Abstract
Serotonin (5-HT), and in particular 5-HT2 receptors, play an important role in cardiorespiratory function within the brainstem. In addition, abnormalities in the 5-HT system have been implicated in many cardiorespiratory disorders, including sudden infant death syndrome. However little is known about the mechanisms of action of 5-HT2 receptors in altering the activity of parasympathetic cardiac neurons in the brainstem. In this study we examined the effects of activation of different subtypes of 5-HT2 receptors on spontaneous and respiratory evoked GABAergic neurotransmission to cardioinhibitory vagal neurons within the nucleus ambiguus as well as rhythmic fictive inspiratory-related activity. A single application of α-Me-5-hydroxytryptamine maleate (α-Me-5-HT), a 5-HT2 receptor agonist, did not significantly alter the frequency of spontaneous or respiratory-evoked GABAergic inhibitory post-synaptic currents (IPSCs) in cardiac vagal neurons. However, repetitive successive applications of α-Me-5-HT elicited a long-lasting (≥ 1 hour) decrease in the frequency of spontaneous as well as inspiratory-related GABAergic IPSCs to cardiac vagal neurons. This study demonstrates multiple, but not single applications of the 5-HT2 receptor agonist α-Me-5-HT caused a long-lasting inhibition of both spontaneous and fictive inspiratory-related GABAergic neurotransmission to CVNs which can be prevented by the 5-HT2B receptor antagonist SB204741, but persisted with the 5-HT2A/2C receptor antagonist ketanserin. The 5-HT2 receptor agonist α-Me-5-HT also reversibly and transiently excited central fictive inspiratory activity which was abolished by ketanserin, but was unaffected by the 5-HT2B receptor antagonist SB204741.
Keywords: ambiguus, 5-HT, serotonin, parasympathetic, cardiac, vagus, SIDS
Introduction
Heart rate is dominated by the activity of premotor cardioinhibitory vagal neurons (CVNs) located in the nucleus ambiguus. Within the nucleus ambiguus (NA) premotor neurons receive a high number of axosomatic serotonin (5-HT) contacts, and the 5-HT contacts surrounding neurons in the nucleus ambiguus are among the most dense in the brainstem (Takeuchi et al., 1983). 5-HT fibers also specifically surround CVNs which have been described as “ensheathed in 5-HT immunoreactive axonal boutons” (Izzo et al., 1993).
A multitude of different 5-HT receptors have been shown to influence cardiorespiratory function in the brainstem. Central 5-HT7 receptors play an important role in the reflex activation of parasympathetic outflow to the heart upon stimulating cardiopulmonary afferent fibers, arterial baroreceptors and chemoreceptor afferents (Kellett et al., 2005). Central 5-HT1A receptors are also involved in mediating both cardiopulmonary and baroreceptor reflex evoked vagal bradycardia, but may not be involved in chemoreceptor elicited responses in cardiac vagal neurons (Skinner et al., 2002). While activation of 5-HT1A receptors potentiate, 5-HT1B/D agonists depress chemoreceptor reflex activation of parasympathetic cardiac neurons (Dando et al., 1998).
Microinjection or ionphoretic application of different 5-HT agonists into the NA has also provided mixed responses. While low doses of the 5-HT1A agonist 8-OH-DPAT generally inhibit CVNs higher doses elicited an excitation of CVNs (Wang and Ramage, 2001). Other work has shown microinjection of the 5-HT1A agonist 8-OH-DPAT excited CVNs to evoke a bradycardia (Chitravanshi and Calaresu, 1992). Additional 5-HT receptor agonists have yet to be tested. A limitation of these microinjection studies is that the sites of action and mechanisms responsible for these responses are unknown. The heart rate responses to microinjection of 5-HT agonists may have been evoked by stimulation of local polysynaptic pathways, interneurons, activation of presynaptic terminals that synapse upon cardiac vagal neurons, modification of postsynaptic synaptic currents, or direct alterations in the membrane properties of CVNs.
In contrast, 5-HT receptors, and in particular 5-HT2 receptors, are critical to respiratory function in the brainstem. 5-HT2 receptors have been shown to be essential in the responses to intermittent hypoxia, and evoked long-term changes of synaptic and central respiratory activity (Fuller et al., 2001a; Chen and Bazan, 2003; Shay et al., 2005). A prolonged 5-HT-dependent augmentation of respiratory motor output, long term facilitation, can be elicited by both episodic hypoxia (Mitchell and Johnson, 2003) and episodic activation of 5-HT2 receptors (Johnson et al., 2001; Bocchiaro and Feldman, 2004).
In summary, despite the strong evidence from anatomic work that 5-HT fibers abundantly surround parasympathetic cardiac vagal neurons (Izzo et al., 1993) as well as the evidence 5-HT2 receptors play an essential role in cardiorespiratory network function, there is a lack of information on the role of acute and intermittent activation of 5-HT2 receptors in modulating the activity of cardiac vagal neurons in the NA. In this study we tested the hypothesis that single as well as multiple applications of 5-HT2 receptor agonists modulate the important respiratory related and spontaneous GABAergic neurotransmission to cardiac vagal neurons, and investigated the relative contribution of different 5-HT2 receptor subtypes (5-HT2B, and 5-HT2A/C).
Experimental Procedures
To identify cardiac vagal neurons in-vitro a two stage procedure was utilized. In an initial surgery, Sprague-Dawley rats (postnatal days 2–6; Hilltop, Scottdale, PA) were anesthetized with hypothermia and received a right thoracotomy. The heart was exposed, and 0.05 ml of rhodamine (Molecular Probes, Eugene, OR) was injected into the pericardial sac to retrogradely label CVNs. The labeling of CVNs using these procedures has been previously described (Bouairi et al., 2006). Specificity of the cardiac vagal labeling has been confirmed by the absence of any labeled neurons in the brainstem when rhodamine is injected either outside the pericardial sac or within the pericardial sac if the cardiac branch of the vagus nerve is sectioned (n=4). Recent work has shown this method does not label neurons in the compacta formation but identifies cardiac vagal neurons localized in the external formation of the NA (Bouairi et al., 2006). In other control experiments (n = 10), intravenous injection of up to 10 mg of rhodamine failed to label any neurons in the medulla except for rare labeling of neurons in the area postrema, an area with a deficient blood-brain barrier. On the day of experiment (2–4 days later), the animals were anesthetized with halothane and sacrificed by rapid cervical dislocation. The brain was submerged in cold (4°C) buffer of the following composition: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 5 mM glucose, and 10 mM HEPES, and continually gassed with 100% O2. Under a dissection microscope, the cerebellum was removed, and the hindbrain was isolated. A single slice of the medulla (800-μm thickness) that included CVNs, the rostral hypoglossal nucleus and rootlets, and the pre-Botzinger complex was obtained and submerged in a recording chamber which allowed perfusion (5–10 ml/min) of artificial cerebrospinal fluid at room temperature containing 125 mM NaCl, 3 mM KCl, 2 mM CaCl2, 26 mM NaHCO3, 5 mM glucose, and 5 mM HEPES equilibrated with carbogen (95% O2 and 5% CO2, pH = 7.4). All animal procedures were performed in compliance with the institutional guidelines at George Washington University and are in accordance with the recommendations of the Panelon Euthanasia of the American Veterinary Medical Association and the National Institutes of Health publication Guide for the Care and Use of Laboratory Animals.
