Abstract
Two “reverse prenyl” hexahydropyrroloindole alkaloids, 5-N-acetylardeemin and 5-N-acetyl-8-demethylardeemin, were evaluated as reversal agents in cells exhibiting a multidrug resistant (MDR) phenotype. These ardeemins (i) reversed drug resistance to vinblastine (VBL) or to taxol as much as 700-fold at relatively noncytotoxic concentrations in vitro; (ii) as a single agent at high concentrations killed MDR cells more efficaciously than the respective parent wild-type cells; and (iii) exhibited strong synergistic effects with doxorubicin (DX) and VBL against the growth of MDR neoplastic cells, and to a lesser extent, of the parent wild-type cells. Mechanistic studies showed that photoaffinity labeling of P-glycoprotein (Pgp) with [3H] azidopine was competitively inhibited by the ardeemins. Resistance to DX in MDR-[Pgp+ and MDR-associated protein (MRP)+], MDR-Pgp+, lung resistance protein (LRP)+-expressing, and wild-type lung cancer cells were reversed 110- to 200-fold, 50- to 66-fold, 7- to 15-fold, and 0.9- to 3-fold, respectively, by 20 μM of the ardeemins. Moreover, these compounds increased the intracellular accumulation of VBL and markedly decreased its efflux. Finally, in vivo combination studies demonstrated that nontoxic doses of the ardeemins with DX significantly improved the chemotherapeutic effects in B6D2F1 mice bearing DX-resistant P388 leukemia, and nude mice bearing human MX-1 mammary carcinoma xenografts. The above features indicate that the ardeemins may have utility in the therapy of cancer.
Innate or acquired resistance in chemotherapy remains a serious impediment toward the use of drugs in the treatment of cancer (1). A pattern of resistance to a variety of chemotherapeutic drugs with unrelated chemical structures and different mechanisms of action is broadly known as multidrug resistance (MDR). Although the etiology of MDR is multifactorial, the overexpression of P-glycoprotein (Pgp), a membrane protein that mediates the transport of MDR drugs, remains the most common alteration underlying MDR in laboratory models (2). Moreover, expression of Pgp has been linked to the development of MDR in human cancer, particularly in the leukemias, lymphomas, multiple myeloma, neuroblastoma, and soft tissue sarcoma (3–7). Recent studies showed that tumor cells expressing MDR-associated protein (MRP) (8) and lung resistance protein (LRP) (9) and mutation of DNA topoisomerase II (10) also may render MDR.
A plethora of agents have been developed that modify, modulate, or reverse the MDR phenotype. Many natural and synthetic products of various structures, including calcium channel blockers [e.g., verapamil (VRPL), nifedipine] (9), calmodulin antagonists (e.g., trifluoperazine, chlorpromazine), various steroids (e.g., progesterone, tamoxifen), quinolines (e.g., chloroquine, quinidine), immunosuppressive drugs (e.g., cyclosporine, rapamycin), antibiotics (e.g., rifapicin, tetracyclines), surfactants (e.g., Tween 80, Cremophor-EL), and yohimbine alkaloids (e.g., reserpine, yohimbine) have been shown to block the function of Pgp (1, 3–7). Unfortunately, most of these compounds are not useful for MDR reversal at a clinically sustainable level. In some instances, there is a lack of potency. Alternatively, the MDR reversal agent may expose the patient to unacceptable side effects or toxicity at doses required for effectiveness (6, 7). These limitations have spurred efforts to search for new, more effective compounds such as dexverapamil (4) and cyclosporin derivatives (6, 11).
In this paper, we describe studies with two hexacyclic indole alkaloids, 5-N-acetylardeemin (NAA) and 5-N-acetyl-8-demethylardeemin (NADMA) (12), recently synthesized in our laboratories (13). These agents strongly enhance the efficacy of several anticancer agents, both in vitro and in mice bearing tumors. To varying extents, they also sensitize parent non-MDR tumors toward certain chemotherapeutic [vinblastine (VBL) and doxorubicin (DX)] agents that are known to be the substrates of Pgp. Furthermore, it has been found that MDR cells exhibit collateral sensitivity to the ardeemins, i.e., the MDR cells are killed by a much lower dose than their parent cells, even in the absence of VBL and DX. In addition, the drug resistance reversing effects of NAA and NADMA in tumor cells expressing MDR-Pgp, MRP, and LRP are compared.
MATERIALS AND METHODS
Chemicals.
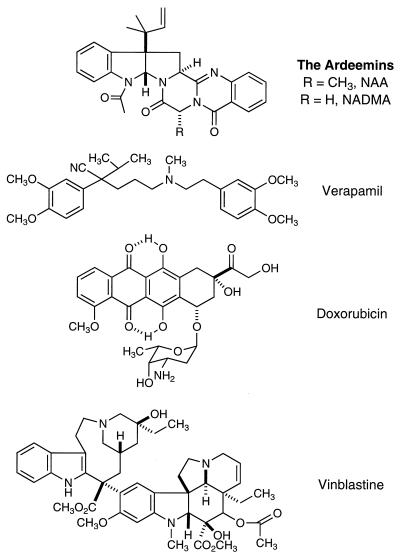
Although NAA is a natural product, presently chemical synthesis provides superior access to this agent. NADMA is available only through chemical synthesis in a related way as described (Fig. 1) (13). VRPL, DX, VBL, etoposide, and teniposide (VM) were purchased from Sigma; [3H]VBL and [3H]DX were obtained from Moravek Biochemicals (Brea, CA), and [3H]azidopine was acquired from Amersham.
Figure 1.
Structures of NAA, NADMA, VRPL, DX, and VBL.
