BF00970521. [DOI] [PubMed] [Google Scholar]
- 8.Bridges R J, Stanley M S, Anderson M W, Cotman C W, Chamberlain A R. J Med Chem. 1991;34:717–725. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- 9.Griffiths R, Dunlop J, Gorman A, Senior J, Grieve A. Biochem Pharmacol. 1994;47:267–274. doi: 10.1016/0006-2952(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 10.Waldmeier P C, Wicki P, Feldtrauer J. Naunyn-Schmiedeberg’s Arch Pharmacol. 1993;348:478–4785. doi: 10.1007/BF00173206. [DOI] [PubMed] [Google Scholar]
- 11.Nonaka S, Hough C J, Chuang D-M. Proc Natl Acad Sci USA. 1998;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]