The thick medullary slice preparation generates rhythmic fictive inspiratory-related motor discharge in hypoglossal cranial nerves. Spontaneous fictive inspiratory-related activity was recorded by monitoring motorneuron population activity from hypoglossal nerve rootlets using a suction electrode. Hypoglossal rootlet activity was amplified 50,000 times, filtered (10–300 HZ bandpass; CWE, Ardmore, PA), and electronically integrated (τ = 50 ms; CWE).
Individual CVNs in the NA were identified by the presence of the fluorescent tracer using a Zeiss Axioskop upright microscope (Carl Zeiss Inc., Thornwood, NY) using a 40× water immersion objective. These identified CVNs were then imaged with differential interference contrast optics, infrared illumination, and infrared-sensitive video detection cameras to gain better spatial resolution. Patch pipettes (2.5–3.5 MΩ) containing 150 mM KCl, 4 mM MgCl2, 2 mM EGTA, 2 mM Na-ATP, 10 mM HEPES (pH = 7.4) were guided to the surface of individual CVNs. This pipette solution resulted in inward Cl− currents upon activation GABA receptors (calculated reversal potential of Cl− = +4 mV) at holding potential of −80 mV. Voltage clamp whole cell recording were made with an Axopatch 200B, and pClamp 8 software (Axon Instruments, Union City, CA).
GABAergic neurotransmission was isolated by continuous focal application of strychnine (1 μM), D-2-amino-5-phosphonovalerate (50 μM), and 6-cyano-7-nitroquinoxaline-2,3-dione (50 μM) to block glycine, NMDA, and non-NMDA receptors, respectively. Continual focal drug applications were performed using a pneumatic picopump pressure system (WPI, Sarasota, FL). Drugs were continuously and focally released throughout the experiments using a picrospritzer and pressure ejected from a patch pipette positioned within 30 μm of the patched CVN. The maximum range of drug application has been determined previously to be 100–120 μm downstream from the drug pipette and considerably less behind the drug pipette (Wang et al., 2002). The following drugs were applied by inclusion in the perfusate: the 5-HT2 receptor agonist α-methyl-5-hydroxytryptamine maleate (α-Me-5-HT, 1 μM); and two 5-HT2 receptor antagonists with differential affinity for different 5-HT2 receptors: 5-HT2B receptor antagonist SB204741 (10 μM) and 5-HT2A/2C receptor antagonist Ketanserin (1 μM). The pK values of the drugs are, respectively on 5-HT2A, 5-HT2B, and 5-HT2C receptors: α-Me-5-HT: 7.4, 8.8, 6.2; SB204741: < 5.3, 7.8, < 6; Ketanserin 8.9, 5.4, 7.0 (Baxter et al., 1995).
Control rhythmic fictive inspiratory-related activity and GABAergic IPSCs were recorded simultaneously for 4 min. Slices were initially exposed to a single 4-min application of α-Me-5-HT and then after a 30-min washout of the agonist were re-exposed to multiple applications of αMe-5-HT (3 applications of 4-min duration every 9 min). In experiments including antagonists, the antagonist was applied for 4 min followed by the same protocol for agonist application. After the end of each experiment the GABAergic IPSCs were abolished by gabazine (25 μM) focal application to the patched CVN. Only one experiment was conducted per preparation. This protocol was designed to mimic previous studies in the literature that examine respiratory responses to intermittent hypoxia and elicit long-term facilitation of respiratory activity.
Synaptic events were detected using MiniAnalysis (version 5.6.12; Synaptosoft, Decatur, GA). The frequency of inhibitory post-synaptic currents that occurred in CVNs was grouped in 1 s bins and cross-correlated with onset of fictive inspiratory-related hypoglossal activity. The 1 s period before the fictive inspiratory burst onset was considered spontaneous activity, while the 1 s period immediately after the fictive inspiratory burst onset was analyzed as the inspiratory period. Burst frequency and duration were measured using pClamp 8 software (Molecular Devices). Data were analyzed during the last 2 min of the control period, during last 2 min of single drug applications, during 2 min immediately after the end of multiple drug applications, and every 2 mins during the washout condition after single and multiple drug applications, as appropriate. The results from studying α-Me-5-HT concentration-dependent responses are presented as mean percentage of control ± SEM and statistically compared with ANOVA and Kruskal-Wallis non-parametric test followed by Dunns post-test. Otherwise results were not presented as percent of control but are presented as mean ± SEM and statistically compared using ANOVA with repeated measures and Tukey’s post-test to examine the responses throughout the time course of the experiments and paired Student’s t-test when comparing the results from control to antagonist applications. Significant difference for all data was set at p < 0.05.