Cell Lines.
The CCRF-CEM human lymphoblastic leukemia cell line and its VBL-selected (CCRF-CEM/VBL100) and VM-selected (CCRF-CEM/VM1) sublines were obtained from W. T. Beck (Univ. of Illinois, Chicago) (14). The DC-3F hamster lung fibroblast cell line and its actinomycin d-selected subline (DC-3F/ADII) were obtained from J. L. Biedler (Sloan-Kettering, New York), and the murine leukemic P388/0 and its DX-selected subline (P388/DX) were obtained from F. A. Schmid (Sloan-Kettering). The small cell lung cancer (SCLC) cell line SHP77 was obtained from A. Koros (Univ. of Pittsburgh, PA). This line was not drug selected and was established before chemotherapy (15, 16). The SHP77 line naturally expresses MDR Pgp, MRP, and LRP (17, 18). This line was grown in RPMI medium 1640 plus 10% fetal bovine serum without any drug selection at 37°C, 5% CO2.
The nonsmall cell lung cancer (NSCLC) cell line SW1573 (parental), SW2R160 (MDR+), and SW2R120 (LRP+) were obtained from Henricus J. Broxterman (Free University Hospital, Amsterdam). The 2R160 and the 2R120 lines were developed from the SW1573 line by stepwise selection with DX (19, 20). The drug-resistant lines were continuously cultured in DX to maintain expression of the drug-resistant markers. The lines were cultured for 1 week in the appropriate concentration of DX, removed from the media, and rested for a minimum of 5 days before each assay. All cell lines were maintained in RPMI medium 1640/10% fetal bovine serum at 37°C, 5% CO2.
Cytotoxicity Assays.
The known anticancer drugs and the ardeemins, individually or in combination, were evaluated for their cytotoxic effects on the growth of various tumor cell lines in vitro under specified conditions.
The cells were cultured in an initial density of 5 × 104 cell/ml. They were maintained in a 5% CO2-humidified atmosphere at 37°C in RPMI medium 1640 (GIBCO/BRL) containing penicillin (100 units/ml), streptomycin (100 mg/ml) (GIBCO-BRL), and 10% heat-inactivated fetal bovine serum. For cell suspension cultures, these were performed by the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-(carboxanilide)-2H-tetrazolium hydroxide (XTT)-microculture tetrazonium method (21) in duplicate in 96-well microtiter plates. XTT was prepared at 1 mg/ml in prewarmed (37°C) medium without serum. Phenazine methosulfate (PMS) and fresh XTT were mixed together to obtain 0.025 mM PMS-XTT solution (25 μl of the stock 5 mM PMS was added per 5 ml of 1 mg/ml XTT). After a 72-h incubation (15), 50 μl of the assay aliquots were added to each well of the cell culture. After incubation at 37°C for 4 h, absorbance at 450 nm and 630 nm was measured with a microplate reader (EL340, Bio-Tek, Winooski, VT).
The cytotoxicity of the ardeemins and selected antitumor agents (VBL and DX) toward the monolayer cell cultures such as (DC-3F, DC-3F/ADII) was determined in 96-well microtiter plates by the sulforhodamine B method described by Skehan et al. (22) for measuring the cellular protein content. Cultures were fixed with trichloroacetic acid and then stained for 30 min with 0.4% sulforhodamine B dissolved in 1% acetic acid. Unbound dye was removed by acetic acid washes, and the protein-bound dye was extracted with an unbuffered Tris base [tris(hydroxy-methyl)aminomethane] for determination of absorbance at 570 nm in a 96-well microtiter plate reader. The experiments were carried out in duplicate. Each run entailed 5–6 concentrations of the drugs being tested. Data were analyzed with previously described computer software (23, 24).
Animals.
Athymic nude mice (nu/nu) were used for MX-1 (human mammary carcinoma) xenografts. Mice were obtained from Taconic Farms (outbred, Swiss background). Male mice 6–10 weeks old, weighing 20–25 g, were used. Transplantable murine tumors (P388 or P388/DX) were maintained in the mouse of origin and transferred to the B6D2F1 mice (DBA/2 and BALB/c × DBA/2 F1) for chemotherapeutic studies. Male mice from Charles River Breeding Laboratories were used. All studies were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Animals and after protocol review by the Institutional Care and Use Committee. For the humane treatment of tumor-bearing animals, mice were euthanized when tumors reached ≥10% of their total body weight.
Membrane Preparation and Photoaffinity Labeling.
To measure Pgp in cells, plasma membrane-enriched microsomal fractions were prepared from CCRF-CEM and CCRF-CEM/VBL100 cells according to the method described by Gerlach et al. (25). Briefly, cells were washed and lysed, and the plasma membrane-enriched microsomal fraction was isolated by ultracentrifugation at 100,000 g for 1 hr at 4°C and resuspended in lysis buffer. Typically, about 100 mg of membrane proteins were obtained from 5 × 107 cells. Pgp was measured by using SDS/PAGE (see below). Photoaffinity labeling of Pgp was performed as previously described (26). Membrane protein (50 μg of protein/assay) was incubated in the presence of the ardeemins or vehicle (dimethyl sulfoxide, DMSO) for 30 min at 20°C in 10 μM Tris⋅HCl, pH 7.5, 0.25 M sucrose. [3H]Azidopine (52 Ci/mmol) was added to a final concentration of 0.5 μM, and the samples were incubated for an additional 20 min. The samples were photolabeled on ice by UV irradiation for 10 min. An equal volume (50 μl) of 2× Laemmli sample buffer was added. The samples were incubated for 5 min at 30°C, after which samples were electrophoresed on 8% polyacrylamide gel and photolabeled bands were detected by autoradiography.
