Abstract
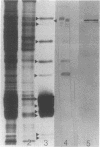
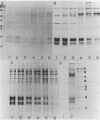
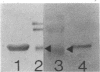
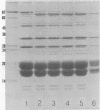
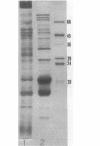
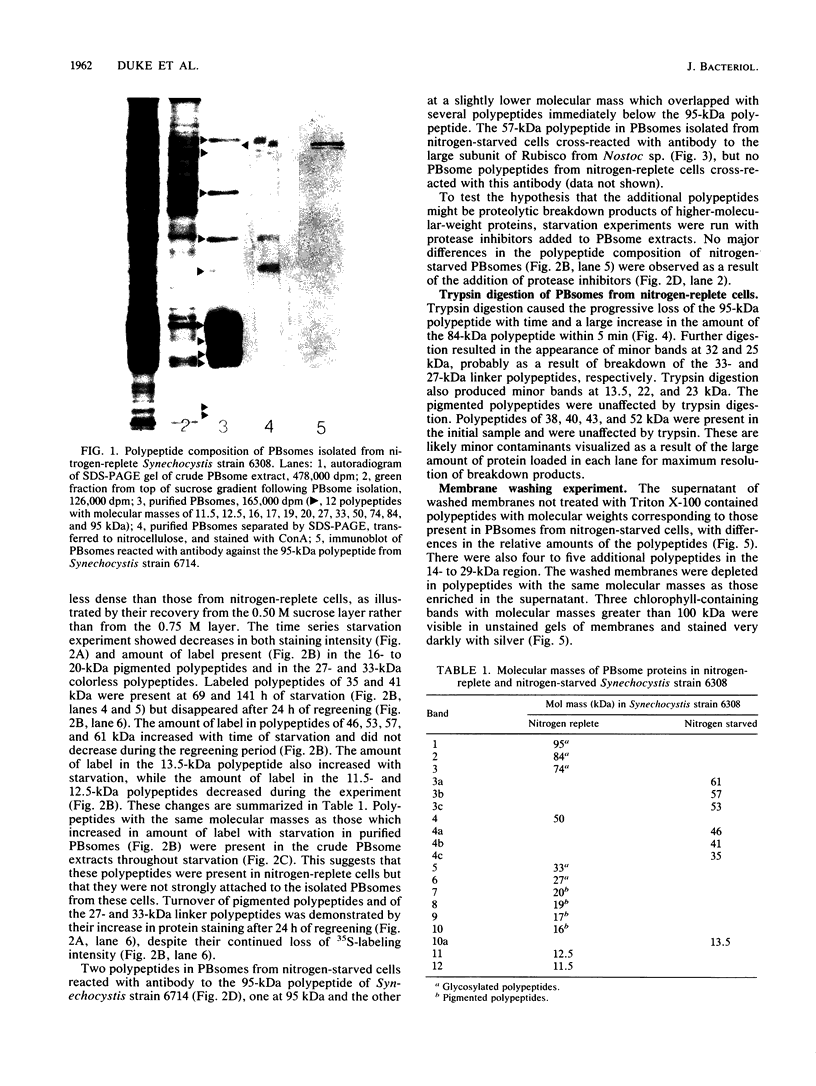
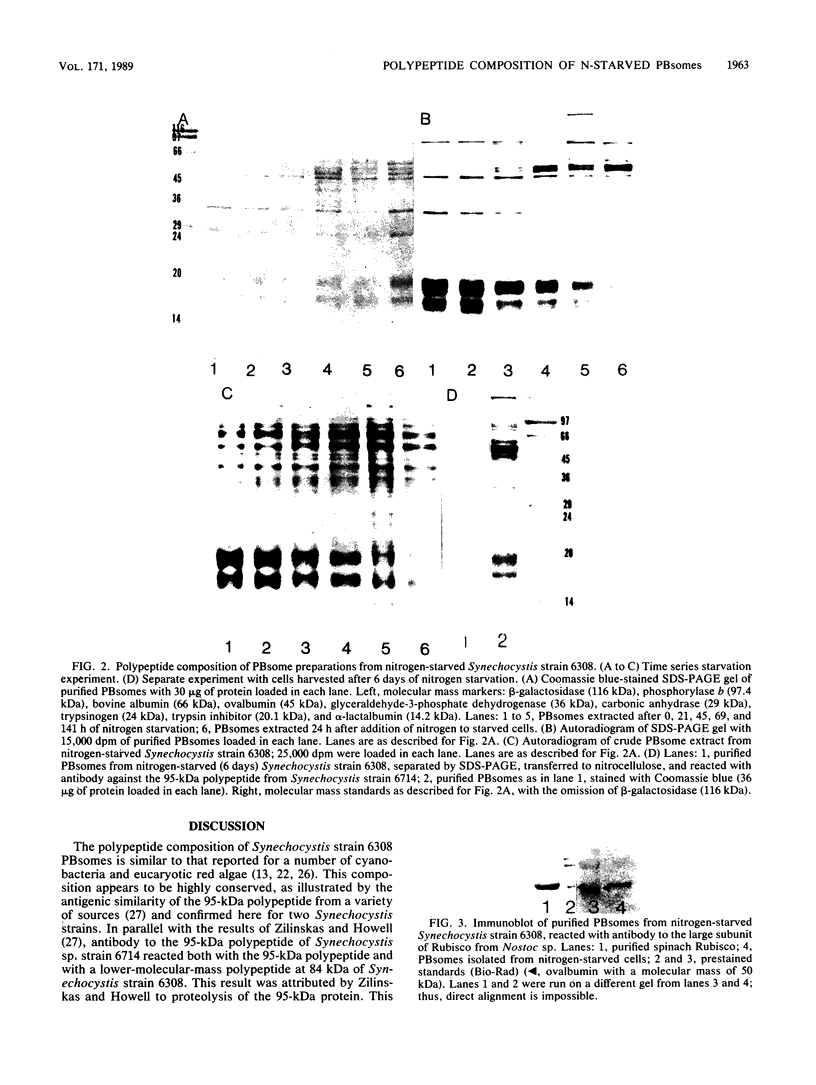
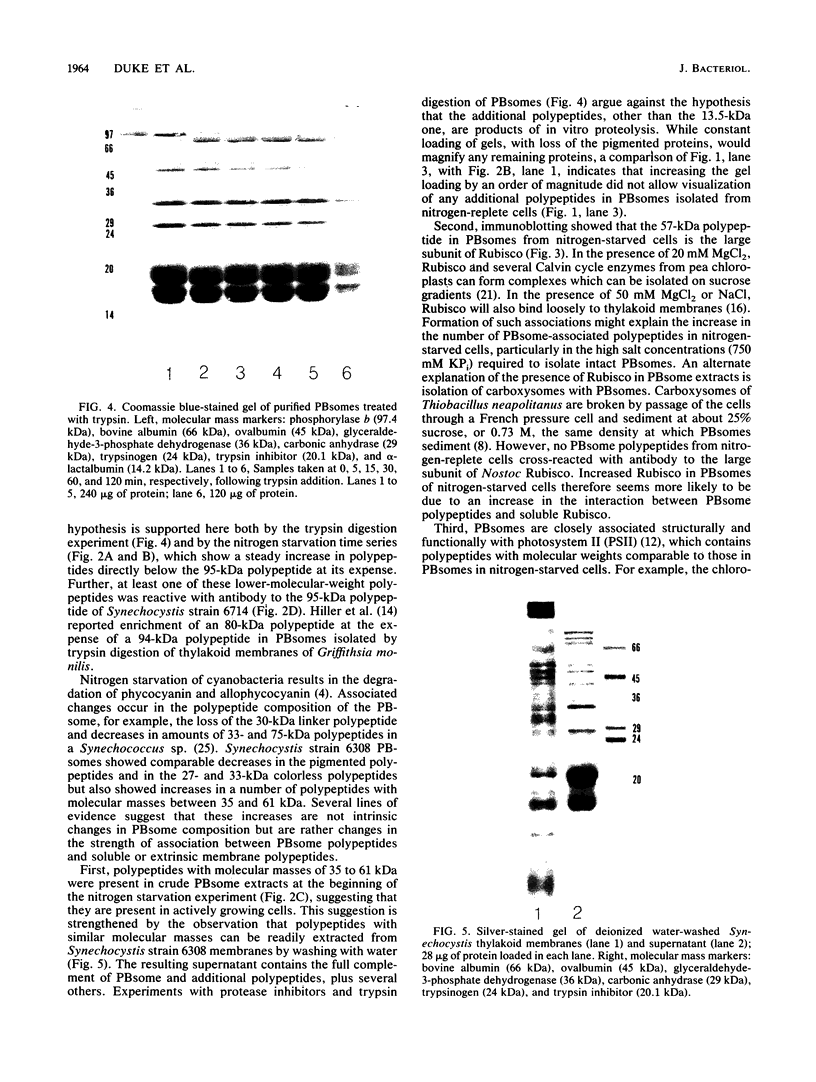
Phycobilisomes isolated from actively growing Synechocystis sp. strain 6308 (ATCC 27150) consist of 12 polypeptides ranging in molecular mass from 11.5 to 95 kilodaltons. The phycobilisome anchor and linker polypeptides are glycosylated. Nitrogen starvation causes the progressive loss of phycocyanin and allophycocyanin subunits with molecular masses between 16 and 20 kilodaltons and of two linker polypeptides with molecular masses of 27 and 33 kilodaltons. Nitrogen starvation also leads to enrichment of four additional polypeptides with molecular masses of 46, 53, 57, and 61 kilodaltons and a transient enrichment of 35- and 41-kilodalton polypeptides in isolated phycobilisomes. The 57-kilodalton additional polypeptide was identified by immunoblotting as the large subunit of ribulosebisphosphate carboxylase/oxygenase. Proteins with the same molecular weights as the additional polypeptides were also coisolated with the 12 phycobilisome polypeptides in the supernatant of nitrogen-replete Synechocystis thylakoid membranes extracted in high-ionic-strength buffer and washed with deionized water. These observations suggest that the additional polypeptides in phycobilisomes from nitrogen-starved cells may be soluble or loosely bound membrane proteins which associate with phycobilisomes. The composition and degree of association of phycobilisomes with soluble and adjacent membrane polypeptides appear to be highly dynamic and specifically regulated by nitrogen availability. Possible mechanisms for