Results
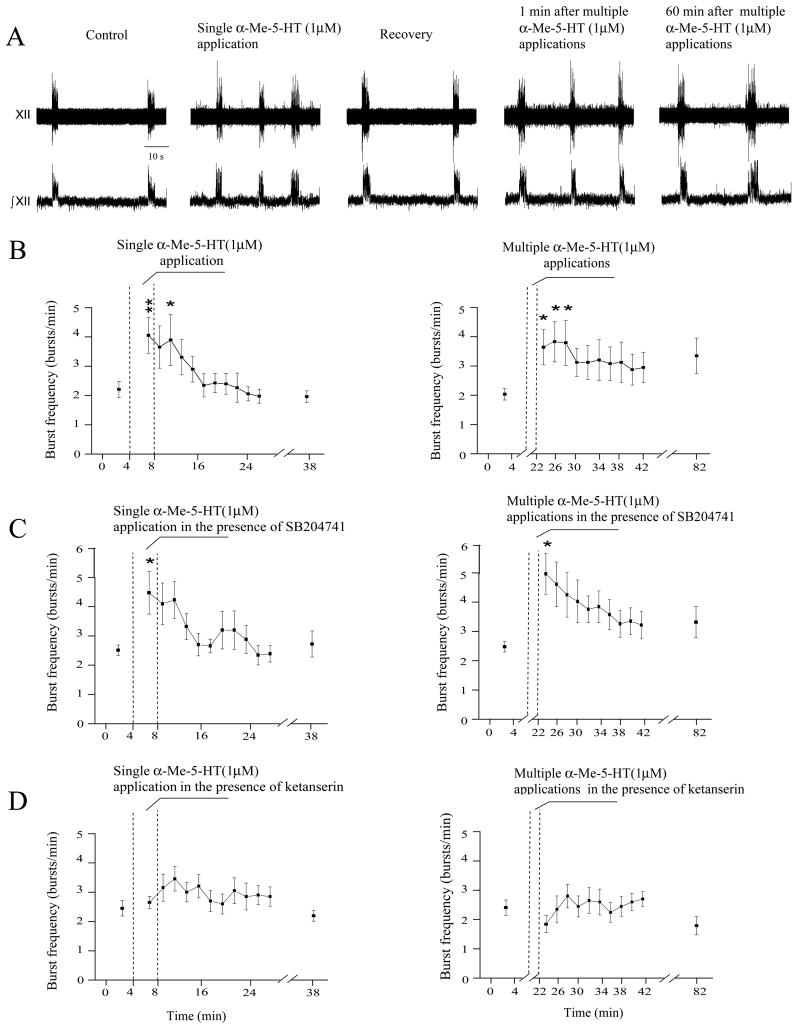
A single application of the 5-HT2 receptor agonist α-Me-5-HT (1 μM) elicited a significant and transient increase in the frequency of fictive inspiratory bursts from 2.2 ± 0.3 bursts/min to 4.0 ± 0.6 bursts/min (p < 0.01, n = 12, Fig. 1, left, A, B). Multiple applications of α-Me-5-HT (1 μM) also significantly increased fictive inspiratory burst frequency from 2.0 ± 0.2 bursts/min to 3.5 ± 0.6 bursts/min (p < 0.05, n = 12, Fig. 1, right, A, B). The fictive inspiratory burst frequency remained significantly elevated for 6 min (Fig. 1, B).
Figure 1.
In a single experiment (A), both single and multiple applications of the 5-HT2 receptor agonist α-Me-5-HT significantly and reversibly increased the hypoglossal fictive inspiratory burst frequency (single application: p < 0.01; multiple applications: p < 0.05). The summary data from 12 preparations are illustrated in B, C and D. As shown in C, the 5-HT2B receptor antagonist SB204741 did not block the excitatory effect of α-Me-5-HT on respiratory activity (n = 11). The 5-HT2A/2C receptor antagonist ketanserin blocked the excitatory effect of α-Me-5-HT, D (n = 10). In this and all subsequent figures: XII denotes the hypoglossal nerve rootlet activity; ∫XII denotes integrated the hypoglossal nerve rootlet activity,* denotes p < 0.05, and ** denotes p < 0.01.
Neither single nor multiple applications of α-Me-5-HT (1 μM) caused a significant change in the fictive inspiratory burst duration (single application: 2.2 ± 0.2 sec versus 2.2 ± 0.1 sec; multiple applications: 2.4 ± 0.2 sec versus 2.1 ± 0.1 sec, n = 12). The absence of a change in burst duration was maintained over a range of α-Me-5-HT concentrations as fictive inspiratory burst duration was not significantly changed by either a single application of α-Me-5-HT (108.9 ± 7.8 %, 99.5 ± 3.8 %, and 94.5 ± 9.5% at concentrations of 0.1 (n=10), 1.0 (n=12) and 5 μM (n=10), respectively), or with multiple applications of α-Me-5-HT (96.3 ± 8.6 %, 97.7 ± 5.4 % and 96.3 ± 8.8 % at concentrations of 0.1 (n=10), 1.0 (n=12) and 5 μM (n = 10) respectively).
Application of the 5-HT2B receptor antagonist SB204741 (10 μM) by itself did not significantly change the frequency of fictive inspiratory bursts (2.7 ± 0.4 bursts/min versus 2.5 ± 0.2 bursts/min; n = 11). SB204741(10 μM) also did not alter the α-Me-5-HT (1 μM) elicited increase in the fictive inspiratory burst frequency, as fictive inspiratory burst frequency increased from 2.5 ± 0.2 bursts/min to 4.5 ± 0.7 bursts/min in response to a single application (p < 0.05, n = 11), and from 2.5 ± 0.2 bursts/min to 5.1 ± 0.7 bursts/min in response to multiple applications (p < 0.05, n = 11), Fig. 1, C.
In contrast, although application of the 5-HT2A/2C receptor antagonist ketanserin (1 μM) did not by itself significantly change the basal frequency of fictive inspiratory bursts (2.7 ± 0.3 bursts/min versus 2.4 ± 0.3 bursts/min; n = 10), ketanserin (1 μM) abolished the facilitation of fictive inspiratory activity evoked by α-Me-5-HT. In the presence of ketanserin (1 μM) applications of α-Me-5-HT (1 μM) failed to significantly change the fictive inspiratory burst frequency (2.4 ± 0.3 versus 2.6 ± 0.2 bursts/min (single application; n = 10), and 2.4 ± 0.3 versus 1.9 ± 0.3 bursts/min (multiple applications; n = 10)), Fig. 1, D.
