Abstract
Accumulating evidences have indicated a link between dopamine signaling and obesity in both animals and humans. We have recently demonstrated heightened avidity to sapid sweet solutions in the obese CCK-1 receptor deficient Otsuka Long Evans Tokushima Fatty (OLETF) rat. To investigate the dopamine dependence and the respective contribution of D1 and D2 receptor subtypes in this phenomenon, real and sham intake of 0.3M sucrose solution were compared between prediabetic, obese OLETF and age-matched lean LETO cohorts following peripheral (IP) administration of equimolar doses (50–800 nmol/kg) of the D1 (SCH23390) and D2 (raclopride) selective receptor antagonists. Both antagonists were potent in reducing sucrose intake in both strains with both drugs suppressing sham intake starting at lower doses than real intake (200 nmol/kg vs. 400 nmol/kg for SCH23390, and 400 nmol/kg vs. 600 nmol/kg for raclopride, respectively). Furthermore, when percent suppression of intake, a measure that controlled for the higher baseline sucrose intake by obese rats was analyzed, OLETF rats expressed an increased sensitivity to raclopride in reducing ingestion of sucrose with 1.7- and a 2.9-fold lower inhibitory dose threshold (ID50) for real and sham intake conditions, respectively, compared to LETO controls. In contrast, SCH23390 caused no differential strain effect with respect to dosage whether sucrose was real or sham fed. These findings demonstrate that D2 receptors are involved in heightened increased consumption of sucrose observed in the OLETF obese rat.
Introduction
Overconsumption induced by the high palatability of the modern diet in general and individual differences in responsivity to palatability in particular may contribute to obesity (Sorensen et al., 2003, Yeomans et al., 2004). This is because highly palatable foods, such as preferred ‘fast foods’ and snacks, are often high in fat and sugar content, and are therefore high in energy density (Drewnowski, 1998, Prentice and Jebb, 2003).
The observation that foods’ palatability is sufficient to stimulate eating beyond homeostatic needs demonstrates the particular importance of food reward as a determinant of excess food intake and development of dietary obesity. Among the most studied putative reward substrates is the mesoaccumbens dopamine (DA) system and considerable evidence implicates its role in natural and drug-related reward processes [for review see (Wise, 2005)]. Similarly, electrophysiological and microdialysis studies have shown increased DA release in nucleus accumbens (NAcc), [for review see (Smith, 2004a)] and stimulation of DA NAcc neuronal activity (Taha and Fields, 2005) as a function of sucrose concentration in various feeding paradigms (Hajnal et al., 2004). Therefore, the role of the dopaminergic system in control of food intake is well established.
In accordance with the proposed role of DA in feeding, selective DA receptor antagonists reduce intake driven by palatable food. Specifically, systemic administration of DA antagonists suppresses both real and sham-feeding of sucrose (Smith, 2004b) as well as oils (Weatherford et al., 1990). Conversely, D1 receptor agonist SKF 38393 enhances preference for high-palatability energy-dense foods (Cooper and Al-Naser, 2006). These findings strongly suggest that DA is important to assign incentive salience to the neural representation of food-reward related stimuli based on palatability.
Despite dopamine’s role in modulating food reward and motivation, its involvement in control of food intake and body weight regulation either directly or by interacting with other neuronal system is much less understood. Evidence of DA’s involvement in obesity, particularly through its interactions with D2Rs, has been reported in both humans (Wang et al., 2002) and animal studies (Cincotta et al., 1997, Szczypka et al., 2000, Pijl, 2003). Furthermore, anatomical and functional evidence demonstrate interactions at multiple levels between the mesencephalic dopamine and cholecystokinin (CCK) system (Hokfelt et al., 1980, Seroogy et al., 1989, Hurd et al., 1992, Hamilton et al., 2000). Specifically, CCK stimulates DA release by acting on CCK-1 receptors (Crawley, 1991). CCK-1 receptors are found in the brain structures linked to the mesolimbic DA system: the VTA and NAcc (Hill et al., 1990, Hamilton and Freeman, 1995). DA and CCK are co-localized in rat midbrain neurons (A10 and medial A9 regions) projecting to several limbic and cortical regions (Hokfelt et al., 1980, Seroogy et al., 1989). Of these regions, the caudal NAcc receives the densest DA/CCK projections (Maidment and Villafranca, 1997). This co-localization may provide the basis for the reported interactions between DA and CCK. Also, DA interacts with CCK to control food intake. For instance, treatment with a D1 or D2 antagonist augments the inhibitory effect of CCK on intraorally infused sucrose (Bednar et al., 1995). These results demonstrate specific involvement of DA D1 and D2 receptors in inhibition of consummatory ingestive behavior by CCK.
The OLETF rat, an out-bred strain of Long Evans, has been established as an animal model of NIDDM and obesity (Kawano et al., 1992). These rats have a 6.8-kb deletion in the CCK-1 receptor gene, resulting in a failure to produce or express functional CCK-1 receptors (Takiguchi et al., 1997). Consistent with this, OLETF rats have deficits in the control of meal size (Moran, 2000) and diminished sensitivity to postingestive satiation signals (Covasa and Ritter, 2001, Moran and Bi, 2006) and vagal responses (Covasa and Ritter, 2005) that are also controlled by CCK-1 receptors. Obesity as well as NIDDM can be greatly reduced by caloric restriction (Okauchi et al., 1995) or exercise (Shima et al., 1996) in this strain, suggesting that obesity and NIDDM in OLETF rats are secondary to their hyperphagia. Relevant to this, we have demonstrated that OLETF rats express increased sham intake of normally preferred sucrose solutions (De Jonghe et al., 2005b) and an increased avidity generalized to various agents that taste sweet to human including non caloric sweetener saccharin and the amino acid alanine (Hajnal et al., 2005). This finding suggests that an increased sensitivity to sweet reward may be the result of disruption in DA/CCK signaling pathways which in turn could contribute to the development of obesity in this strain. Therefore, this study was designed to investigate DA’s involvement in increased intake of sucrose in obese, prediabetic CCK-1 deficient OLETF rats and to delineate the respective contributions of D1, and D2 receptor subtypes.
