Abstract
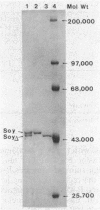
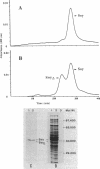
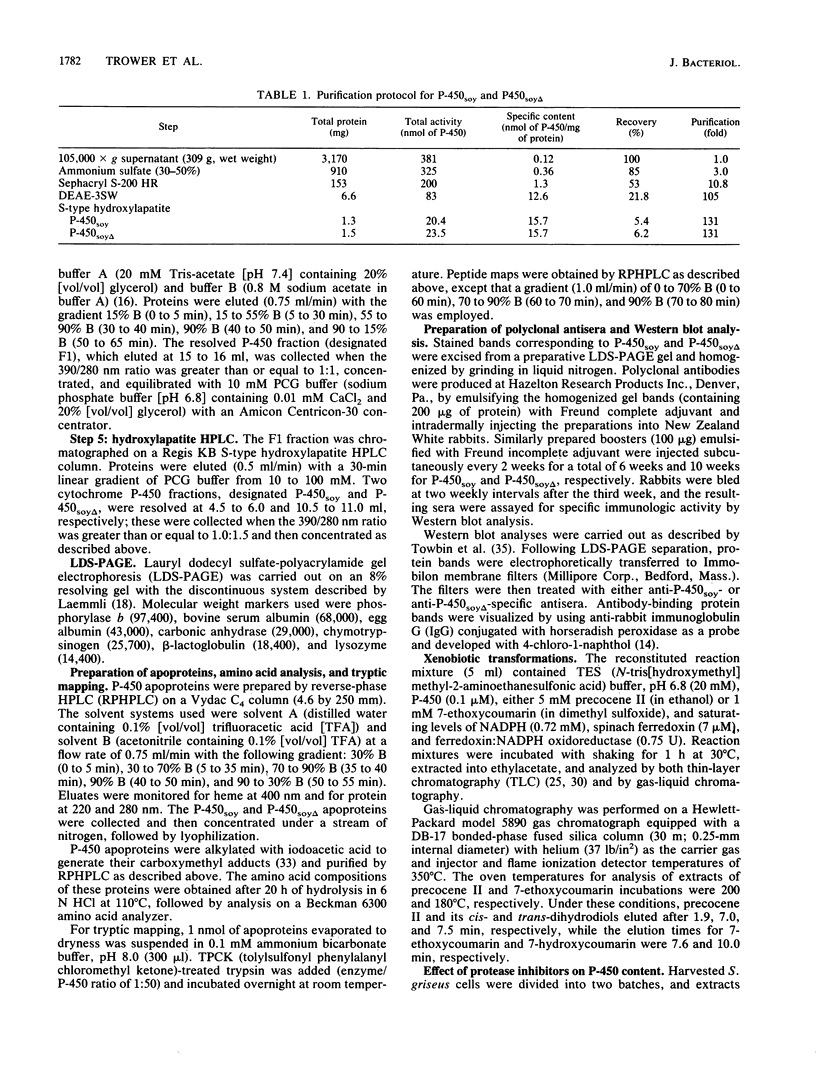
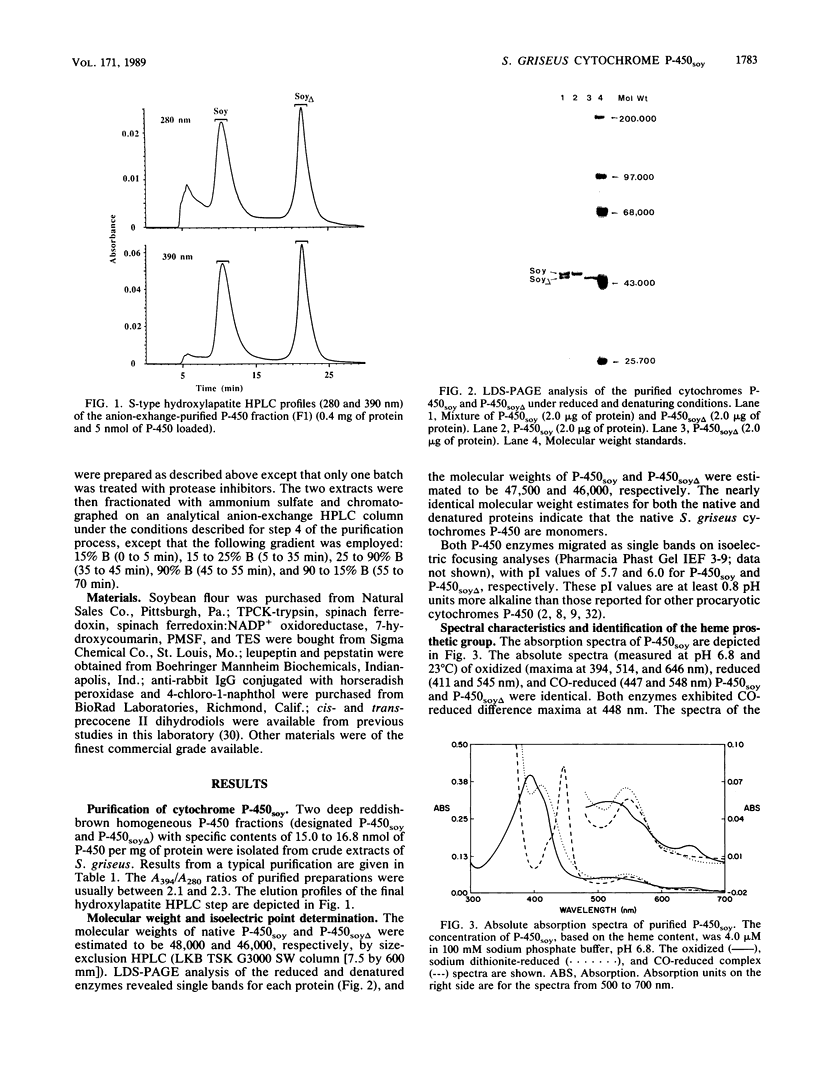
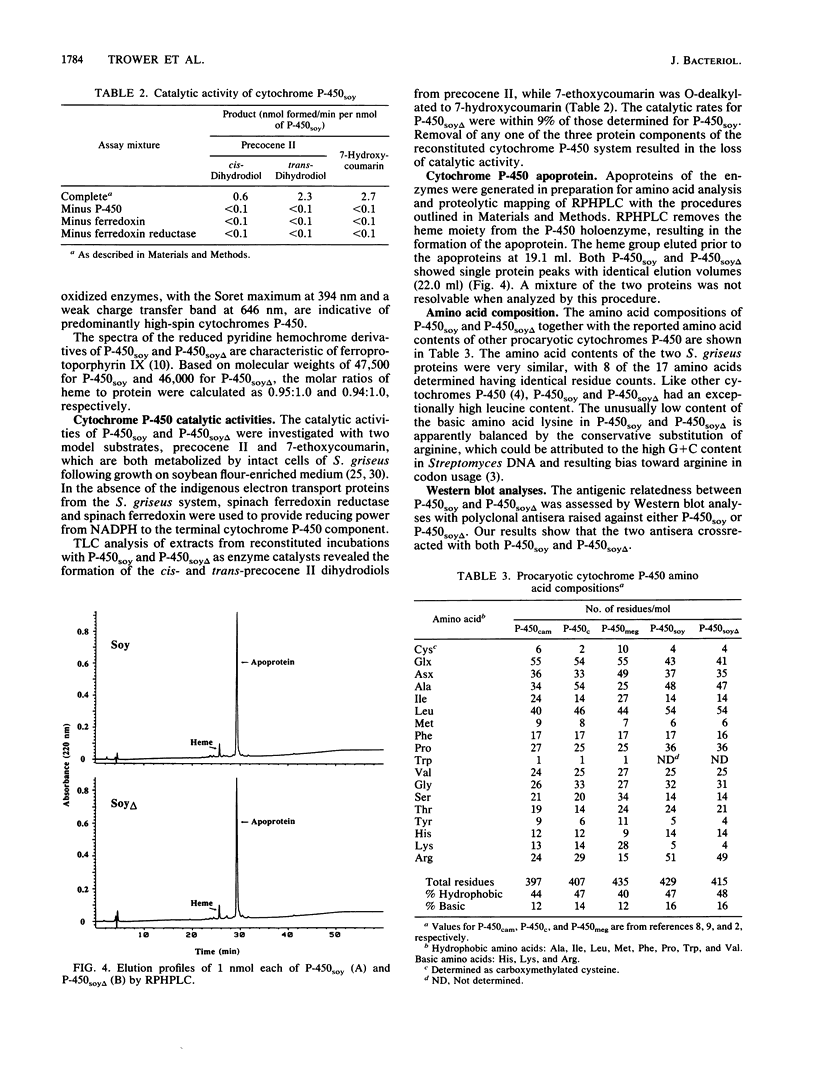
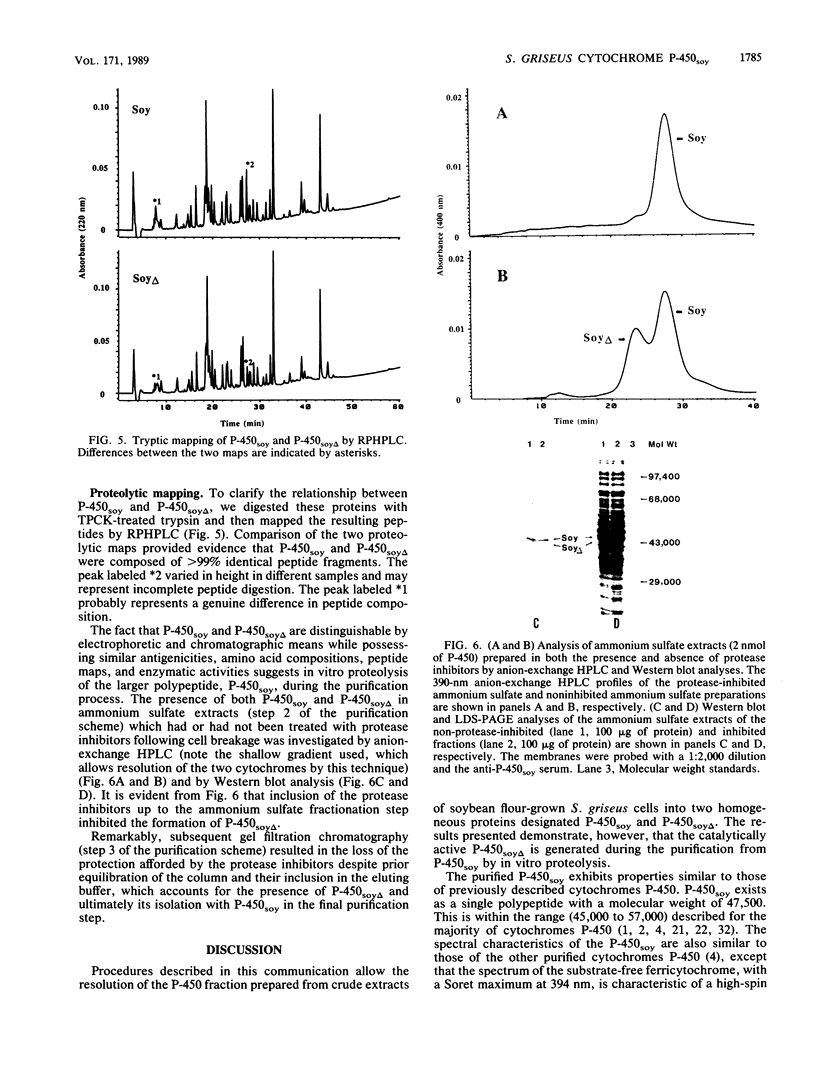
A soybean flour-induced, soluble cytochrome P-450 (P-450soy) was purified 130-fold to homogeneity from Streptomyces griseus. Native cytochrome P-450soy is a single polypeptide, with a molecular weight of 47,500, in association with one ferriprotoporphyrin IX prosthetic group. Oxidized P-450soy exhibited visible absorption maxima at 394, 514, and 646 nm, characteristic of a high-spin cytochrome P-450. The CO-reduced difference spectrum of P-450soy had a Soret maximum at 448 nm. When reconstituted with spinach ferredoxin and spinach ferredoxin:NADP+ oxidoreductase, purified cytochrome P-450soy catalyzed the NADPH-dependent oxidation of the xenobiotic substrates precocene II and 7-ethoxycoumarin. In vitro proteolysis of cytochrome P-450soy generated a stable and catalytically active cytochrome P-450, designated P-450soy delta.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Purification of Rhizobium cytochromes P-450. Methods Enzymol. 1978;52:157–166. doi: 10.1016/s0076-6879(78)52018-1. [DOI] [PubMed] [Google Scholar]
- Berg A., Ingelman-Sundberg M., Gustafsson J. A. Purification and characterization of cytochrome P-450meg. J Biol Chem. 1979 Jun 25;254(12):5264–5271. [PubMed] [Google Scholar]
- Bibb M. J., Findlay P. R., Johnson M. W. The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene. 1984 Oct;30(1-3):157–166. doi: 10.1016/0378-1119(84)90116-1. [DOI] [PubMed] [Google Scholar]