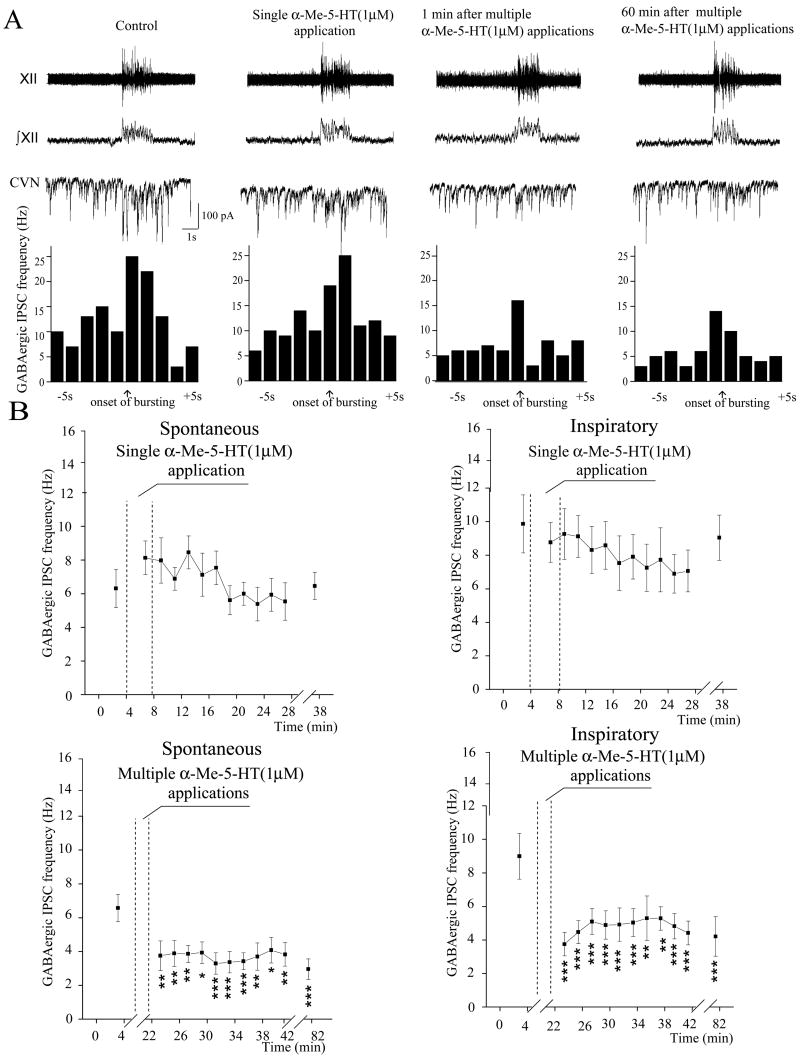
Consistent with previous reports (Neff et al., 2004; Huang et al., 2006) the frequency of GABAergic IPSCs increased during fictive inspiratory bursts (Fig. 2, A; Fig. 4, A; Fig. 5, A). A single application of α-Me-5-HT (1 μM) did not produce a significant change in the frequency of either spontaneous or fictive inspiratory-related GABAergic IPSCs (6.4 ± 1.1 Hz versus 8.0 ± 1.0 Hz; and 9.9 ± 1.7 Hz versus 8.8 ± 1.2 Hz, respectively; n = 10 CVNs), Fig. 2, A, B. In contrast, multiple applications α-Me-5-HT (1 μM) caused a significant and long-lasting inhibition in the frequency of both spontaneous and fictive inspiratory-related GABAergic IPSCs. The frequency of spontaneous GABAergic IPSCs decreased from 6.4 ± 0.8 Hz (control) to 3.8 ± 0.9 Hz (1–2 min after applications, p < 0.01, n = 10) and remained significantly decreased for at least 60 min after the applications of α-Me-5-HT (3.0 ± 0.6 Hz; p < 0.001, n = 10), Fig. 2, A, B. Similar to spontaneous GABAergic neurotransmission, the frequency of fictive inspiratory-related GABAergic IPSCs decreased from 9.1 ± 1.4 Hz (control) to 3.9 ± 0.7 Hz (1–2min after applications; p < 0.001, n = 10) and remained significantly decreased for at least 60 min after the applications of α-Me-5-HT (4.3 ± 1.2 Hz; p < 0.001, n = 10), Fig. 2, A, B.
Figure 2.
A single application of the 5-HT2 receptor agonist α-Me-5-HT did not significantly alter the frequency of spontaneous or respiratory-related GABAergic IPSCs in CVNs; a typical example is shown in A, and the summary data (n = 10 CVNs) in B. In contrast, multiple applications of α-Me-5HT evoked a significant decrease in the frequency of spontaneous (p < 0.01 n = 10) and fictive inspiratory-related (p < 0.001 n = 10) GABAergic IPSCs, that persisted for at least 1 hour, A, C. *** denotes p < 0.001 in this and all subsequent figures.
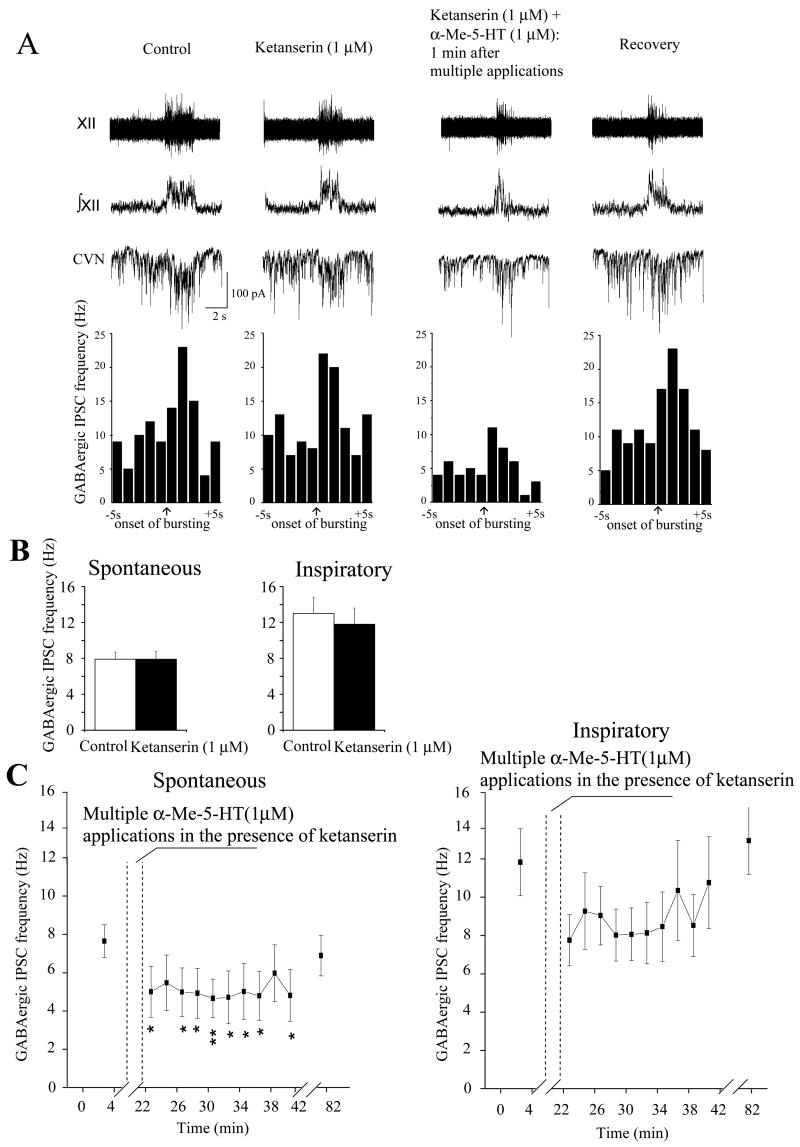
Figure 4.