Materials and methods
Subjects
Thirty-six, male OLETF and LETO rats were obtained as a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan. All rats were individually housed in mesh-floored, stainless-steel, hanging cages in a temperature-controlled vivarium while constantly maintained on a 12:12-h light-dark cycle (lights on at 0600 hr, off at 1800 hr). At the beginning of the experiments, rats were twelve weeks old with an initial body weight (mean ± SE) of 426 ± 15.0 g vs. 334 ± 6.0 g for OLETF and LETO rats, respectively. Rats were handled daily for a minimum of one week prior to the onset of experimental procedures. Tap water and pelleted rat chow (Purina 5001) were available ad libitum throughout experiments except where indicated. All protocols used were conducted in accordance with the National Institute of Health Guide for the Use of Laboratory Animals (NIH Publications No. 80-23) and approved by The Pennsylvania State University Institutional Animal Care and Use Committee. The authors further attest that all efforts were made to minimize the number of animals used and their suffering.
Gastric cannulation surgery
Rats designated for sham feeding studies (n=8 per strain) were fasted overnight and anesthetized prior to surgery via intramuscular injection with 1.0 ml/kg mixture of ketamine HCl (50.0 mg/ml), xylazine (5.0 mg/ml), and acepromazine maleate (10.0 mg/ml), obtained from Burns Veterinary Supply, Rockville Centre, NY and surgically implanted with chronic gastric cannulas as previously described (De Jonghe et al., 2005b). A minimum of 10 days were allowed for postsurgical recovery, before sham feeding training commenced.
Real feeding sucrose intake following dopamine receptor antagonism
In order to assess whether altered dopamine receptor sensitivity contributes to enhanced sucrose intake in OLETF rats, an equal number of age-matched OLETF and LETO rats were divided in 2 independent groups (n=5 per strain), one receiving D1 and the other D2 blockers prior to sucrose intake (i.e. rats in D1 antagonist group never received D2 antagonists, and vice-versa). For these experiments, the D1 antagonist SCH23390 (SCH23390 hydrochloride, Sigma-Aldrich, St. Louis, MO) and the D2 antagonist raclopride (s(−)-raclopride (+)-tartarate, Sigma-Aldrich, St. Louis, MO) were used.
Briefly, at 0800 hr each morning all rats had chow removed from home cages. At 0945 hr, water was removed and rats were intraperitoneally (IP) injected with either SCH23390 or raclopride. Fifteen minutes later, at 1000hr, rats were allowed 60 min ad libitum access to 0.3M (10.26% (w/v)) sucrose via a calibrated glass drinking burette. Following sucrose access, chow and water were returned. Doses of receptor antagonist administered were: 50, 200, 400, 600, 800 nmol/kg: for both SCH23390 and raclopride, and tested in ascending order. These doses were chosen based on previous studies showing inhibition of intake subsequent to IP administration of these drugs within these ranges (e.g. (Weatherford et al., 1990, Hsiao and Smith, 1995, Baker et al., 2001). Each dose was repeated a minimum of two occasions, with each drug day bracketed by a control, 0.9% saline injection day such that drugs were administered 48 hr apart. There were no significant difference between drug tests, therefore data were pooled for analysis. Sucrose intake was measured to the nearest 0.1 ml.
Sham feeding of sucrose after pretreatment with D1 and D2 antagonists
Twenty-three week old rats were used over a period of 4 weeks. Animal body weights for this experimental period were, at study start, 553 ± 13.2 g vs. 456 ± 6.1 g for OLETF and LETO rats, respectively; and upon study end, 569.3 ± 13.9 g vs. 441 ± 10.8 g, for OLETF and LETO rats, correspondingly.
Daily procedures began at 1000hr, when following a brief 2 hr fast, the stainless steel screws occluding the gastric fistulae were removed, and stomach contents were lavaged with warm tap water to ensure minimal gastric volume upon start of sham feeding. Rats were placed into Plexiglas sham feeding boxes and acclimated to the sham feeding procedure by presenting them with 0.3M sucrose for 60 min over several sessions until stable baseline intake was reached. Intakes were deemed stable when amount consumed on successive days varied by less than 10%. Stable baselines were established for all rats within the first 3–4 sham drinking sessions. Rats were then grouped into independent D1 or D2 antagonist-receiving groups as in the previous experiment. All procedures of drug administration were identical to the previous experiment except that doses of antagonists administered were: 50, 200, 400, 800 nmol/kg for both SCH23390 and raclopride. Sham intake was measured to the nearest 0.1 ml every 5 min. To ensure complete sham feeding, gastric drainage was collected in plastic graduated cylinders placed beneath the cages and the volume recorded at experiment termination.
Oral Glucose Tolerance Test (OGTT)
An oral glucose tolerance test was performed in a subset of rats (n=5, per strain) both before and after each set of real (10 and 17 weeks of age) and sham (20 and 27 weeks of age) feeding experiments. The test was administered following a 16hr fast, when an oral glucose load (2g/kg) was delivered to each rat via latex gavage. Blood glucose was measured before and at 30, 60, 90, and 120 min post-glucose loading using a standard glucometer (LifeScan, One-Touch Basic). Animals were classified as diabetic if the peak level of plasma glucose was ≥300 mg/dL and a peak glucose level at 120 min > 200 mg/dl (Kawano et al., 1992).