Immunofluorescence Staining and Flow Cytometry.
For MDR-1, 5 × 105 cells were stained with the mAb 4E3 (IgG2A) (Signet Laboratories, Dedham, MA), HYB241 (IgG1) (Hybritech), the isotype matched control MOPC-21 (IgG1), or UPC-10 (IgG2a) (Sigma). The cells were counterstained with goat anti-mouse IgG2a-fluorescein isothiocyanate (FITC) or goat anti-mouse IgG1-PE (Southern Biotechnology Associates). For MRP and LRP staining, 5 × 105 cells were fixed in 10% (vol/vol) lysing solution G (Becton-Dickinson) at room temperature for 10 min. The cells then were washed out of the lysing solution G and then incubated with a 1:20 dilution of MRPm6 (IgG1) (Kamiya Biomedical, Tukwila, WA), LRP-56 (IgG2b) (a generous gift from Rik Scheper, Free University Hospital, Amsterdam), or the isotype matched control MOPC-21 or MOPC-141 (IgG2b) (Sigma) at 4°C for 1 hr. The cells then were counterstained with goat anti-mouse IgG1-FITC or goat anti-mouse IgG2b-FITC (Southern Biotechnology Associates). All stained samples for MDR, MRP, and LRP were analyzed by flow cytometry by using the EPICS XL-MCL (Coulter) to determine the % positive and mean fluorescence intensity.
Intracellular Accumulation of [3H]VBL.
Uptake and intracellular accumulation of [3H]VBL was measured by a rapid oil-layer separation technique described by Wohlhueter et al. (27). A mixture of mineral oil (Sigma) and silicon oil (J. T. Baker), which had a final density of 1.032 g/ml was used to separate medium and cells after rapid centrifugation (12,000 g) by using an Eppendorf model 5415C microcentrifuge (27, 28). The [3H]VBL-containing cells rapidly moved to the bottom of the oil layer whereas the [3H]VBL-containing medium remained on top. The radioactivity in the cell packs were determined by a liquid scintillation counter.
Mathematical Analysis of Drug Combinations.
To determine whether synergistic, additive, or antagonistic antitumor effects were achieved in vitro, cell lines were treated singly and with two-drug combinations. The median-effect plot and the combination index-isobologram method of Chou and Talalay (29, 30) and a computer software program (23, 24) were used to analyze the experimental data. The dose-reduction index denotes the folds of dose reduction allowed for each drug in the combination when compared with each drug alone, at a given effect level (30).
RESULTS
Cytotoxicity and Collateral Sensitivity.
In vitro studies with NAA and NADMA showed moderate cytotoxicity toward two hamster lung cell lines, DC-3F and DC-3F/ADII, with IC50 values of 1.6–26 μM and between 21 and 44 μM for the three human leukemia lines (Table 1). NAA and NADMA inhibited the growth of an actinomycin d-resistant cell line to a greater extent than the parental cell line. The magnitude of this differential cytotoxicity or collateral sensitivity was between 1.4- and 3.1-fold for the ardeemins. The exception was the topoisomerase II-resistant cell line, CCRF-CEM/VM1, which showed weak resistance to the ardeemins relative to the parent.
Table 1.
Cytotoxicity and collateral sensitivity of NAA in wild-type and MDR-Pgp cell lines
| Compound | Cytotoxicity–IC50, μM*
|
||||
|---|---|---|---|---|---|
| Hamster lung cells
|
Human lymphoblastic leukemic cells
|
||||
| DC-3F | DC-3F/ADII | CCRF-CEM | CCRF-CEM/VBL100 | CCRF-CEM/VM1 | |
| NAA | 5.06 | 1.59 | 29.8 | 20.8 | 44.2 |
| NADMA | 26.1 | 14.6 | 22.7 | 32.9 | 35.8 |
| DX | 0.36 | 2.14 | 0.55 | 3.31 | 1.42 |
| VBL† | 0.00083 | 0.632 | 0.00045 | ||
| VBL† + NAA (15 μM) | 0.00021 | 0.00011 | 0.00004 | ||
| VBL† + NADMA (15 μM) | 0.00035 | 0.00017 | 0.00002 | ||
| VBL† + VRPL (75 μM) | 0.00015 | 0.00010 | 0.00016 | ||
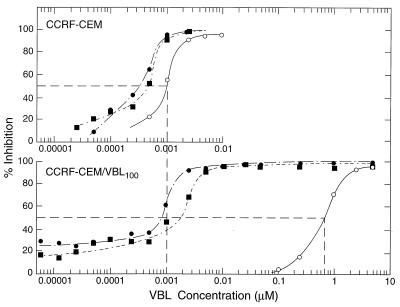
On the other hand, drug combination studies demonstrated strong synergism of the ardeemins with VBL and significant synergism with DX. For VBL with and without NAA or NADMA, dose-effect curves were constructed by using CCRF-CEM and CCRF-CEM/VBL100 cell lines (Fig. 2). In each experiment, concentrations of about one-half of the IC50 for the ardeemins were used. In the parent CCRF-CEM cell line, addition of NAA or NADMA shifted the VBL dose-effect curve about one-half a log unit lower (curves shifted to the left); whereas in the CCRF-CEM/VBL100 cell line, the same concentrations of NADMA or NAA shifted the VBL dose-effect curves to the left 2.5–2.9 log units, respectively. Thus, VBL resistance is essentially nullified by the addition of the ardeemins.
Figure 2.