Although the 5-HT2A/2C receptor antagonist ketanserin did not alter the frequency of either spontaneous or fictive inspiratory-related GABAergic IPSCs to CVNs, A, B, in the presence of ketanserin multiple applications of α-Me-5-HT evoked a significant decrease in the frequency of spontaneous (p < 0.05, n = 7) but not inspiratory-related (n = 7) GABAergic IPSCs to CVNs, C.
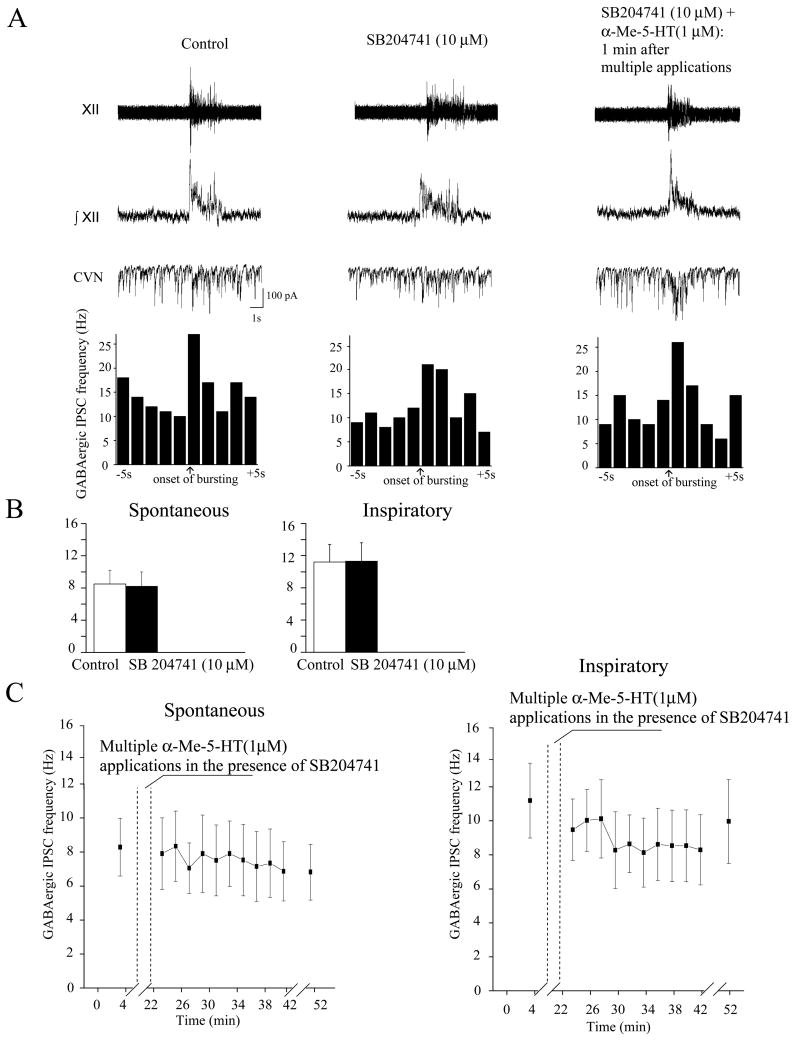
Figure 5.
The 5-HT2B receptor antagonist SB204741 did not evoke any significant change in the basal frequency of either spontaneous or fictive inspiratory-related GABAergic IPSCs but SB204741 blocked the responses to multiple applications of α-Me-5HT (n = 7). A single experiment is shown in A, whereas the summary data are demonstrated in B, C.
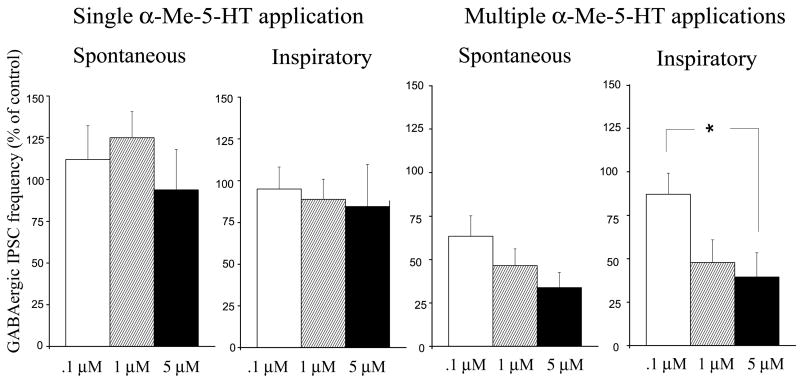
The lack of responses of GABAergic frequency to a single α-Me-5-HT application were consistent over a range of different concentrations of α-Me-5-HT including 0.1, 1.0 and 5 μM, Fig. 3. Neither spontaneous GABAergic frequency (112 ± 20 %, 125 ± 16% and 94 ± 24 % at concentrations of 0.1 (n=6), 1 (n=10) and 5 μM (n=6), respectively) or fictive inspiratory related GABAergic frequency (95±13 %, 89 ± 12%, and 85 ± 25% at concentrations of 0.1 (n=6), 1 (n=10) and 5 μM (n=6), respectively), were changed by a single application of α-Me-5-HT, Fig. 3.