Statistical Analysis
Two-way repeated measures ANOVAs were performed with strain and drug dosage as main effects. Separate analyses were run for each drug administered. Percent suppression of sucrose intake from baseline due to dopamine receptor antagonism was calculated using the following formula: percent suppression = 1−(experimental/baseline) × 100. Linear regression analyses were performed for each drug with dose as independent variable and percent suppression scores as dependent variable to determine the dose that would inhibit sucrose intake by 50% (ID50). A sensitivity ratio was determined based on the potency fold shift of DA antagonists’ inhibition of sucrose intake and compared between strains using t-tests. Blood glucose concentrations following OGTT tests were compared using planned t-tests. For all experiments, ANOVA results were subsequently analyzed by Tukey's honestly significant difference (HSD) post-hoc tests where appropriate. All data were expressed as means ± SEM. Differences were considered statistically significant if P<0.05. Statistical analyses were computed with PC-SAS (version 8.02, SAS Institute, Carey, NC).
Results
Real feeding sucrose intake following dopamine receptor antagonism
Figure 1 depicts reductions in 0.3M sucrose intake in response to pre-treatment with the D1 antagonist SCH 23390 or the D2 antagonist raclopride. In OLETF rats, a significant drug effect was observed to reduce sucrose intake [F(5,20)= 22.5; P<0.0001]. Post-hoc analyses revealed the threshold dose of SCH 23390 required to reduce sucrose intake in OLETF rats was 400 nmol/kg (P<0.05), with all lower doses resulting in no significant effect on sucrose intake (all P’s=ns, Fig. 1A). We also observed significant intake reductions in OLETF rats at the 600 and 800 nmol/kg doses (P<0.0001 for both drug doses). LETO rats significantly decreased sucrose intake in response to D1 antagonism [F(5,20)= 18.4; P<0.0001]. In contrast to OLETF rats, however, the threshold dose of intake reduction in LETO rats was observed at the 600 nmol/kg dose (P<0.001), with sucrose intake at 800 nmol/kg also significantly lower than baseline (P<0.001). All other doses tested did not significantly alter sucrose intake in LETO rats (all P’s=ns).
Fig. 1.
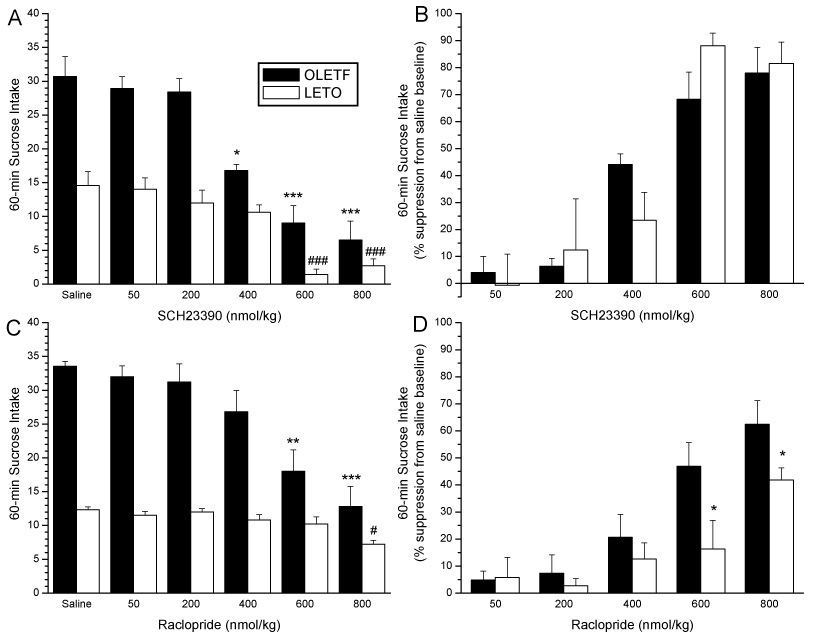
Absolute real intake of 0.3 M sucrose following SCH 23390 (A) or raclopride (C) administration in OLETF and LETO rats. In OLETF rats, the threshold dose of SCH 23390 required to reduce sucrose intake was 400 nmol/kg, whereas, the threshold dose of intake reduction in LETO rats was observed at the 600 nmol/kg dose (A). The threshold dose of raclopride reducing sucrose intake in OLETF rats was 600 nmol/kg, however in LETO rats, the threshold dose of intake reduction was observed at the 800 nmol/kg dose (C). (*P<0.05, **P<0.001, ***P<0.001 for differences in sucrose intake from baseline in OLETF rats. #P<0.05, ### P<0.001 differences in intake from baseline in LETO rats.) Panels B and D depict percent suppression of 0.3M real sucrose intake in response DA receptor antagonism in OLETF and LETO rats. OLETF and LETO rats show no differential responsiveness to D1 antagonism when suppressing real intake (B), whereas, an enhanced suppression of sucrose intake is observed in OLETF rats compared to control LETO rats after D2 antagonism (D). (*P<0.05,**P<0.01 for strain differences in percent suppression of baseline sucrose intake)
When the effect of SCH 23390 on percent suppression of intake from the saline baseline was calculated (Fig. 1B), ANOVAs showed a significant main effect of SCH23390 dose [F(4,32)=31.0, P<0.001], however no main effect for strain [F(1,8)=1.8; NS], or strain x drug dose interaction [F(3.42)=0.3, NS] was observed. Similarly, there was no statistical difference between strains when sensitivity ratio calculated from the ID50 values were compared for SCH 23390’s ability to inhibit sucrose intake.