variation in the strength of association between phycobilisomes and other polypeptides are suggested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M., Hutchison F., Weathers P. J. Cyanophycin granule polypeptide formation and degradation in the cyanobacterium Aphanocapsa 6308. J Bacteriol. 1980 Feb;141(2):687–693. doi: 10.1128/jb.141.2.687-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. M., Smith A. J. Nitrogen chlorosis in blue-green algae. Arch Mikrobiol. 1969;69(2):114–120. doi: 10.1007/BF00409755. [DOI] [PubMed] [Google Scholar]
- Anderson L. K., Rayner M. C., Sweet R. M., Eiserling F. A. Regulation of Nostoc sp. phycobilisome structure by light and temperature. J Bacteriol. 1983 Sep;155(3):1407–1416. doi: 10.1128/jb.155.3.1407-1416.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullerjahn G. S., Riethman H. C., Sherman L. A. Organization of the thylakoid membrane from the heterotrophic cyanobacterium, Aphanocapsa 6714. Biochim Biophys Acta. 1985 Nov 27;810(2):148–157. doi: 10.1016/0005-2728(85)90130-6. [DOI] [PubMed] [Google Scholar]
- Bullerjahn G. S., Sherman L. A. Immunological characterization of photosystem II chlorophyll-binding proteins from the cyanobacterium, Aphanocapsa 6714. J Bioenerg Biomembr. 1986 Aug;18(4):285–293. doi: 10.1007/BF00743049. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Clement-Metral J. D., Gantt E. Characterization of a Purified Photosystem II-Phycobilisome Particle Preparation from Porphyridium cruentum. Plant Physiol. 1985 Mar;77(3):626–629. doi: 10.1104/pp.77.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chereskin B. M., Gantt E. Enrichment of a 50-kilodalton polypeptide in a photosystem II-phycobilisome particle from Porphyridium cruentum. Arch Biochem Biophys. 1986 Nov 1;250(2):286–293. doi: 10.1016/0003-9861(86)90729-0. [DOI] [PubMed] [Google Scholar]