Figure 3.
There was no significant difference in the frequency of either spontaneous or inspiratory-related GABAergic IPSCs during a single α-Me-5-HT application at concentrations of 0.1 (n = 6), 1 (n = 10), or 5 μM (n = 6). Similarly, the decrease of the frequency of spontaneous GABAergic IPSCs 2 min after multiple applications of α-Me-5-HT was not found to be concentration dependent, (n = 6, 10, and 6 CVNs at concentrations of .1, 1, and 5 μM, respectively). However, the inhibition of frequency of fictive inspiratory-related GABAergic IPSCs 2 min after multiple applications of α-Me-5-HT at a concentration of 5 μM (n = 6) was significantly lower (p < 0.05) than the responses at a concentration of 0.1 μM (n = 6) but not significantly different from the responses at a concentration of 1 μM (n = 10).
The inhibition of spontaneous GABAergic IPSCs 59–60 min after multiple applications of α-Me-5-HT were similar with α-Me-5-HT applied at a range of different concentrations including 0.1, 1.0 and 5 μM, Fig. 3. The inhibition of spontaneous GABAergic IPSCs after multiple applications of α-Me-5-HT were 63± 12%, 47 ± 10%, and 34± 9% at concentrations of 0.1 (n=6), 1 (n=10) and 5 μM (n = 6), Fig. 3. However, the inhibition of inspiratory related GABAergic IPSCs after multiple applications of α-Me-5-HT did show some dose-dependence as the most profound decrease in the GABAergic IPSCs frequency was produced by applications of α-Me-5-HT at a concentration of 5 μM (39% ± 14 %, n = 6), which was significantly greater (p < 0.05) than the inhibition elicited by a concentration of 0.1 μM (82 ± 12 %, n = 6) but not significantly different from the responses at a concentration at 1 μM (48 ± 13 %, n = 10), Fig. 3.
Application of the 5-HT2A/2C receptor antagonist ketanserin, over a range of concentrations including 1, 10 and 100 μM, did not significantly change the basal frequency of spontaneous GABAergic IPSCs (control: 8±1 Hz, 1 μM: 8±1 Hz, n=7; control: 5±1 Hz, 10 μM: 6±1 Hz, n=7; control: 4±1 Hz, 100 μM: 4±1 Hz, n=6). Similarly, ketanserin, over a range of concentrations including 1, 10 and 100 μM, did not significantly alter the inhibitory responses to multiple applications of α-Me-5-HT (1 μM) as the spontaneous GABAergic IPSCs decreased upon multiple exposures of α-Me-5-HT with ketanserin concentrations of 1, 10 and 100 μM from 8±1 Hz to 5 ±1 Hz (p<0.05, n=7), from 6±1 Hz to 2 ±1 Hz (p<0.01, n=7), and from 4±1 Hz to 0.6 ±0.2 Hz (p<0.01, n=6), respectively (only the responses in the presence of 1 μM ketanserin are shown in Fig. 4,A,C).
Ketanserin did, however, prevent the α-Me-5-HT mediated inhibition of inspiratory evoked GABAergic activity upon multiple applications of α-Me-5-HT. The long lasting inhibition of inspiratory related GABAergic activity that occurs with multiple applications of α-Me-5-HT exposure was prevented by 1 μM ketanserin as the frequency of inspiratory-related GABAergic IPSCs remained statistically unchanged from control levels after α-Me-5-HT application in the presence of ketanserin (1 μM, 12±2 vs 8±2 Hz, n = 7, Fig. 4, A, C).
Application of the 5-HT2B receptor antagonist SB204741 did not evoke any significant change in the frequency of spontaneous or inspiratory-related GABAergic IPSCs at SB204741 concentrations of both 1 and 10 μM (from 4±1 to 4±1 (n=7), and 9±2 to 8±2 (n=7), respectively). Similarly inspiratory related GABAergic IPSCs responses to a single application of α-Me-5-HT were not altered by SB204741 at a concentration of 10 μM, Fig. 5B (from 11±2 to 11±2 Hz, n=7).
However, the inhibition of spontaneous GABAergic IPSCs with multiple applications of α-Me-5-HT was completely prevented by SB204741 at a concentration of 10 μM, Fig. 5A, C, but SB204741 at a concentration of 1 μM did not alter the inhibition of spontaneous GABAergic IPSCs by multiple applications of α-Me-5-HT. Spontaneous GABAergic IPSC frequency decreased from 4±1 to 0.8±0.2 (p<0.0001, n=7) in presence of 1μM SB204741 after multiple applications of α-Me-5-HT, but in the presence of 10 μM SB204741 the inhibition of GABAergic IPSCS by multiple applications of α-Me-5-HT was prevented (control: 8±2; SB204741: 8±2 Hz, n=7). Similarly, the α-Me-5-HT mediated inhibition of inspiratory related GABAergic IPSCs was prevented by 10 μM SB204741 (control: 11±2, SB204741 9±2 Hz, n=7), Fig. 5,A,C.
Discussion
There are two major findings from this study: 1) Multiple, but not single applications of the 5-HT2 receptor agonist α-Me-5-HT caused a long-lasting inhibition of both spontaneous and fictive inspiratory-related GABAergic neurotransmission to CVNs within the NA. The 5-HT2-mediated inhibitory effect was completely prevented by the 5-HT2B receptor antagonist SB204741, whereas the 5HT2 mediated inhibition of spontaneous GABAergic neurotransmission persisted with the 5-HT2A/2C receptor antagonist ketanserin, although ketanserin abolished the fictive inspiratory-related GABAergic inhibition evoked by α-Me-5-HT. 2) Both single and multiple applications of the 5-HT2 receptor agonist α-Me-5-HT reversibly and transiently excited central fictive inspiratory activity. The excitatory effect on fictive inspiratory activity was abolished by ketanserin, a 5-HT2A/2C receptor antagonist, but was unaffected by the 5-HT2B receptor antagonist SB204741.
