Abstract
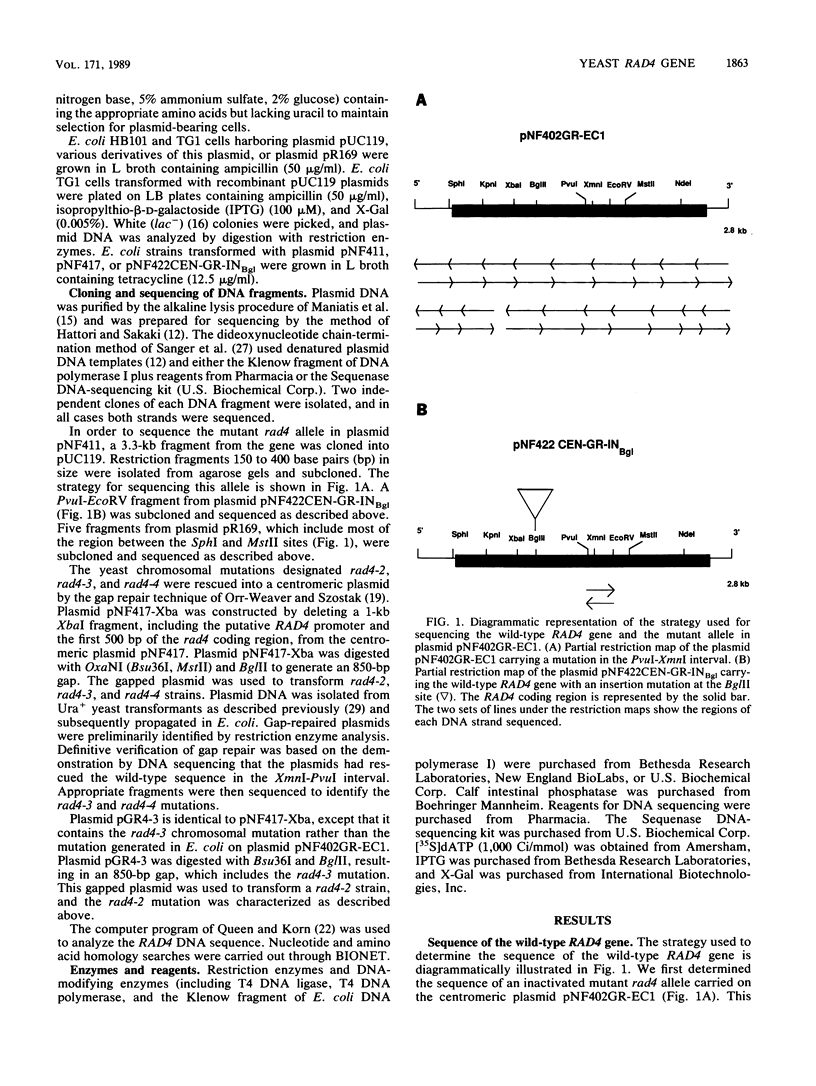
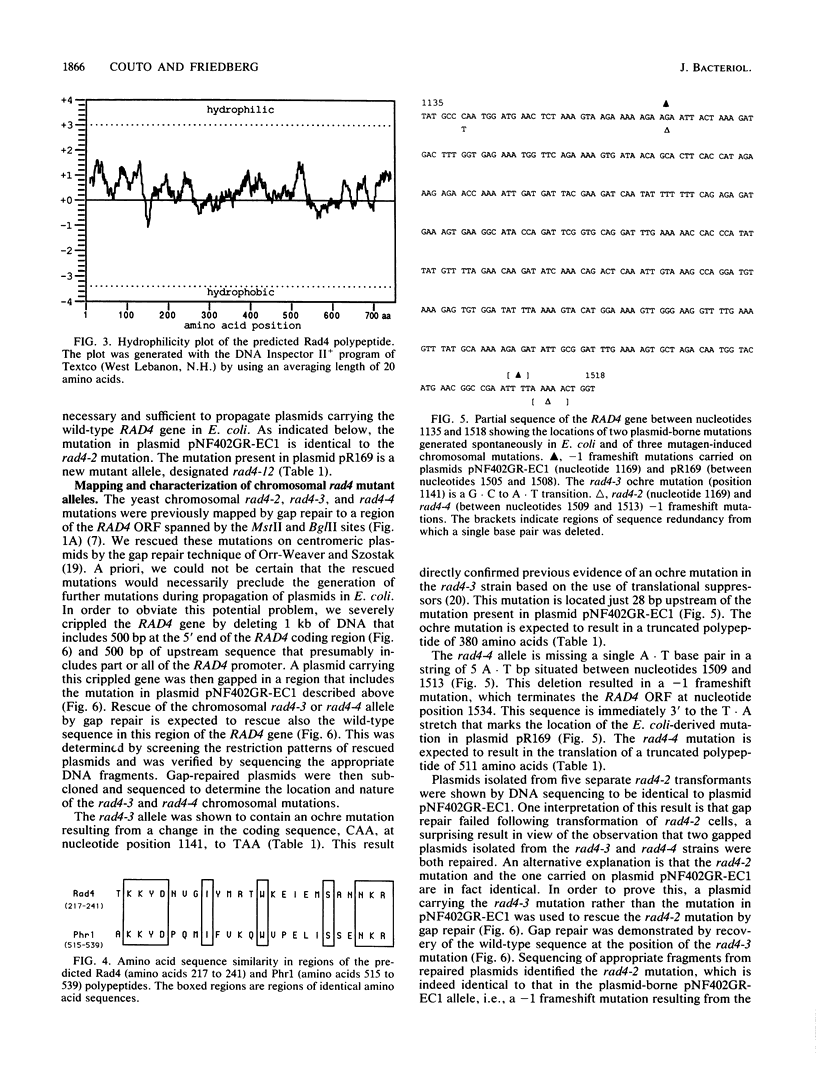
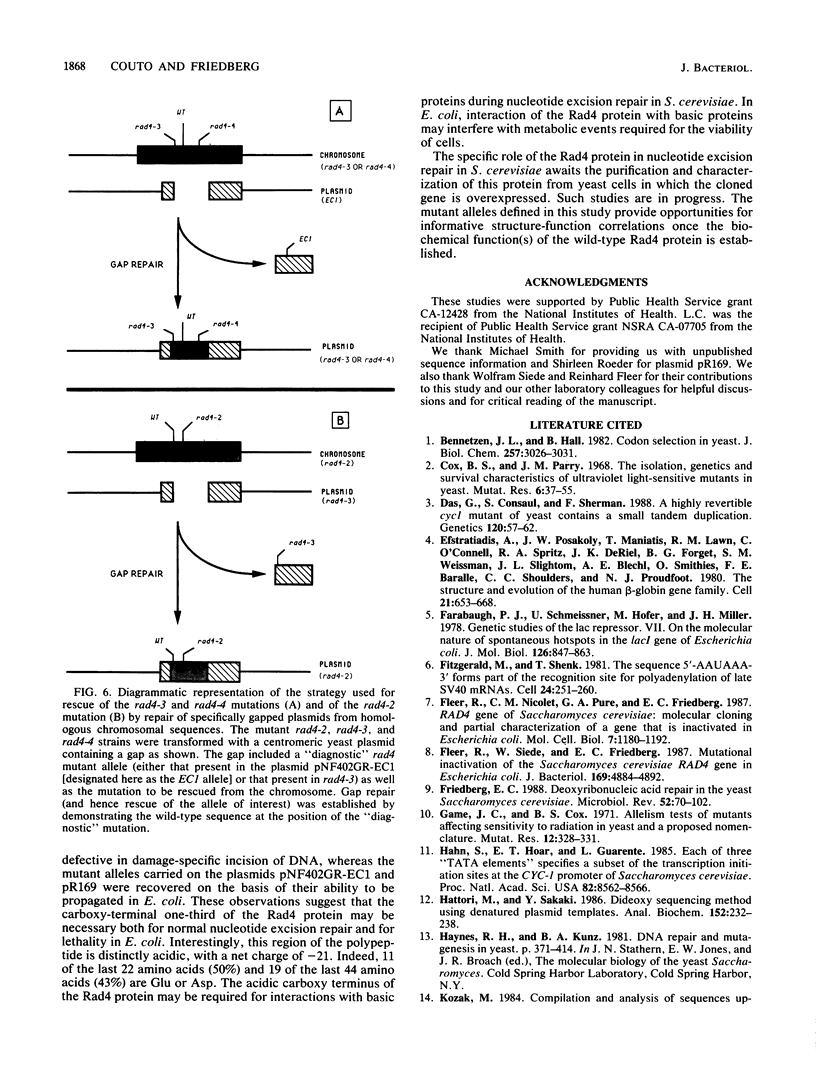
Shuttle plasmids carrying the wild-type RAD4 gene of Saccharomyces cerevisiae cannot be propagated in Escherichia coli (R. Fleer, W. Siede, and E. C. Friedberg, J. Bacteriol. 169:4884-4892, 1987). In order to determine the nucleotide sequence of the cloned gene, we used a plasmid carrying a mutant allele that allows plasmid propagation in E. coli. The wild-type sequence in the region of this mutation was determined from a second plasmid carrying a different mutant rad4 allele. We established the locations and characteristics of a number of spontaneously generated plasmid-borne RAD4 mutations that alleviate the toxicity of the wild-type gene in E. coli and of several mutagen-induced chromosomal mutations that inactivate the excision repair function of RAD4. These mutations are situated in very close proximity to each other, and all are expected to result in the expression of truncated polypeptides missing the carboxy-terminal one-third of the Rad4 polypeptide. This region of the gene may be important both for the toxic effect of the Rad4 protein in E. coli and for its role in DNA repair in S. cerevisiae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Cox B. S., Parry J. M. The isolation, genetics and survival characteristics of ultraviolet light-sensitive mutants in yeast. Mutat Res. 1968 Jul-Aug;6(1):37–55. doi: 10.1016/0027-5107(68)90101-2. [DOI] [PubMed] [Google Scholar]
- Das G., Consaul S., Sherman F. A highly revertible cyc1 mutant of yeast contains a small tandem duplication. Genetics. 1988 Sep;120(1):57–62. doi: 10.1093/genetics/120.