Results of reductions in 0.3M sucrose intake after pre-treatment with the D2 antagonist raclopride are depicted in Figure 1C. OLETF rats significantly reduced sucrose intake after raclopride treatment [F(5,24)= 31.4; P<0.0001]. The threshold raclopride dose able to lower sucrose intake occured at the 600 nmol/kg dose (P<0.001), with all lower doses unable to significantly affect sucrose intake (all P’s=ns). We also observed significant reductions in intake at the 800 nmol/kg dose (P<0.0001). Similarly, LETO rats also showed a significant decrease in sucrose intake following D1 antagonism [F(5,24)= 4.30; P<0.05]. In contrast to OLETF rats, however, in LETO rats the threshold dose of intake reduction was observed at the 800 nmol/kg dose (P<0.05). All lower doses did not significantly decrease sucrose intake in LETO rats (all P’s=ns).
Figure 1 D depicts data from comparisons of percent suppression of sucrose intake evoked by raclopride. In contrast to SCH 23390, for raclopride two way ANOVAs showed significant main effects of drug [F(4,32)=41.3, P<0.0001] and strain [F(1,8)=11.6; P<0.01] as well as a significant strain by x time interaction [F(4,43)=5.8, P<0.01]. Intake suppression in OLETF rats was significantly higher than in LETO rats following 600 and 800 nmol/kg doses of raclopride. Furthermore, whereas the effective dose of raclopride to suppress intake was 400 nmol/kg for OLETF rats, only the highest dose (800 nmol/kg) reduced sucrose consumption in LETO rats. ID50 values for raclopride’s ability to inhibit sucrose consumption showed a significantly increased sensitivity in OLETF compared to LETO rats (ID50: 680 nmol/kg vs. 1165 nmol/kg). This represents a 1.7-fold shift in sensitivity between obese and lean animals.
Sham feeding sucrose intake following dopamine receptor antagonism
Figure 2 depicts 0.3M sucrose intake after pre-treatment with SCH 23390 or raclopride. Similar to the previous experiment, OLETF and LETO rats showed a significant decrease in sham sucrose intake following SCH 23390 administration [F(4,20)= 52.5; P<0.0001 vs. F(4,28)= 43.7; P<0.0001, for OLETF and LETO rats, respectively]. Post-hoc analyses in Figure 2A show a lower threshold dose of SCH 23390 was able to attenuate sucrose intake in OLETF relative to LETO rats. In OLETF rats, sham sucrose intake was significantly reduced after 200, 400 and 800 nmol/kg doses (P<0.0001 for all three doses) of SCH 23390, while in LETO rats, only 400 (P<0.01) and 800 (P<0.0001) nmol/kg doses significantly reduced sham sucrose intake.
Fig. 2.
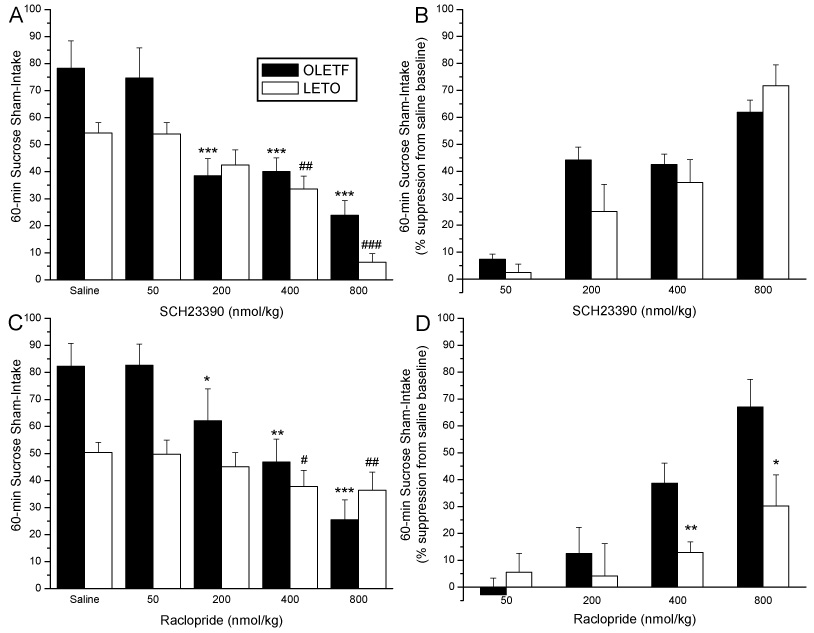
Absolute sham intake of 0.3 M sucrose following SCH 23390 (A) or raclopride (C) administration in OLETF and LETO rats. In OLETF rats, the threshold dose of both SCH 23390 and raclopride required to reduce sucrose intake was 200 nmol/kg, while in LETO rats, threshold intake reduction was observed at the 400 nmol/kg dose (*P<0.05, **P<0.001,***P<0.001 for differences in sucrose intake from baseline in OLETF rats. #P<0.05, ## P<0.001 ### P<0.0001 differences in intake from baseline in LETO rats.) Panels B and D depict percent suppression of 0.3M sham sucrose intake in response DA receptor antagonism in OLETF and LETO rats. OLETF and LETO rats show no differential responsiveness to D1 antagonism when suppressing sham intake (B), however, an enhanced suppression of sucrose sham intake is observed in OLETF rats compared to control LETO rats after D2 antagonism (D). (*P<0.05,**P<0.01 for strain differences in percent suppression of baseline sucrose intake)
In Figure 2B, the effect of SCH 23390 on percent suppression of sham intake is shown. Similar to real intake tests, ANOVA revealed no strain effect. A significant main effect of drug dose [F(3,42)=29.1, P<0.0001], but no main effect for strain [F(1,13)=1.1; NS], or strain by drug dose interaction [F(3,42)=0.1, NS] was noted. The effective dose of SCH 233390 that significantly reduced sham intake in both OLETF and LETO was 200 nmol/kg (p<0.01, and p<0.05, respectively; not shown), that is one dose lower than the one needed to suppress real intake in the previous tests. Similar to the real intake tests, however, ID50 values did not differ between strains.