Dose-effect curves for VBL against the cell growth of human leukemic cells in the absence and presence of MDR reversing agents where ○, varying [VBL] alone; •, varying [VBL] with constant [NAA] (15 μM); ▪, varying [VBL] with constant [NADMA] (15 μM). (Upper) CCRF-CEM lymphoblastic leukemic cells. (Lower) CCRF-CEM/VBL100 leukemic cells 760-fold resistant to VBL. The concentrations of the ardeemins used were about one-half of their IC50 values toward CCRF-CEM cells (see Table 1).
The IC50 values of VBL in CCRF-CEM and in CCRF-CEM/VBL100 were 0.83 nM and 632 nM, respectively (Table 1). The ardeemins (at 15 μM) sensitized the parent cells to VBL about 2.4- to 3.9-fold. CCRF-CEM/VM1 cells were also collaterally sensitive to VBL. However, in the presence of NAA or NADMA at 15 μM, the IC50 values in the CCRF-CEM/VBL100 line ranged from 0.1 to 0.2 nM, which is approximately half the VBL required for the parent cell line. Thus, both NAA and NADMA reversed VBL resistance 760-fold to a level wherein the resistant cells were now more sensitive than the parent cells to VBL. Similar reversal effects were observed for VRPL at 75 μM (Table 2). Experiments for the reversal of resistance to taxol in CCRF-CEM/VBL100 cells by NAA were carried out at a noncytotoxic concentration of NAA (10 μM). As shown in Table 2, the CCRF-CEM/VBL100 cells’ originally 1,372-fold resistance to taxol was reversed to only 3.8-fold resistance in the presence of NAA. For the CCRF-CEM parental cells, taxol effects were enhanced 2.3-fold by NAA, whereas in CCRF-CEM/VBL100 cells, taxol effect was enhanced 839-fold.
Table 2.
Reversal of resistance to taxol in CCRF-CEM/VBL100 cells by NAA
As further evidence toward the generality of the reversing effects of the ardeemins, combinations of NAA and DX (5:1 ratio) also were studied in vitro in the murine leukemia P388/0 cell line and its subline resistant to DX (P388/DX, 9.1-fold resistant) (Table 3). Combination index (CI) values of <1, =1, or >1 indicate synergism, additivism, or antagonism, respectively (27, 28). DX showed a steeper (m > 1, sigmoidal) dose-effect curve for P388/0 cells than the P388/DX cells (m = 1, hyperbolic), whereas NAA showed shallower curves in both cell lines. In combination, the dose-effect curves for both cell lines became steeper and sigmoidal as indicated by the m values (Table 3). The CI values (at IC50–IC95) for the DX and NAA combinations (1:5) were 0.41–0.28 for P388/0 and 0.23–0.10 for P388/DX, which indicates strong synergistic effects for both cell lines with stronger synergism evident in the DX-resistant cell line. At the IC50, the dose-reduction index values (27, 28) indicate that in P388/0, the DX and NAA concentration may be reduced 2.7- and 104-fold, respectively, whereas in P388/DX, they may be reduced 5.1- and 31.7-fold, respectively (Table 3). Similar experiments also were carried out combining NAA with etoposide or taxol. Again synergistic effects were noted.
Table 3.
Synergism between DX and NAA in combination against P388/0 and P388 cell growth
| Tumor cells | Drug | IC50 μM | CI values*
|
Dose reduction index† | Parameters‡
|
||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | IC75 | IC90 | IC95 | m | r | ||||
| P388/0 | DX | 0.23 ± 0.034 | 2.71 | 1.5 ± 0.053 | 0.99 ± 0.005 | ||||
| NAA | 44 ± 20 | 104 | 0.87 ± 0.45 | 0.99 ± 0.015 | |||||
| Combination§ | 0.084 + 0.42 | 0.41 | 0.36 | 0.31 | 0.28 | 1.9 ± 0.56 | 0.92 ± 0.079 | ||
| DX + NAA | ±0.025 | ±0.095 | ±0.16 | ±0.20 | |||||
| P388/DX | DX | 2.1 ± 0.089 | 5.14 | 1.0 ± 0.017 | 0.97 ± 0.013 | ||||
| NAA | 66 ± 1.1 | 31.7 | 0.88 ± 0.059 | 0.95 ± 0.005 | |||||
| Combination§ | 0.42 + 2.1 | 0.23 | 0.17 | 0.12 | 0.099 | 1.4 ± 0.085 | 0.99 ± 0.004 | ||
| DX + NAA | ±0.068 | ±0.056 | ±0.046 | ±0.040 | |||||
CI < 1, synergism; CI > 1, antagonism; CI = 1, additivism, as described (29).
Folds of dose reduction allowed for each drug caused by synergism at a given effect level (30) (e.g., the dose-reduction index values at IC50 effect level for this table).
m, the slope of the median-effect plot (29) signifying the shapes of dose-effect curves (i.e., m = 1, >1, and <1 indicates hyperbolic, sigmodal and shallow sigmoidal shapes, respectively. r, the linear correlation coefficient of the medium-effect plot (29) signifying the conformity of the dose-effect data to the method of data analysis.
NAA/DX 5:1 ratio.
Mechanistic Studies.
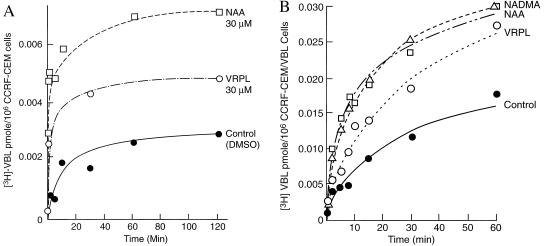
Increased accumulation of MDR drugs. To study the intracellular accumulation of MDR drugs, an oil-layer rapid microcentrifuge method (23) was used. This method allows quick separation of the labeled cells from the radioactive medium. With the parent CCRF-CEM cell line, intracellular concentrations of [3H]VBL were increased in the presence of 30 μM of NAA and 30 μM of VRPL (Fig. 3A). However, in CCRF-CEM/VBL100 cells, where intracellular VBL is much lower, both NAA and NADMA increase [VBL] about 100% to a concentration just under that found in the parent line (Fig. 3B).