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Clement-Metral J. D., Gantt E., Redlinger T. A photosystem II-phycobilisome preparation from the red alga, Porphyridium cruentum: oxygen evolution, ultrastructure, and polypeptide resolution. Arch Biochem Biophys. 1985 Apr;238(1):10–17. doi: 10.1016/0003-9861(85)90135-3. [DOI] [PubMed] [Google Scholar]
- Egelhoff T., Grossman A. Cytoplasmic and chloroplast synthesis of phycobilisome polypeptides. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3339–3343. doi: 10.1073/pnas.80.11.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McNeil P. H., Walker D. A. The effect of magnesium and other ions on the distribution of ribulose 1,5-bisphosphate carboxylase in chloroplast extracts. Arch Biochem Biophys. 1981 Apr 15;208(1):184–188. doi: 10.1016/0003-9861(81)90138-7. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., Riethman H. C., Sherman L. A. Organization of pigment proteins in the photosystem II complex of the cyanobacterium Anacystis nidulans R2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6903–6907. doi: 10.1073/pnas.82.20.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sainis J. K., Harris G. C. The association of ribulose-1,5-bisphosphate carboxylase with phosphoriboisomerase and phosphoribulokinase. Biochem Biophys Res Commun. 1986 Sep 30;139(3):947–954. doi: 10.1016/s0006-291x(86)80269-8. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood N. B., Haselkorn R. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol. 1980 Mar;141(3):1375–1385. doi: 10.1128/jb.141.3.1375-1385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- Zilinskas B. A., Howell D. A. The immunologically conserved phycobilisome-thylakoid linker polypeptide. Plant Physiol. 1986 Apr;80(4):829–833. doi: 10.1104/pp.80.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]