In Figure 2C, results of raclopride administration on sham sucrose intakes are shown. Both OLETF and LETO rats showed a significant main effect [F(4,32)= 60.1; P<0.0001 vs. F(4,28)= 38.1; P<0.0001, for OLETF and LETO rats, respectively]. Post-hoc analyses displayed in Figure 2A show that OLETF rats significantly reduced sham intake following 200 (P<0.05), 400 (P<0.001) and 800 (P<0.0001) nmol/kg doses of raclopride. In LETO rats, in contrast, the 400 (P<0.05) and 800 (P<0.001), but not 200 nmol/kg doses were able to significantly reduce sham sucrose intake.
Figure 2D summarizes the effect of raclopride on percent suppression of sham sucrose intake. ANOVA revealed significant main effects of dose [F(3,39)=44.7, P<0.0001] and strain [F(1,13)=5.4; P<0.001] as well as a significant strain by x dose interaction [F(3,39)=6.5, P<0.01]‥ Post hoc analysis showed that intake suppression in OLETF again was significantly higher than in LETO following 400 and 800 nmol/kg doses of raclopride. Furthermore, whereas the effective dose of raclopride resulting in suppression was 400 nmol/kg for OLETF rats, only the highest dose tested (800 nmol/kg) was effective in reducing sucrose consumption in LETO rats. ID50 of raclopride-induced inhibition of sucrose sham-intake was lower in OLETF than in LETO rats (490 nmol/kg vs. 1400 nmol/kg), indicating a 2.9-fold increase in sensitivity in obese relative to lean rats.
Oral Glucose Tolerance Test
OLETF rats showed increased blood glucose levels relative to LETO rats in response to acute oral glucose challenge at all ages tested (Fig. 3)]. At 10 weeks (Fig. 3A) and 17 weeks (Fig. 3B), corresponding to animals ages before and after real feeding sucrose experiments, significant glucose increases were observed in OLETF rats at 30 min and 60 min (P<0.01 for both time points) compared to LETO rats, with the highest blood glucose peak occurring at 30 min at both ages. Similarly, in 20 week old (Fig. 3C) and 27 week old (Fig. 3D) OLETF and LETO rats used in sham sucrose feeding studies, blood glucose levels were significantly higher in OLETF rats (P<0.01 for all time points), with the highest blood glucose peaks again occurring at 30 min for both strains. Based on the glucose data and on the criteria for overt diabetes in this rat model, OLETF rats were not diabetic at any time point tested (Kawano et al., 1992).
Fig. 3.
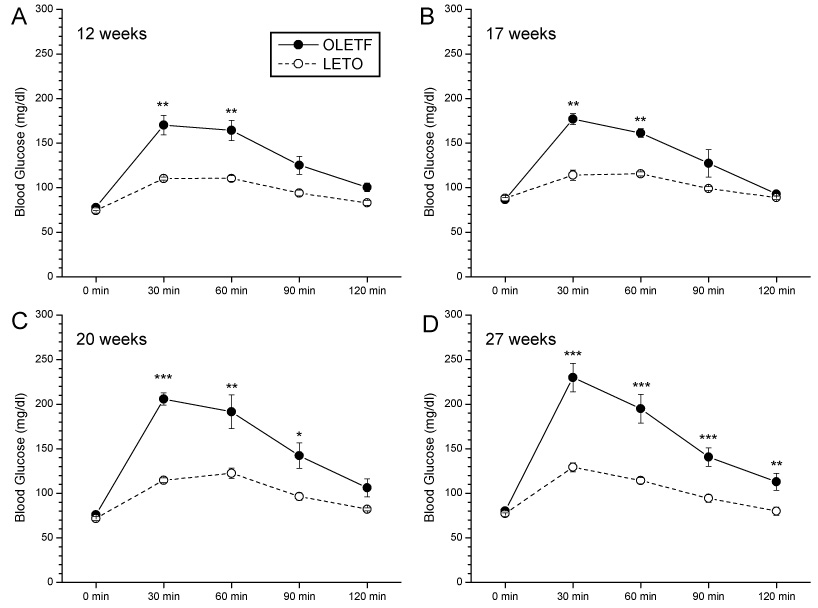
OLETF rats showed increased blood glucose levels relative to LETO rats in response to acute oral glucose challenge. At 10 weeks old (A) and 17 weeks old (B), corresponding to animals ages before and after real feeding sucrose experiments, significant increases were observed in OLETF rats at 30 min and 60 min (P<0.01 for both time points) compared to LETO rats, with highest blood glucose peak at 30 min at both ages. Similarly, in 20 week old (C) and 27 week old [Fig.3D] OLETF and LETO rats used in sham sucrose feeding studies, blood glucose measured post-glucose challenge were significantly higher in OLETF rats (P<0.01 for all time points), with highest blood glucose peaks again occurring at 30 min for both strains. (**P<0.01,***P<0.001 for strain differences in blood glucose).