The results in this study demonstrate that multiple, but not a single, application of the 5-HT2 receptor agonist α-Me-5-HT inhibits both spontaneous and fictive inspiratory-related GABAergic neurotransmission to CVNs within the NA, and this inhibition persisted for at least 1 hour. The mechanisms of this long-lasting inhibition are unknown. 5-HT2 receptors have been demonstrated to have a role in synaptic plasticity (Fuller et al., 2001a; Chen and Bazan, 2003; Shay et al., 2005), and repetitive 5-HT2 receptor activation is hypothesized to initiate intracellular signaling events leading to the rapid synthesis of proteins, particularly brain-derived neurotrophic factor (BDNF), necessary to maintain long-term plasticity (Mitchell et al., 2001; Feldman et al., 2003). It is possible BDNF contributes to the long-lasting inhibition of GABAergic neurotransmission observed in this study since BDNF mRNA is expressed in neurons located within the NA (Zaidi et al., 2005) and BDNF has been demonstrated to suppress GABAergic synaptic neurotransmission (Henneberger et al., 2002) in other neurons. Since αMe-5-HT does not discriminate between subtypes of 5-HT2 receptors (Baxter et al., 1995) we used two 5-HT2 receptor antagonists to isolate the subtypes of the 5-HT2 receptors involved: ketanserin which is a specific 5-HT2A/2C antagonist and SB204741, a selective 5-HT2B antagonist. The inhibitory effect of α-Me-5-HT was blocked by 5-HT2B receptor antagonist SB204741 suggesting a critical role of 5-HT2B receptors in 5-HT2-mediated inhibition of GABAergic neurotransmission, while the 5-HT2A/2C receptor antagonist ketanserin blocked only the fictive inspiratory-related GABAergic inhibition evoked by α-Me-5-HT but ketanserin did not abolish the 5HT2 mediated inhibition of spontaneous GABAergic neurotransmission. Interestingly, in the presence of ketanserin the inhibitory effect of α-ME-5-HT on the spontaneous IPSCs to CVNs did not persist 60 min suggesting that 5-HT2A and 5-HT2C receptors likely contribute to the long-lasting, but not initial, 5-HT2-mediated inhibition of spontaneous GABAergic neurotransmission to CVNs. This work indicates 5-HT2A/2C receptors are likely involved in selectively activating the GABAergic neurotransmission to cardiac vagal neurons that occurs during inspiration, and blocking these 5-HT2A/2C receptors would reduce respiratory sinus arrhythmia and heart rate variability. The site of action with bath-applied 5-HT2 agonist and antagonists in this study is unknown. Since 5-HT2B receptors modulated GABAergic neurotransmission to CVNs but did not influence central fictive inspiratory burst frequency it is likely that there are different sites of 5-HT actions for CVNs and respiratory neurons, including hypoglossal motorneurons.
In addition to 5-HT2 modulation of cardiac vagal neurons, multiple lines of evidence suggest excitatory 5-HT2 receptors are also important in respiratory function in the brainstem. In decerebrate adult cats (Rose et al., 1995), anesthetized adult rats (Fenik and Veasey, 2003), and decerebrate newborn rats ((Khater-Boidin et al., 1999; Glerant et al., 2005) 5-HT induced an increase in hypoglossal activity due to activation of 5-HT2 receptors. The results in this study are in agreement with most other studies since a single application of the 5-HT2 receptor agonist α-Me-5-HT produced a significant increase in fictive inspiratory burst frequency, similar to that observed in a majority of other studies (Schwarzacher et al., 2002). The excitatory effect on fictive inspiratory activity was prevented by ketanserin, but not altered by SB204741. The site(s) of action of α-Me-5-HT is not fully known. α-Me-5-HT may act directly on hypoglossal motorneurons (Berger et al., 1992), in addition α-Me-5-HT may also act on respiratory neurons within pre-Botzinger complex (Schwarzacher et al., 2002).
In addition to the rapid responses, 5-HT2 receptors may be involved in long-term changes of synaptic and central respiratory activity (Fuller et al., 2001a; Chen and Bazan, 2003; Shay et al., 2005). A prolonged 5-HT-dependent augmentation of respiratory motor output is often referred to as long term facilitation (LTF) (Mitchell and Johnson, 2003). LTF can be elicited by episodic hypoxia in anesthetized and conscious animals (Mitchell and Johnson, 2003) and episodic activation of 5-HT receptors (Johnson et al., 2001; Bocchiaro and Feldman, 2004). In this study multiple applications of the 5-HT2 receptor agonist α-Me-5-HT (1 μM) produced a significant increase in hypoglossal nerve burst frequency that only persisted for 6 minutes. These results are different from the results from Bocchiaro and Feldman (Bocchiaro and Feldman, 2004), who, using medullary slice preparation from neonatal rats, have shown that multiple applications of α-Me-5-HT induced persistent (>1 hour) increases in hypoglossal nerve activity. However this LTF may be dependent on the strain of rats used since there appears to be substantial differences in hypoglossal LTF between substrains of Sprague Dawley rats (Fuller et al., 2001b; Feldman et al., 2003). Indeed, hypoglossal LTF has been shown to be present in Charles River Laboratories/Sasco but not Harlan rats (Fuller et al., 2001b). In this study we used Sprague Dawley rats from a Hilltop supplier. Since (Fuller et al., 2001b) did not mention in their study which substrain of Sprague Dawley rats were used, differences between strains of animals may be responsible for the differences in the duration of LTF evoked by the 5-HT2 agonist.
The long-lasting withdrawal of GABAergic neurotransmission to CVNs elicited by repetitive 5-HT2 receptor activation provides a potential neurochemical mechanism that may be important in SIDS. Episodic hypoxia, often resulting from an unstable breathing pattern during sleep would likely result in repetitive activation of raphe 5-HT neurons (Bodineau and Larnicol, 2001; Feldman et al., 2003), which could lead to repetitive 5-HT2 receptor activation and persistent inhibition of spontaneous and fictive inspiratory-related GABAergic neurotransmission to parasympathetic CVNs evoking a bradycardia. Progressive bradycardia and respiratory failure are considered to be the most likely causes of death in SIDS (Meny et al., 1994; Thach, 2005). These responses may be exaggerated in SIDS victims as these infants have a higher number of 5-HT neurons (Paterson et al., 2006). The cerebrospinal fluids of infant victims of sudden death show a very significant increase in the metabolites of 5-HT (Caroff et al., 1992), and evidence from infants that succumb to SIDS suggests that the mechanisms of death involves a progressive bradycardia even in the presence of continued breathing movements (Meny et al., 1994). In addition, a recent report demonstrates that gasping activity is dependent on 5-HT2A receptors (Tryba et al., 2006). It is possible that 5-HT2 receptors are responsible for the bradycardia that occurs with hypoxia and that an exaggeration of these 5-HT2 receptor-mediated responses increases the excitation of CVNs and the risk of SIDS.