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Farabaugh P. J., Schmeissner U., Hofer M., Miller J. H. Genetic studies of the lac repressor. VII. On the molecular nature of spontaneous hotspots in the lacI gene of Escherichia coli. J Mol Biol. 1978 Dec 25;126(4):847–857. doi: 10.1016/0022-2836(78)90023-2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M., Shenk T. The sequence 5'-AAUAAA-3'forms parts of the recognition site for polyadenylation of late SV40 mRNAs. Cell. 1981 Apr;24(1):251–260. doi: 10.1016/0092-8674(81)90521-3. [DOI] [PubMed] [Google Scholar]
- Fleer R., Nicolet C. M., Pure G. A., Friedberg E. C. RAD4 gene of Saccharomyces cerevisiae: molecular cloning and partial characterization of a gene that is inactivated in Escherichia coli. Mol Cell Biol. 1987 Mar;7(3):1180–1192. doi: 10.1128/mcb.7.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleer R., Siede W., Friedberg E. C. Mutational inactivation of the Saccharomyces cerevisiae RAD4 gene in Escherichia coli. J Bacteriol. 1987 Nov;169(11):4884–4892. doi: 10.1128/jb.169.11.4884-4892.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Deoxyribonucleic acid repair in the yeast Saccharomyces cerevisiae. Microbiol Rev. 1988 Mar;52(1):70–102. doi: 10.1128/mr.52.1.70-102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Hoar E. T., Guarente L. Each of three "TATA elements" specifies a subset of the transcription initiation sites at the CYC-1 promoter of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8562–8566. doi: 10.1073/pnas.82.24.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Okada Y., Streisinger G., Owen J. E., Newton J., Tsugita A., Inouye M. Molecular basis of a mutational hot spot in the lysozyme gene of bacteriophage T4. Nature. 1972 Apr 14;236(5346):338–341. doi: 10.1038/236338a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4417–4421. doi: 10.1073/pnas.80.14.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Sherman F. Mutagenic specificity: reversion of iso-1-cytochrome c mutants of yeast. J Mol Biol. 1973 Sep 5;79(1):65–82. doi: 10.1016/0022-2836(73)90270-2. [DOI] [PubMed] [Google Scholar]