Discussion
The present study was performed to investigate DA dependency of the heightened sucrose intake in the OLETF rat model of obesity lacking CCK-1 receptors, by using DA antagonists on real and sham intake of a sucrose solution known to activate the mesoaccumbens DA system (Hajnal and Norgren, 2001, Hajnal et al., 2004, Smith, 2004b). The results show that whereas both D1 and D2 antagonists reduced sucrose ingestion in both the lean and the obese rats, the D2 specific antagonist raclopride but not the D1 antagonist SCH23390 showed increased potency to reduce sucrose intake in sucrose-preferring obese OLETF rats. This effect was identical whether sucrose was sham or real fed. The significance of these findings unfolds at multiple levels. First, they demonstrate that altered DA signaling is involved in the increased potency of palatable sucrose to maintain ingestion in this obese strain. Second, the differential effect with regards to DA receptor subtype reveals a dominant role for D2 over D1-type receptors in this effect. Lastly, our results indicate that orosensory effects of sucrose are sufficient to produce a differential pharmacological effect across strains. These observations further support the notion that DA’s role in enhanced sucrose intake is exerted via mechanisms that are primarily involved in mediation of orosensory components of sweet reward whereas postabsorptive negative feedback appears to exert negligible effect on D2-mediated mechanisms of sucrose intake.
Overall suppression of sucrose intake by DA antagonists
In concert with DA’s proposed role in sweet reward reviewed in the Introduction, administration of receptor antagonists reduced both real and sham intake of palatable sucrose in both strains. However, the potency of the receptor blockers to reduce intake was higher in sham compared to real feeding condition. This conclusion is based on the observation that lower doses of both SCH 23390 and raclopride were required to significantly reduce sucrose consumption from baseline levels. This effect may be explained by the potency of postabsorptive and metabolic consequences of sucrose ingestion to recruit more regulatory and transmitter systems than the orosensory effects may exert. We consider this finding as an additional support for the notion that DA’s role in increased sucrose intake is related to assignment of an actual rewarding value of sucrose primarily based on its sensory stimulatory effects. The magnitude of supression of both sham and real intake by either D1 or D2 antagonists in both strains were very similar when the respective first effective doses are compared (i.e. percent reduction of real intake to SCH 23390 at 400 nmol/kg in Fig. 1B vs reduction of sham intake to SCH 23390 at 200 nmol/kg in Fig. 2B; and raclopride at 600 nmol/kg in Fig. 1D compared to raclopride at 400 nmol/kg in Fig. 2D). It has to be noted, however, that the present experiment was exclusively aimed at the DA systems and did not investigate all aspects of sucrose hedonics that might implicate other neurochemical systems (for reviews see (Kelley and Berridge, 2002, Levine et al., 2003). Moreover, since OLETF rats used in this experiment already developed reduced insulin sensitivity as seen from reduced glucose tolerance, it cannot be excluded that prediabetes might contribute to their increased sensitivity to D2 receptor antagonism.
One potential caveat of peripheral administration of lipophylic drugs based on total body mass not normalized with respect to body composition is to underestimate dosage that ultimately affects CNS functions. Therefore, due to increased adipose mass, the brain tissues of the OLETF rats would have experienced lower dosages of the receptor antagonists. If that was correct then OLETF rats should have reduced intake less than LETO rats. However, this was not the case. In fact, OLETF rats suppressed sucrose intake more than LETO rats. This strongly argues against the possibility that more of the injected receptor antagonists would have become sequestered in the fat in these rats.
Differential effects by DA receptor subtypes
The major finding of the present study was that OLETF rats were more sensitive to the suppressive effect of D2 antagonist raclopride but not D1 antagonist SCH23390 on sucrose ingestion. Based on linear regression values (i.e., ID50), OLETF rats displayed a 1.7-fold and a 2.9-fold increased sensitivity to raclopride-induced reduction of sucrose intake in real-, and sham-fed conditions, respectively. In contrast, there was no strain difference observed in sensitivity to D1 antagonism-induced suppression of sucrose intake in real or sham-feeding condition.
In regards to the underlying mechanism, there have been numerous studies showing differential involvement of DA receptor subtypes in unconditioned food preferences. It is well established that D1 antagonist, SCH 23390, and D2 antagonist, raclopride, each dose-dependently decrease sucrose intake (Schneider, 1989, Hsiao and Smith, 1995, Yu et al., 2000). Using reverse microdialysis of sulpiride into the NAcc of normal, lean rats prior to access to 0.3M sucrose solution we showed that D2 antagonist increased both DA release and concurrent intake of sucrose (Hajnal and Norgren, 2001). Systemic administration of DA receptor antagonists, however, may act on multiple brain regions, and also on various domains of DA regulation. Therefore, the specific site of action of the DA antagonists in the present study cannot be determined.
Altered DA functions in the context of obesity
Brain imaging studies have documented reductions in D2 receptors in the striatum of obese individuals (Wang et al., 2002). In obese but not in controls subjects, D2 receptors levels were inversely related to the body mass index (BMI). In contrast, PET studies in patients with anorexia nervosa have reported higher than normal striatal D2 receptors availability (Frank et al., 2005). These and other observations suggest an intricate relationship between DA signalling and the pathology of eating and body weight maintenance (Volkow and Swanson, 2003). The findings of the present study add new information to the growing body of evidence showing a functional relationship between altered D2 receptor signalling and sweet preference. Since, in our experiment, obese rats were more sensitive to lower doses of raclopride, one may assume that the number of available binding sites for the antagonist was also reduced. Even with the binding capacity unaltered, less competition from endogenous DA due to a reduced basal DA levels would explain an increased effect to raclopride at lower doses. In fact, in separate autoradiographic study we have observed a 18% reduction in sulpiride-labelled RTI-55 binding in the NAcc of 14 weeks old OLETF rats compared to age-matched LETO controls (Margas et al., 2005). These findings are in concert with the notion that obesity is accompanied by an overall reduced DA signaling (Orosco et al., 1995, Meguid et al., 2000) that may result in increased reward sensitivity, and increased avidity to sweet could be a manifestation of this effect. An analogy between drug-taking in addicts and overeating in obese has been proposed as means to temporarily compensate for a decreased sensitivity of DA D2 receptor-regulated reward circuits based on similarities in PET imaging findings (Wang et al., 2004).