Acknowledgments
Supported by grant HL 59895 to DM
Abbreviations
- 5-HT
Serotonin
- CVNs
cardioinhibitory vagal neurons
- NA
nucleus ambiguous
- α-Me-5-HT
α-Me-5-hydroxytryptamine maleate
- IPSCs
Inhibitory post-synaptic currents
- LTF
long term facilitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter G, Kennett G, Blaney F, Blackburn T. 5-HT2 receptor subtypes: a family reunited? Trends Pharmacol Sci. 1995;16:105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- Berger AJ, Bayliss DA, Viana F. Modulation of neonatal rat hypoglossal motoneuron excitability by serotonin. Neurosci Lett. 1992;143:164–168. doi: 10.1016/0304-3940(92)90257-8. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodineau L, Larnicol N. Brainstem and hypothalamic areas activated by tissue hypoxia: Fos-like immunoreactivity induced by carbon monoxide inhalation in the rat. Neuroscience. 2001;108:643–653. doi: 10.1016/s0306-4522(01)00442-0. [DOI] [PubMed] [Google Scholar]
- Bouairi E, Kamendi H, Wang X, Gorini C, Mendelowitz D. Multiple types of GABAA receptors mediate inhibition in brain stem parasympathetic cardiac neurons in the nucleus ambiguus. J Neurophysiol. 2006;96:3266–3272. doi: 10.1152/jn.00590.2006. [DOI] [PubMed] [Google Scholar]
- Caroff J, Girin E, Alix D, Cann-Moisan C, Sizun J, Barthelemy L. Neurotransmission and sudden infant death. Study of cerebrospinal fluid. C R Acad Sci III. 1992;314:451–454. [PubMed] [Google Scholar]
- Chen C, Bazan NG. Acetaminophen modifies hippocampal synaptic plasticity via a presynaptic 5-HT2 receptor. Neuroreport. 2003;14:743–747. doi: 10.1097/00001756-200304150-00017. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Calaresu FR. Additive effects of dopamine and 8-OH-DPAT microinjected into the nucleus ambiguus in eliciting vagal bradycardia in rats. J Auton Nerv Syst. 1992;41:121–127. doi: 10.1016/0165-1838(92)90134-3. [DOI] [PubMed] [Google Scholar]
- Dando SB, Skinner MR, Jordan D, Ramage AG. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br J Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik P, Veasey SC. Pharmacological characterization of serotonergic receptor activity in the hypoglossal nucleus. Am J Respir Crit Care Med. 2003;167:563–569. doi: 10.1164/rccm.200202-107OC. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001a;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001b;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Glerant JC, Khater-Boidin J, Salzmann F, Duron B. Vagal pulmonary afferents and central respiratory effects of 5-HT in newborn rats. Eur J Neurosci. 2005;22:2249–2256. doi: 10.1111/j.1460-9568.2005.04417.x. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Juttner R, Rothe T, Grantyn R. Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J Neurophysiol. 2002;88:595–603. doi: 10.1152/jn.2002.88.2.595. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Bouairi E, Mendelowitz D. Differential control of central cardiorespiratory interactions by hypercapnia and the effect of prenatal nicotine. J Neurosci. 2006;26:21–29. doi: 10.1523/JNEUROSCI.4221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo PN, Deuchars J, Spyer KM. Localization of cardiac vagal preganglionic motoneurones in the rat: immunocytochemical evidence of synaptic inputs containing 5-hydroxytryptamine. J Comp Neurol. 1993;327:572–583. doi: 10.1002/cne.903270408. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JE, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro. J Appl Physiol. 2001;91:2703–2712. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Central 5-HT7 receptors are critical for reflex activation of cardiac vagal drive in anaesthetized rats. J Physiol. 2005;563:319–331. doi: 10.1113/jphysiol.2004.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khater-Boidin J, Rose D, Glerant JC, Duron B. Central effects of 5-HT on respiratory rhythm in newborn rats in vivo. Eur J Neurosci. 1999;11:3433–3440. doi: 10.1046/j.1460-9568.1999.00762.x. [DOI] [PubMed] [Google Scholar]
- Meny RG, Carroll JL, Carbone MT, Kelly DH. Cardiorespiratory recordings from infants dying suddenly and unexpectedly at home. Pediatrics. 1994;93:44–49. [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Rose D, Khater-Boidin J, Toussaint P, Duron B. Central effects of 5-HT on respiratory and hypoglossal activities in the adult cat. Respir Physiol. 1995;101:59–69. doi: 10.1016/0034-5687(95)00008-2. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Gunther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Shay BL, Sawchuk M, Machacek DW, Hochman S. Serotonin 5-HT2 receptors induce a long-lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J Neurophysiol. 2005;94:2867–2877. doi: 10.1152/jn.00465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MR, Ramage AG, Jordan D. Modulation of reflexly evoked vagal bradycardias by central 5-HT1A receptors in anaesthetized rabbits. Br J Pharmacol. 2002;137:861–873. doi: 10.1038/sj.bjp.0704941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Kojima M, Matsuura T, Sano Y. Serotonergic innervation on the motoneurons in the mammalian brainstem. Light and electron microscopic immunohistochemistry. Anat Embryol (Berl) 1983;167:321–333. doi: 10.1007/BF00315670. [DOI] [PubMed] [Google Scholar]
- Thach BT. The role of respiratory control disorders in SIDS. Respir Physiol Neurobiol. 2005;149:343–353. doi: 10.1016/j.resp.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Irnaten M, Venkatesan P, Evans C, Baxi S, Mendelowitz D. Synaptic activation of hypoglossal respiratory motorneurons during inspiration in rats. Neurosci Lett. 2002;332:195–199. doi: 10.1016/s0304-3940(02)00957-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ramage AG. The role of central 5-HT(1A) receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SI, Jafri A, Doggett T, Haxhiu MA. Airway-related vagal preganglionic neurons express brain-derived neurotrophic factor and TrkB receptors: implications for neuronal plasticity. Brain Res. 2005;1044:133–143. doi: 10.1016/j.brainres.2005.02.037. [DOI] [PubMed] [Google Scholar]