Figure 3.
Intracellular accumulation of [3H]VBL in (A) CCRF-CEM with •, DMSO control; □, 30 μM NAA; ○, 30 μM VRPL, and in (B) CCRF-CEM/VBL100 cells with •, DMSO control; ▵, 30 μM NADMA; □, 30 μM NAA, and ○, 30 μM VRPL. Cells were preloaded with [3H]VBL at 37°C for 30 min. Without washing, MDR reversing agents were added at 0 min and incubated at 25°C for various times. At each time point, intracellular drug concentrations were determined by the oil-layer microfuge method and with scintillation counting (27, 28).
Retardation of MDR drug efflux.
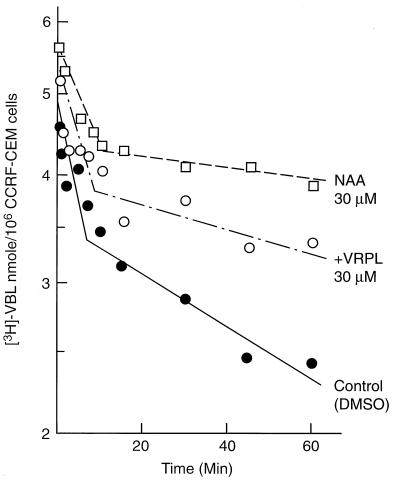
To study the effect of NAA on net efflux of VBL, CCRF-CEM cells were preloaded with [3H]VBL and then transferred to fresh medium. The time course of the decrease of intracellular drug is shown in Fig. 4. Drug elimination was biphasic with an initial half-life of 12 min and a β-phase half-life of 105 min. In the presence of 30 μM VRPL, the half-lives for the two phases were prolonged to 18 min and 210 min, respectively. In the presence of 30 μM NAA, these half-lives were extended to 29 min and 380 min, respectively. Thus, NAA not only resulted in a higher intracellular concentration of VBL, but also prolonged the half-life of the preloaded VBL with a higher intracellular concentration of VBL than did either VRPL or the control.
Figure 4.
Efflux kinetics for preloaded [3H]VBL in CCRF-CEM cells in the presence and absence of MDR reversing agents: •, control (DMSO vehicle, 0.2% vol/vol); ○, 30 μM VRPL, and □, 30 μM NAA. [3H]VBL was preloaded for 30 min at 37°C, washed twice at 4°C with fresh medium. NAA or VRPL was added at 0 min and incubated at 37°C for various time periods.
Inhibition of azidopine photoaffinity labeling of Pgp.
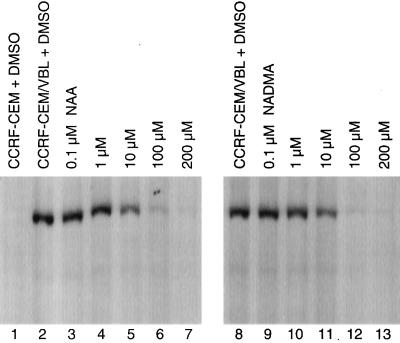
Evidence that NAA interacts directly with the transporter, Pgp, was obtained by examining its effect on the inhibition of photoaffinity labeling of [3H]azidopine, a photoactivatable substrate of Pgp. The membrane fractions from CCRF-CEM parent cells showed little or no [3H]azidopine binding (Fig. 5, lane 1). Membrane fractions isolated from CCRF-CEM/VBL100 cells expressing Pgp were used for the displacement studies (Fig. 5, lanes 2–13). Both NAA and NADMA inhibited [3H]azidopine binding to Pgp in a dose-dependent manner. Partial inhibition was observed for both drugs at 10 μM (Fig. 5, lanes 5 and 11), and nearly complete inhibition was observed at 100 μM (Fig. 5, lanes 6 and 12) when compared with the control (DMSO, Fig. 5, lanes 2 and 8).
Figure 5.
Effect of NAA or NADMA on [3H]azidopine photoaffinity labeling of Pgp. DMSO control with CCRF-CEM Pgp only (lane 1) and CCRF-CEM/VBL100 Pgp only as controls (lanes 2 and 8); CCRF-CEM/VBL100 Pgp with 0.1, 1, 10, 100, and 200 μM of NAA (lanes 3–7), and with 0.1, 1, 10, 100, and 200 μM of NADMA (lanes 9–13) are shown.
Ardeemin-enhanced efficacy of DX in tumor cells expressing different MDR phenotypes.