Disruptions in DA/CCK signaling pathways
Due to missing stimulation from the CCK-1 receptors, it is possible that OLETF rats express a reduced tonic DA levels in the shell region of the NAcc and normal or even elevated DA levels in other terminal fields of the VTA projections, such as in the core of the NAcc or in the prefrontal cortex where less CCK-1 receptors are expressed. This may result from a reduced negative feedback from the NAcc shell to the VTA which could explain complex DA-related deficits described in OLETF rats, such as learning, memory, motor, and sensory-motor gating problems (Nomoto et al., 1999, Feifel et al., 2001, Baldo et al., 2002, De Jonghe et al., 2005a). In addition, an unbalanced CCK control on DA regulation in the NAcc and VTA has been implicated in CCK’s role in behavioral sensitization to psychostimulants (Beinfeld, 2003). Alternatively, a suppressed basal DA tone in the shell of the OLETF rats may make the stimulus-bound, i.e. phasic DA signalling more effective. Such an effect may, in turn, augment rewarding properties of a stimulus and facilitate reinforcement. A similar mechanism has been proposed in reward sensitization to drug of abuse (Di Chiara, 1998), and in attention deficit hyperactivity disorder (ADHD) (Volkow and Swanson, 2003). Experiments using reverse microdialysis of DA receptor antagonists directly in the NAcc shell or core in OLETF rats will help address some of the proposed above mechanisms.
Conclusions
In summary, the present data corroborated prior results showing that the D1 antagonist SCH23390 and D2 antagonist raclopride suppresses intake of sweet solutions, and revealed two new findings. The first is that OLETF rats are differentially sensitive to these effects, with obese rats being more sensitive to blockade of D2, whereas the effect of D1 antagonist remains unchanged. The second major finding is that the increased sensitivity to suppression of sucrose intake by D2 receptor blockade in obese OLETF rats can be evoked even when postingestive effects of sucrose are greatly reduced. This finding further supports the notion that DA plays a role in maintenance of sweet preference determined by orosensory effects. In light of growing interest in D2 receptor functions in obesity, the present finding also add to the growing evidence demonstrating a functional relationship between altered DA signaling and increased avidity for overeating on palatable foods. Such an effect may, in turn, contribute to the development of obesity not only in the investigated animal model but also in human subjects predisposed to develop dietary obesity.
Acknowledgements
The authors wish to thank Otsuka Pharmaceutical Co. (Tokushima, Japan) for the generous donation of the OLETF and LETO animals used to perform this research. This research was supported by National Institute of Diabetes & Digestive & Kidney Diseases Grant DK065709.
LIST OF ABBREVIATIONS
- D1R
Dopamine D1-like receptor
- D2R
Dopamine D2-like receptor
- DA
Dopamine (2-(3,4-dihydroxyphenyl)ethylamine)
- IP
intraperitoneal
- LETO
Long-Evans Tokushima Otsuka
- NAcc
nucleus accumbens
- OLETF
Otsuka Long Evans Tokushima Fatty
- SCH 23390
R-(+) 7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker RW, Osman J, Bodnar RJ. Differential actions of dopamine receptor antagonism in rats upon food intake elicited by either mercaptoacetate or exposure to a palatable high-fat diet. Pharmacol Biochem Behav. 2001;69:201–208. doi: 10.1016/s0091-3057(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Bednar I, Carrer H, Qureshi GA, Sodersten P. Dopamine D1 or D2 antagonists enhance inhibition of consummatory ingestive behavior by CCK-8. Am J Physiol. 1995;269:R896–R903. doi: 10.1152/ajpregu.1995.269.4.R896. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC. What we know and what we need to know about the role of endogenous CCK in psychostimulant sensitization. Life Sci. 2003;73:643–654. doi: 10.1016/s0024-3205(03)00384-9. [DOI] [PubMed] [Google Scholar]
- Cincotta AH, Tozzo E, Scislowski PW. Bromocriptine/ SKF38393 treatment ameliorates obesity and associated metabolic dysfunctions in obese (ob/ob) mice. Life Sci. 1997;61:951–956. doi: 10.1016/s0024-3205(97)00599-7. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Al-Naser HA. Dopaminergic control of food choice: contrasting effects of SKF 38393 and quinpirole on high-palatability food preference in the rat. Neuropharmacology. 2006;50:953–963. doi: 10.1016/j.neuropharm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–1348. doi: 10.1016/s0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Reduced CCK-induced Fos expression in the hindbrain, nodose ganglia, and enteric neurons of rats lacking CCK-1 receptors. Brain Res. 2005;1051:155–163. doi: 10.1016/j.brainres.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- De Jonghe BC, Di Martino C, Hajnal A, Covasa M. Brief intermittent access to sucrose differentially modulates prepulse inhibition and acoustic startle response in obese CCK-1 receptor deficient rats. Brain Res. 2005a;1052:22–27. doi: 10.1016/j.brainres.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonghe BC, Hajnal A, Covasa M. Increased oral and decreased intestinal sensitivity to sucrose in obese, prediabetic CCK-A receptor-deficient OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2005b;288:R292–R300. doi: 10.1152/ajpregu.00481.2004. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. A motivational learning hypothesis of the role of mesolimbic dopamine in compulsive drug use. J Psychopharmacol. 1998;12:54–67. doi: 10.1177/026988119801200108. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Energy density, palatability, and satiety: implications for weight control. Nutr Rev. 1998;56:347–353. doi: 10.1111/j.1753-4887.1998.tb01677.x. [DOI] [PubMed] [Google Scholar]
