Abstract
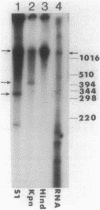
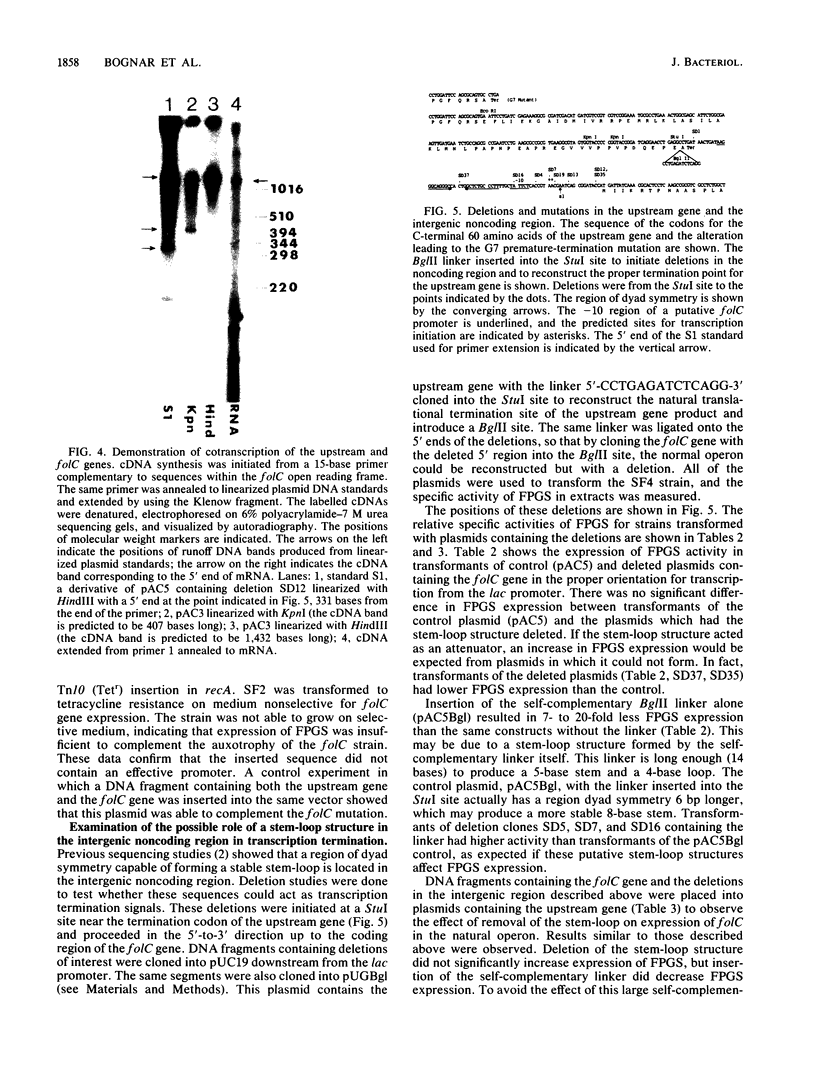
The folC gene of Escherichia coli is cotranscribed with an upstream gene from two promoters located in the noncoding region 5' to the coding sequence of the upstream gene. Virtually all of the expression of the folC gene product, folylpolyglutamate synthetase-dihydrofolate synthetase, is therefore due to the upstream gene promoters. No promoter activity was found in the coding sequence of the upstream gene or in the 72-base-pair noncoding region between the two genes. It is shown that a third gene, which may overlap the coding sequence of the folC gene by 8 base pairs at the 3' end, nevertheless, has an promoter independent from that of the upstream gene-folC operon. These results contrast with those presented by Nonet et al. (M. L. Nonet, C. C. Marvel, and D. Tolan, J. Biol. Chem., 262:12209-12217, 1987), who concluded that folC was cotranscribed with the gene at its 3' end and the gene upstream to folC was cotranscribed with the gene(s) further upstream. A stable stem-loop structure resembling a rho-independent terminator is present within the noncoding region between the upstream gene and the folC gene. Folypolyglutamate synthetase expression is 6- to 15-fold lower than that of the upstream gene product, suggesting that the stem-loop terminates some of the transcription from the upstream gene promoter. We found by deletion mutagenesis and cloning sequences containing the stem-loop structure into a termination reporter plasmid that this stem-loop does not act as an effective terminator of transcription. We also found that the stem-loop does not protect the upstream gene message from degradation, since expression of the upstream gene product in maxicell experiments is the same whether the stem-loop structure is present or deleted.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bognar A. L., Osborne C., Shane B. Primary structure of the Escherichia coli folC gene and its folylpolyglutamate synthetase-dihydrofolate synthetase product and regulation of expression by an upstream gene. J Biol Chem. 1987 Sep 5;262(25):12337–12343. [PubMed] [Google Scholar]
- Bognar A. L., Osborne C., Shane B., Singer S. C., Ferone R. Folylpoly-gamma-glutamate synthetase-dihydrofolate synthetase. Cloning and high expression of the Escherichia coli folC gene and purification and properties of the gene product. J Biol Chem. 1985 May 10;260(9):5625–5630. [PubMed] [Google Scholar]
- Bognar A. L., Shane B. Purification and properties of Lactobacillus casei folylpoly-gamma-glutamate synthetase. J Biol Chem. 1983 Oct 25;258(20):12574–12581. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Revised interpretation of the origin of the pSC101 plasmid. J Bacteriol. 1977 Nov;132(2):734–737. doi: 10.1128/jb.132.2.734-737.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- McNeil J. B., Friesen J. D. Expression of the Herpes simplex virus thymidine kinase gene in Saccharomyces cerevisiae. Mol Gen Genet. 1981;184(3):386–393. doi: 10.1007/BF00352510. [DOI] [PubMed] [Google Scholar]
- McNeil J. B., Storms R. K., Friesen J. D., Smith M. Efficient expression of the Escherichia coli leuB gene in yeast. Curr Genet. 1985;9(8):653–660. doi: 10.1007/BF00449818. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Marvel C. C., Tolan D. R. The hisT-purF region of the Escherichia coli K-12 chromosome. Identification of additional genes of the hisT and purF operons. J Biol Chem. 1987 Sep 5;262(25):12209–12217. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. Cloning of uvrA, lexC and ssb genes of Escherichia coli. Biochem Biophys Res Commun. 1979 Sep 12;90(1):123–129. doi: 10.1016/0006-291x(79)91598-5. [DOI] [PubMed] [Google Scholar]
- Sancar A., Wharton R. P., Seltzer S., Kacinski B. M., Clarke N. D., Rupp W. D. Identification of the uvrA gene product. J Mol Biol. 1981 May 5;148(1):45–62. doi: 10.1016/0022-2836(81)90234-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane B. Pteroylpoly(gamma-glutamate) synthesis by Corynebacterium species. Purification and properties of folypoly(gamma-glutamate) synthetase. J Biol Chem. 1980 Jun 25;255(12):5655–5662. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wong H. C., Chang S. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc Natl Acad Sci U S A. 1986 May;83(10):3233–3237. doi: 10.1073/pnas.83.10.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]