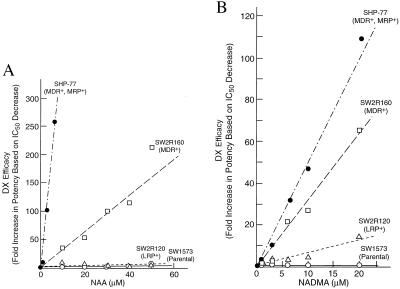
Enhancement of DX cytotoxicity by NAA (1–50 μM, Fig. 6A) and by NADMA (1–20 μM, Fig. 6B) in human lung cancer cell lines expressing different MDR phenotypes were studied in cell culture. For SHP-77 (MDR-Pgp+ + MRP+) cells, the IC50 of DX (2.35 μM) was reduced 260-fold to 0.009 μM by 6 μM NAA. In contrast, for SW1573 (parental), SW2R120 (LRP+), and SW2R160 (MDR-Pgp+) cells, the DX IC50 values of 0.021 μM, 0.270 μM, and 2.04 μM, respectively, were reduced 3-fold, 7-fold, and 50-fold, respectively, by 20 μM of NAA (Fig. 6A). Similarly for SHP-77 (MDR-Pgp+ + MRP+) cells, the DX IC50 value was reduced 110-fold, to 0.021 μM by 20 μM NADMA. For SW1573 (parental), SW2R120 (LRP+), and SW2R160 (MDR-Pgp+) cells, the DX IC50 values were reduced 0.9-fold, 15-fold, and 66-fold by 20 μM NADMA, respectively (Fig. 6B). Therefore, both NAA and NADMA markedly enhanced the efficacy of DX against SHP-77 (MDR-Pgp+ + MRP+) and SW2R160 (MDR-Pgp+) cells, but had little or no effect in enhancing the efficacy of DX against SW2R120 (LRP+) or SW1573 (parental) cells. The IC50 for NADMA alone in these cell lines ranged from 96 ± 34 to 156 ± 31 μM and from 71 ± 18 to 137 ± 45 μM, respectively.
Figure 6.
Enhancement of DX cytotoxicity by (A) NAA and (B) NADMA. Six concentrations of DX were tested for cytotoxicity in (•), SHP77 (MDR+ + MRP+); (▵), SW2R120 (wild type); (□), SW2R160 (MDR+), and (○), SW1573 (LRP+) cells in the presence and absence of NAA or NADMA. The concentrations required for 50% inhibition of cell growth (IC50 in μM) were determined by the median-effect plot using computer software (23, 24). Fold of increase in efficacy of DX is based on fold of decrease in IC50. NAA or NADMA alone showed little or no cytotoxicity in these cell lines.
In Vivo Studies of DX and the Ardeemins.
The antitumor effects of percent increase in life span (% ILS) in B6D2F1 mice bearing either parental P388/0 or resistant P388/DX leukemia cells were evaluated in two parallel experiments both of which included treatment with DX alone, NAA alone, and the two drugs in combination (Table 4) relative to DMSO control groups. Daily i.p. injection of NAA alone at 70 mg/kg for 4 days per week for 2 weeks provided no beneficial therapeutic effects in terms of % ILS. For the P388/0 line, the same treatment schedule of DX alone (0.5–2 mg/kg) increased the life span 128–178% in a dose-dependent manner. The combination of DX and NAA increased the life span further to 144–189%, also in a dose-dependent manner. For P388/DX leukemia, DX alone increased the life span 49–106%, whereas the combination increased the life span 69–151%. In the P388/0 experiment, NAA significantly improved the therapeutic effects of DX at 0.5 and 1.0 mg/kg dosages but not significantly at a 2.0 mg/kg dosage. In contrast, for the P388/DX experiment, NAA significantly enhanced the therapeutic effects of DX at 0.1, 1.0, and 2.0 mg/kg DX dosages.
Table 4.
Therapeutic effects of DX and NAA in B6D2F1 mice bearing P388/0 and P388/DX tumors
| Tumor | Dosage, mg/kg
|
Median survival time, days ± SEM* | % increase in life span | |
|---|---|---|---|---|
| DX | NAA | |||
| P388/0 | 0 | 0 | 7.58 ± 0.32 | 0 |
| 0.5 | 17.3 ± 0.80 | +128 | ||
| 1.0 | 19.1 ± 0.87 | +152 | ||
| 2.0 | 21.1 ± 0.66 | +178 | ||
| 70 | 7.75 ± 0.65 | +2 | ||
| 0.5 | 70 | 18.5 ± 0.65‡ | +144 | |
| 1.0 | 70 | 20.8 ± 1.14§ | +174 | |
| 2.0 | 70 | 21.9 ± 1.02¶ | +189 | |
| P388/DX | 0 | 7.55 ± 0.38 | 0 | |
| 0.5 | 11.3 ± 0.91 | +49 | ||
| 1.0 | 13.3 ± 0.76 | +75 | ||
| 2.0 | 15.6 ± 0.54 | +106 | ||
| 70 | 7.55 ± 0.67 | +0 | ||
| 0.5 | 70 | 12.8 ± 0.86‡ | +69 | |
| 1.0 | 70 | 15.2 ± 0.55‡ | +101 | |
| 2.0 | 70 | 19.0 ± 0.82† | +151 | |
Male B6D2F1 mice were inoculated with tumor cells, 106/mouse, i.p. on day 0, and treated by DX and/or NAA starting on day 1, daily for 4 days per week for 2 weeks. There were five mice per dosage and 10 mice in the control group.
Survival time was recorded at the time of death with the following decimal values: 8 a.m. (0.0), 1 p.m. (0.25), and 5 p.m. (0.5). Comparing the median-survival time for treatment with DX for the same tumor in the presence and absence of NAA: †, P < 0.005; ‡, P < 0.01; §, P < 0.05; ¶, not significant.
Additional in vivo experiments of NAA with DX using nude mice bearing MX-1 human mammary carcinoma xenografts were conducted (Table 5). To ensure effective tumor incorporation, treatment was withheld until the seventh day after implantation. The control mice each received 40 μl of DMSO vehicle. When DX was injected alone (1.5 mg/kg), average tumor volume was reduced 44% by day 27 relative to the control with a manifested toxicity. With twice daily injections of NAA alone (50 mg/kg), tumor volume showed a slight increase rather than a decrease over the same period. When these doses were administered in combination, average tumor volume decreased 75% by day 27 and resulted in two cures by day 49 with similar toxicity to mice as DX alone.
