Abstract
The objective of this study was to prospectively examine the comparative importance of body mass index (BMI) and metabolic syndrome (MS)-related risk factors in predicting future risk of cardiovascular disease (CVD) in women. Among 25,626 women who were aged ≥ 45 years and were free of CVD, cancer, and diabetes at baseline in the Women’s Health Study, we classified all women into six groups according to 3 BMI categories (<25 kg/m2, 25–29.9 kg/m2, and ≥30 kg/m2) and the presence or absence of the MS as defined by a modified criteria of the National Cholesterol Education Program’s Adult Treatment Program III. During a median of 10-year follow-up, 724 incident CVD events were documented. Compared with lean women without the MS, the multivariate RRs of CVD adjusting for age, physical activity, and other covariates were 2.40 (95% CI, 1.71–3.37) for lean women who had the MS, 1.08 (95% CI, 0.87–1.33) for overweight women who had no MS, 3.01 (95% CI, 2.30–3.94) for overweight women with the MS, 1.58 (95% CI, 1.21–2.08) for obese women without the MS, and 2.89 (95% CI, 2.19–3.80) for obese women with the MS. Similar associations were also evident for total coronary heart disease but were not significant for total stroke. Overall, while CRP concentrations added additional prognostic information beyond BMI and the MS, CRP did not fully account for the observed high risk of CVD associated with MS. In conclusion, MS may largely account for the elevated risk of CVD associated with BMI among apparently healthy women.
Keywords: body mass index, metabolic syndrome, cardiovascular disease, C-reactive protein, and women
Body mass index (BMI), as a commonly used surrogate for overall adiposity, has been strongly associated with increased risk of cardiovascular disease (CVD) 1,2. A clustering of related metabolic abnormalities including insulin resistance or diabetes, hypertension, and dyslipidemia, defined as the metabolic syndrome (MS), has been recognized as a major contributor to the development of CVD 3–5 and is also thought to be an underlying mechanism for the adverse consequences of adiposity 6. Although there is a well-defined gradient relationship between the level of BMI and the probability of having MS 7,8, most obese individuals do not have MS and, conversely, many individuals with the MS are not overweight or obese 6,7. In this study we examined joint associations of BMI with individual MS-associated risk factors and the MS as an entity in relation to the incidence of CVD over 10 years of follow-up of a large cohort of initially healthy women. We also investigated whether subclinical inflammation, as measured by plasma C-reactive protein (CRP) concentrations, could modify these associations.
Methods
The Women’s Health Study was a randomized, double-blind, placebo-controlled trial designed to evaluate the balance of benefits and risks of low-dose aspirin and vitamin E in the primary prevention of CVD and cancer 9. As described previously, participants provided detailed information on behavioral, lifestyle, and demographic risk factors at baseline 10. In total, 39,876 female health professionals aged 39 to 89 years who were free of coronary heart disease, stroke, and cancer (except for non-melanoma skin cancer) have been followed up. Of them, 28,345 provided baseline blood samples. We excluded individuals with incomplete data on individual metabolic syndrome components, which left 25,626 women for the analysis. All participants in the Women’s Health Study provided written informed consent and the study protocol was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, Mass).
As described previously 11,12, the primary end point for this analysis was incident CVD, which included nonfatal myocardial infarction, stroke, percutaneous transluminal coronary angioplasty, coronary artery bypass graft, and fatal CVD that occurred during the follow-up period. Coronary heart disease was defined to include nonfatal myocardial infarction, fatal coronary events, percutaneous transluminal coronary angioplasty, and coronary artery bypass graft. Diagnoses were confirmed by a committee of cardiologists and one neurologist.
Blood samples for the Women’s Health Study were stored in liquid nitrogen and were thawed before assay. As previously described 13, these samples underwent lipid analysis and evaluation for high-sensitivity CRP in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program.
The metabolic syndrome (MS) was defined using a modification of the criteria proposed by the Adult Treatment Program III of the National Cholesterol Education Program 14. Women with 3 or more of the following conditions were defined as having the metabolic syndrome (excluding obesity criterion): 1) triglycerols ≥150 mg/dL; 2) high density lipoprotein cholesterol <50 mg/dL; 3) blood pressure ≥135/85 mm Hg; and 4) abnormal glucose metabolism as defined by a fasting glucose ≥ 110 mg/dL. Baseline waist circumference and fasting glucose concentrations were not available. We instead used the diagnosis of incident type 2 diabetes during an average of 8.8 years follow-up as an alternative measure of baseline abnormal glucose metabolism. The validity of self-reported diabetes has been confirmed by a validation study, as previously reported 15.