- Pure G. A., Robinson G. W., Naumovski L., Friedberg E. C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J Mol Biol. 1985 May 5;183(1):31–42. doi: 10.1016/0022-2836(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Beard C., Smith M., Keranen S. Isolation and characterization of the SPT2 gene, a negative regulator of Ty-controlled yeast gene expression. Mol Cell Biol. 1985 Jul;5(7):1543–1553. doi: 10.1128/mcb.5.7.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R. Frameshift mutations. Annu Rev Genet. 1974;8:319–346. doi: 10.1146/annurev.ge.08.120174.001535. [DOI] [PubMed] [Google Scholar]
- Sancar G. B. Sequence of the Saccharomyces cerevisiae PHR1 gene and homology of the PHR1 photolyase to E. coli photolyase. Nucleic Acids Res. 1985 Nov 25;13(22):8231–8246. doi: 10.1093/nar/13.22.8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. Mutants of yeast sensitive to ultraviolet light. J Bacteriol. 1967 Sep;94(3):571–575. doi: 10.1128/jb.94.3.571-575.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wilcox D. R., Prakash L. Incision and postincision steps of pyrimidine dimer removal in excision-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1981 Nov;148(2):618–623. doi: 10.1128/jb.148.2.618-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chaleff D. T., Valent B., Fink G. R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984 Jun;107(2):179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui A., Langeveld S. A. Homology between the photoreactivation genes of Saccharomyces cerevisiae and Escherichia coli. Gene. 1985;36(3):349–355. doi: 10.1016/0378-1119(85)90190-8. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]