- Boström H., Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of subfractions with different substrate specificity from cytochrome P-450LM4. J Biol Chem. 1982 Oct 10;257(19):11755–11759. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dus K., Goewert R., Weaver C. C., Carey D. P-450 hemeproteins of Rhizobium japonicum. Purification by affinity chromatography and relationship to P-450CAM and P-450LM-2. Biochem Biophys Res Commun. 1976 Mar 22;69(2):437–445. doi: 10.1016/0006-291x(76)90541-6. [DOI] [PubMed] [Google Scholar]
- Dus K., Katagiri M., Yu C. A., Erbes D. L., Gunsalus I. C. Chemical characterization of cytochrome P-450cam. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1423–1430. doi: 10.1016/0006-291x(70)90026-4. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of liver microsomal cytochromes p-450: electrophoretic, spectral, catalytic, and immunochemical properties and inducibility of eight isozymes isolated from rats treated with phenobarbital or beta-naphthoflavone. Biochemistry. 1982 Nov 9;21(23):6019–6030. doi: 10.1021/bi00266a045. [DOI] [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Properties of electrophoretically homogeneous phenobarbital-inducible and beta-naphthoflavone-inducible forms of liver microsomal cytochrome P-450. J Biol Chem. 1976 Dec 25;251(24):7929–7939. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hsia M. T., Grossman S., Schrankel K. R. Hepatotoxicity of the anti-juvenile hormone precocene II and the generation of dihydrodiol metabolites. Chem Biol Interact. 1981 Nov;37(3):265–277. doi: 10.1016/0009-2797(81)90113-7. [DOI] [PubMed] [Google Scholar]