Table 5.
Combination therapy of NAA and DX in swiss (nu/nu) mice bearing human MX-1 mammary carcinoma xenograft
| Dose, mg/kg
|
Average tumor volume, mm3, mean ± SEM on day
|
Total no. of mice (n) | Mice died of toxicity | Mice tumor-free on day 49 | ||||
|---|---|---|---|---|---|---|---|---|
| DX* | NAA* | D12 | D17 | D22 | D27 | |||
| 0 | 0 | 47.7 ± 11.1 | 98.1 ± 28.6 | 200.7 ± 49.4 | 353.9 ± 63.3 | 7 | 0 | 0 |
| 1.5 | 32.3 ± 6.3 | 80.0 ± 29.2 | 145.7 ± 30.5 | 198.4 ± 57.6 | 8 | 2 | 0 | |
| 50 | 42.6 ± 12.1 | 140.6 ± 45.6 | 282.7 ± 86.1 | 465.5 ± 86.3 | 7 | 0 | 0 | |
| 1.5 | 50 | 39.5† ± 7.9 | 60.6† ± 16.0 | 97.9‡ ± 23.9 | 90.0‡ ± 19.7 | 8 | 1 | 2 |
MX-1 tumor tissue 50 μl/mouse was implanted s.c. on day 0. Treatment started on day 7, i.p., DX daily for 5 days; NAA twice daily for 5 days (at 15 min and 90 min after DX injection). Thus, nude mice were treated on days 6–10 and days 13–17.
Control group received the solvent vehicle DMSO, 40 μl/mouse.
†, not significant; ‡, P < 0.05 when compared with tumor volume treated with DX in the absence of NAA.
Again, NAA significantly increased the therapeutic effects of DX without markedly increasing its overall systemic toxicity.
DISCUSSION
The two ardeemins, NAA and NADMA, enhance significantly the effects of a few widely used chemotherapeutic drugs. Cytotoxicity assays suggested that their enhancing power is associated with heightening the activity of VBL and DX in parent cell lines, such as CCRF-CEM and P388, respectively, while resensitizing the resistant cell line relatives CCRF-CEM/VBL100 and P388/DX. For example, as shown in Table 1, the addition of NAA or NADMA (15 μM) to VBL increases the latter’s potency 2- to 4-fold in the parent cell line and 3,700- to 5,700-fold in the VBL-resistant line. The latter cases are noteworthy as the measured IC50s are brought to lower levels than in the parent cell lines by the simple addition of the ardeemins.
These studies also described strong levels of synergism between the chemotherapeutic agents and the ardeemins, which lead to significant dose-reduction index values. For instance, addition of NAA to DX in a 5:1 ratio against P388/DX increases the potency of DX five times.
In vivo studies of NAA with DX prolonged the survival life of tumor-bearing B6D2F1 mice. Depending on the dosage, increases as high as 189% were found for those inoculated with P388 cells or 151% with P388/DX. Other studies of NAA with DX in nude mice bearing human MX-1 mammary carcinoma xenografts led to some cures and substantially greater tumor volume reduction than with the chemotherapeutic agents alone.
The exact mechanism(s) of action exerted by the ardeemins in the cell are unclear. Both NAA and NADMA completely inhibit the photoaffinity labeling of Pgp by [3H]azidopine at 100 μM and partial inhibition was noticeable at 10 μM. Also, NAA increases the uptake and decreases the efflux of VBL in human leukemia cell lines. Thus, through several effects, the ardeemins prolong the time these drugs spend in cells. Further studies with lung cancer cells expressing different MDR phenotypes indicated that the ardeemins markedly enhanced DX efficacy against MDR-Pgp+ and MRP+ cells, but with much less enhancement in LRP+ cells.
Another important property of the ardeemins worth noting is their low toxicity in mice. Quantities as high as 300 mg/kg per day i.p. of NADMA for 3 days or 150 mg/kg per day i.p. for 8 days have been tolerated by mice, without notable weight loss (data not shown). This is in marked contrast to VRPL in which 150 mg/kg per day for 3 days led to 40% lethality to mice. The remarkably low level of toxicity of the ardeemins coupled with their potent MDR-reversing activity at nontoxic concentrations as demonstrated above, renders these compounds appropriate for further study and development.
Acknowledgments
We are grateful to Dr. Joseph R. Bertino and Dr. Kathleen Scotto for many helpful discussions regarding this manuscript. We thank Quen-Hui Tan, Luan-Ing Chen, and Xiuguo Zhang for technical assistance and Yehudah Rubenstein for assisting the preparation of the manuscript. This work was supported by National Institutes of Health Grant HL25848. We also acknowledge the Pfizer Corporation for a Pfizer Predoctoral Fellowship (K.M.D).