- Feifel D, Priebe K, Shilling PD. Startle and sensorimotor gating in rats lacking CCK-A receptors. Neuropsychopharmacology. 2001;24:663–670. doi: 10.1016/S0893-133X(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, Mathis CA, Wagner A, Hoge J, Ziolko S, Barbarich-Marsteller N, Weissfeld L, Kaye WH. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, pre-diabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001;904:76–84. doi: 10.1016/s0006-8993(01)02451-9. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Hamilton ME, Freeman AS. Effects of administration of cholecystokinin into the VTA on DA overflow in nucleus accumbens and amygdala of freely moving rats. Brain Res. 1995;688:134–142. doi: 10.1016/0006-8993(95)00518-u. [DOI] [PubMed] [Google Scholar]
- Hamilton ME, Redondo JL, Freeman AS. Overflow of dopamine and cholecystokinin in rat nucleus accumbens in response to acute drug administration. Synapse. 2000;38:238–242. doi: 10.1002/1098-2396(20001201)38:3<238::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hill DR, Shaw TM, Graham W, Woodruff GN. Autoradiographical detection of cholecystokinin-A receptors in primate brain using 125I-Bolton Hunter CCK-8 and 3H-MK-329. J Neurosci. 1990;10:1070–1081. doi: 10.1523/JNEUROSCI.10-04-01070.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Smith GP. Raclopride reduces sucrose preference in rats. Pharmacol Biochem Behav. 1995;50:121–125. doi: 10.1016/0091-3057(95)00315-n. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Lindefors N, Brodin E, Brene S, Persson H, Ungerstedt U, Hokfelt T. Amphetamine regulation of mesolimbic dopamine/cholecystokinin neurotransmission. Brain Res. 1992;578:317–326. doi: 10.1016/0006-8993(92)90264-a. [DOI] [PubMed] [Google Scholar]
- Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Kotz CM, Gosnell BA. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78:834S–842S. doi: 10.1093/ajcn/78.4.834S. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Villafranca NP. Persistence of the releasable pool of CCK in the rat nucleus accumbens and caudate-putamen following lesions of the midbrain. Brain Res. 1997;747:290–296. doi: 10.1016/s0006-8993(96)01238-3. [DOI] [PubMed] [Google Scholar]
- Margas WM, Acharya NK, Covasa M, Hajnal A. Obese OLETF rats show augmented ingestive and motor responses to dopamine D2 receptor stimulation; Society for the Study of Ingestive Behavior: Annual Meeting; 2005. pp. 329–392. Appetite. [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16:858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–367. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- Nomoto S, Miyake M, Ohta M, Funakoshi A, Miyasaka K. Impaired learning and memory in OLETF rats without cholecystokinin (CCK)-A receptor. Physiol Behav. 1999;66:869–872. doi: 10.1016/s0031-9384(99)00033-5. [DOI] [PubMed] [Google Scholar]
- Okauchi N, Mizuno A, Yoshimoto S, Zhu M, Sano T, Shima K. Is caloric restriction effective in preventing diabetes mellitus in the Otsuka Long Evans Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Diabetes Res Clin Pract. 1995;27:97–106. doi: 10.1016/0168-8227(95)01029-d. [DOI] [PubMed] [Google Scholar]
- Orosco M, Gerozissis K, Rouch C, Meile MJ, Nicolaidis S. Hypothalamic monoamines and insulin in relation to feeding in the genetically obese Zucker rat as revealed by microdialysis. Obes Res. 1995;3 Suppl 5:655S–665S. doi: 10.1002/j.1550-8528.1995.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol. 2003;480:125–131. doi: 10.1016/j.ejphar.2003.08.100. [DOI] [PubMed] [Google Scholar]
- Prentice AM, Jebb SA. Fast foods, energy density and obesity: a possible mechanistic link. Obes Rev. 2003;4:187–194. doi: 10.1046/j.1467-789x.2003.00117.x. [DOI] [PubMed] [Google Scholar]
- Schneider LH. Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. 1989;575:307. doi: 10.1111/j.1749-6632.1989.tb53252.x. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Schalling M, Brene S, Dagerlind A, Chai SY, Hokfelt T, Persson H, Brownstein M, Huan R, Dixon J, et al. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- Shima K, Shi K, Mizuno A, Sano T, Ishida K, Noma Y. Exercise training has a long-lasting effect on prevention of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima Fatty rats. Metabolism. 1996;45:475–480. doi: 10.1016/s0026-0495(96)90222-x. [DOI] [PubMed] [Google Scholar]
- Smith GP. Accumbens Dopamine is a Physiological Correlate of the Rewarding and Motivating Effects of Food. In: Stricker EMW, S C, editors. Neurobiology of Food and Fluid Intake, vol. 14 of Handbook of Behavioral Neurobiology. New York: Kluwer Academic / Plenum Publishers; 2004a. [Google Scholar]
- Smith GP. Accumbens dopamine mediates the rewarding effect of orosensory stimulation by sucrose. Appetite. 2004b;43:11–13. doi: 10.1016/j.appet.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Sorensen LB, Moller P, Flint A, Martens M, Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. Int J Obes Relat Metab Disord. 2003;27:1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Rainey MA, Palmiter RD. Dopamine is required for hyperphagia in Lep(ob/ob) mice. Nat Genet. 2000;25:102–104. doi: 10.1038/75484. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J Neurosci. 2005;25:1193–1202. doi: 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/s0378-1119(97)00259-x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Greenberg D, Gibbs J, Smith GP. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmacol Biochem Behav. 1990;37:317–323. doi: 10.1016/0091-3057(90)90341-e. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Blundell JE, Leshem M. Palatability: response to nutritional need or need-free stimulation of appetite? Br J Nutr. 2004;92 Suppl 1:S3–S14. doi: 10.1079/bjn20041134. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav. 2000;65:635–647. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]