- Kotake A. N., Funae Y. High-performance liquid chromatography technique for resolving multiple forms of hepatic membrane-bound cytochrome P-450. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6473–6475. doi: 10.1073/pnas.77.11.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz D. A., Reddy G. S., Vatvars A. Identification of transformation products arising from bacterial oxidation of codeine by Streptomyces griseus. Appl Environ Microbiol. 1985 Oct;50(4):831–836. doi: 10.1128/aem.50.4.831-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan E. T., Koop D. R., Coon M. J. Catalytic activity of cytochrome P-450 isozyme 3a isolated from liver microsomes of ethanol-treated rabbits. Oxidation of alcohols. J Biol Chem. 1982 Dec 10;257(23):13951–13957. [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- O'Keeffe D. H., Ebel R. E., Peterson J. A. Purification of bacterial cytochrome P-450. Methods Enzymol. 1978;52:151–156. doi: 10.1016/s0076-6879(78)52017-x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Patten C. J., Ning S. M., Lu A. Y., Yang C. S. Acetone-inducible cytochrome P-450: purification, catalytic activity, and interaction with cytochrome b5. Arch Biochem Biophys. 1986 Dec;251(2):629–638. doi: 10.1016/0003-9861(86)90373-5. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Eckenrode F. M., Beale J. M., Jr, Rosazza J. P. Formation of a reactive iminium derivative by enzymatic and chemical oxidations of 16-O-acetylvindoline. J Med Chem. 1984 Jun;27(6):749–754. doi: 10.1021/jm00372a008. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., Kunz D. A. Induction of cytochrome P-450 in Streptomyces griseus by soybean flour. Biochem Biophys Res Commun. 1986 Dec 15;141(2):405–410. doi: 10.1016/s0006-291x(86)80187-5. [DOI] [PubMed] [Google Scholar]
- Sariaslani F. S., McGee L. R., Ovenall D. W. Microbial transformation of precocene II: oxidative reactions by Streptomyces griseus. Appl Environ Microbiol. 1987 Aug;53(8):1780–1784. doi: 10.1128/aem.53.8.1780-1784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslani F. S., Rosazza J. P. Biotransformations of 1',2'-dihydrorotenone by Streptomyces griseus. Appl Environ Microbiol. 1985 Feb;49(2):451–452. doi: 10.1128/aem.49.2.451-452.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariaslani F. S., Rosazza J. P. Novel Biotransformations of 7-Ethoxycoumarin by Streptomyces griseus. Appl Environ Microbiol. 1983 Aug;46(2):468–474. doi: 10.1128/aem.46.2.468-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S. L., Shires T. K. Purification and characterization of a previously unreported form of cytochrome P-448 from the liver of 3-methylcholanthrene-pretreated rats. Biochem J. 1986 May 1;235(3):859–868. doi: 10.1042/bj2350859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee A., Hutchinson C. R. Macrolide antibiotic biosynthesis: isolation and properties of two forms of 6-deoxyerythronolide B hydroxylase from Saccharopolyspora erythraea (Streptomyces erythreus). Biochemistry. 1987 Sep 22;26(19):6204–6210. doi: 10.1021/bi00393a037. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]