ABBREVIATIONS
- MDR
multidrug resistance
- Pgp
P-glycoprotein
- MRP
MDR-associated protein
- LRP
lung resistance protein
- CI
combination index
- NAA
5-N-acetylardeemin
- NADMA
5-N-acetyl-8-demethylardeemin
- VRPL
verapamil
- DX
doxorubicin
- VBL
vinblastine
- VM
teniposide
- DMSO
dimethyl sulfoxide
References
- 1.Laing N M, Tew K D. In: Encyclopedia of Cancer. Bertino J R, editor. Vol. 1. San Diego: Academic; 1997. pp. 560–570. [Google Scholar]
- 2.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 3.Fan D, Beltran P J, O’Brian C A. In: Reversal of Multidrug Resistance in Cancer. Kellen J A, editor. Boca Raton, FL: CRC; 1994. pp. 93–125. [Google Scholar]
- 4.Pirker R, Zochbauer S, Kupper H, Lassmann A, Gsur A, Frass M, Krajnik G, Knobl P, Lechner A. Eur J Cancer. 1991;27:1639–1642. doi: 10.1007/BF02351067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirker R, Keilhauer G, Raschack M, Lechner C, Ludwig H. Int J Cancer. 1989;45:916–919. doi: 10.1002/ijc.2910450523. [DOI] [PubMed] [Google Scholar]
- 6.Ross D D, Wooten P J, Sidhara R, Ordonez J V, Lee E J, Schiffer C A. Blood. 1994;82:1288–1299. [PubMed] [Google Scholar]
- 7.Malayeri R, Filipits M, Suchomel R W, Zochbauer S, Lechner K, Pirker R. Leukemia Lymphoma. 1996;23:451–458. doi: 10.3109/10428199609054853. [DOI] [PubMed] [Google Scholar]
- 8.Cole S P C, Bhardwaj G, Gerlach J H, Mackie J E, Grant C E, Almquist K C, Stewart A J, Kurz E V, Duncan A M V, Deeley R G. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 9.Scheffer G L, Wijngaard P L J, Flens M J, Inzquierdo M A, Slovak M L, Pinedo H M, Meijer C J L M, Clevers H C, Scheper R J. Nat Med. 1995;1:578–582. doi: 10.1038/nm0695-578. [DOI] [PubMed] [Google Scholar]
- 10.Beck W T. J Natl Cancer Inst. 1989;81:1683–1685. doi: 10.1093/jnci/81.22.1683. [DOI] [PubMed] [Google Scholar]
- 11.Slate L D, Bruno A N, Casey M S, Zutshi N, Garvin J L, Wu H, Pfister R J. Anticancer Res. 1995;15:811–814. [PubMed] [Google Scholar]
- 12.Karnowski J P, Jackson M, Rasmussen R R, Humphrey P E, Poddig J B, Kohl W L, Scherr M H, Kadam S, McAlpine J B. J Antibiot. 1993;46:374–386. doi: 10.7164/antibiotics.46.374. [DOI] [PubMed] [Google Scholar]
- 13.Marsden S P, Depew K M, Danishefsky S J. J Am Chem Soc. 1994;116:11143–11144. [Google Scholar]
- 14.Beck W T, Mueller T J, Tanzer L R. Cancer Res. 1979;39:2027–2076. [PubMed] [Google Scholar]
- 15.Fisher E R, Faulson J D. Cancer Res. 1978;38:3830–38356. [PubMed] [Google Scholar]
- 16.Koros A M, Klein E C, Pan S, Atchison R W, Lakomy R, Bahnson A, Shever C. Cancer Res. 1985;45:2725–2731. [PubMed] [Google Scholar]
- 17.Chan D, Chuang N, Jewett P, Kirpotin D, Helfrich B, Bunn P., Jr Proc Am Assoc Cancer Res. 1993;34:323. (Abstr.) [Google Scholar]
- 18.Chan D, Helfrich B, Helm K, Chou T, Bunn P. Proc Am Assoc Cancer Res. 1997;38:591. (Abstr.). [Google Scholar]
- 19.Keizer H, Schuurhuis G J, Broxterman H J, Lankelma J, Schounen W, Vani Rijin J, Pinedo H M, Joenje H. Cancer Res. 1989;49:2988–2993. [PubMed] [Google Scholar]
- 20.Kuiper C M, Broxterman H J, Baas F, Schuurhuis G J, Haisma H J, Scheffer G L, Lankelma J, Pinedo H M. J Cell Pharmacol. 1990;1:35–41. [Google Scholar]
- 21.Scudiero D A, Shoemaker R H, Paull K D, Monks A, Tierney S, Nofziger T H, Currens M J, Seniff D, Boyd M R. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren J T, Bokesch H, Kenny S, Boyd M R. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Chou J, Chou T-C. Dose-Effect Analysis with Microcomputers: Quantitation of ED 50, Synergism, Antagonism, Low-Dose Risk, Reception-Ligand Binding and Enzyme Kinetics. Cambridge, U.K.: Biosoft; 1987. [Google Scholar]
- 24.Chou T-C, Hayball M. CalcuSyn for Windows 3.1 and Windows 95: Multiple Drug Dose-Effect Analyzer and Manual for IBM-PC. Cambridge, U.K.: Biosoft; 1996. [Google Scholar]
- 25.Gerlach J H, Bell D R, Karakousis C, Slocum H K, Kartner N, Rustum Y M, Ling V, Baker R M. J Clin Oncol. 1987;5:1452–1460. doi: 10.1200/JCO.1987.5.9.1452. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Sachs C W, Fine R L, Casey P J. J Biol Chem. 1994;269:15973–15976. [PubMed] [Google Scholar]
- 27.Wohlhueter R M, Marz R, Graff J C, Plagemann R G W. Methods Cell Biol. 1978;20:211–236. doi: 10.1016/s0091-679x(08)62020-8. [DOI] [PubMed] [Google Scholar]
- 28.Kolassa N, Paterson A R P, Chou T-C. Cancer Treatment Rep. 1983;67:51–58. [PubMed] [Google Scholar]
- 29.Chou T-C, Talalay P T. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Chou T C. In: Synergism and Antagonism in Chemotherapy. Chou T-C, Rideout D C, editors. San Diego: Academic; 1991. pp. 61–102. [Google Scholar]