Each participant contributed follow-up time from the date of return of the baseline questionnaire to the data of the first diagnosis of a CVD end point, death, or the latest date of adjudicated follow-up through March 31, 2004, whichever came first. Participants were divided into six different groups according to levels of body mass index (BMI in kg/m2) (<25, 25–29.9, and ≥30), and the MS (yes/or no). We used Cox proportional hazards models to estimate the hazard rate ratio (relative risks) and 95% confidence intervals (CIs) of developing CVD. The first Model was adjusted for age (in years), and randomized assignments (aspirin and vitamin E). The second multivariable model added total energy intake (quintiles), smoking (current, past, and never), exercise (rarely/never, <1/week, 1–3 times/week, and ≥4 time/week), alcohol intake (rarely/never, 1–3 drinks/month, 1–6 drinks/week, and ≥1 drink/day), postmenopausal hormone use (never, past, and current), use of multivitamin supplements (never, past, and current), and parental history of myocardial infarction before age 60 (yes/no). We made further adjustment for BMI or CRP in the second model to assess their potential impacts on the observed associations.
Joint effects among BMI, MetS, and CRP were evaluated by performing the same analytic approach as above by adding 11 comparison groups stratified by BMI (<25, 25–29.9, and ≥30), the MetS (yes or no), and the concentrations of CRP (≤3 mg/L and >3 mg/L) when compared with women having a BMI<25, the absence of the MetS, and CRP concentrations ≤3 mg/L. Additional joint effects were also evaluated according to BMI (<25, 25–29.9, ≥30) and the presence of individual metabolic factors (yes vs. no) including baseline hypertension, dyslipidemia (defined as low HDL and/or high triglycerols), and incident diabetes, or at least 1 or at least 2 factors. Further adjustment for other features of MetS in the models was made for individual metabolic risk factors. All statistical analyses were conducted using SAS (version 8.0; SAS Institute, Cary, NC). All P values were two-tailed.
RESULTS
Overall, the prevalences of the MS were 4.31% among lean women (BMI<25), 14% among overweight women (BMI 25–29.9), and 31% among obese women (BMI≥30). As expected, women with the MS had a higher likelihood of having cardiovascular risk factors than those without the MS within each BMI category (Table 1).
TABLE 1.
Baseline Characteristics of 25,626 Apparently Healthy Participants in the Women’s Health Study
| BMI (kg/m2) | <25 | 25–29.9 | ≥30 | |||
|---|---|---|---|---|---|---|
| Metabolic Syndrome * | No (n=12,943) | Yes (n=583) | No (n=6,730) | Yes (n=1,104) | No (n=2,925) | Yes (n=1,341) |
| Variables † | ||||||
| Age (years) | 54±7.1 | 59±7.8 | 55±7.0 | 57±7.4 | 54±6.3 | 55±6.9 |
| BMI (kg/m2) | 22±1.7 | 23±1.5 | 27±1.4 | 28±1.4 | 34±3.8 | 35±4.3 |
| Alcohol (g/d) | 5.42±8.71 | 4.37±8.17 | 4.34±8.37 | 3.20±6.98 | 3.07±7.24 | 2.27±6.29 |
| Physical activity (kcal/wk) | 1042±1191 | 920±1147 | 985±1222 | 897±1088 | 919±1486 | 781±1145 |
| Systolic blood pressure (mmHg) | 119±12 | 137±10 | 123±13 | 137±11 | 128±13 | 139±12 |
| Diastolic blood pressue (mmHg) | 74±8.5 | 84±7.0 | 77±8.4 | 85±7.0 | 80±8.4 | 85±7.2 |
| Total Cholesterol (mg/dL) | 207±41 | 225±41 | 214±42 | 229±42 | 211±42 | 218±41 |
| High density lipoprotein cholesterol (mg/dL) | 59±15 | 41±6.7 | 53±13 | 40±6.7 | 49±12 | 39±7.2 |
| Low density lipoprotein cholesterol (mg/dL) | 119±33 | 132±37 | 127±34 | 136±36 | 128±34 | 129±34 |
| Triglycerols (mg/dL) | 115±66 | 251±111 | 142±82 | 257±118 | 146±80 | 241±109 |
| High sensitivity CRP (mg/L) | 2.31±4.54 | 3.80±4.40 | 3.73±5.28 | 4.55±4.69 | 6.06±6.69 | 7.37±7.18 |
| Ethnicity (white) | 96% | 95% | 95% | 95% | 94% | 97% |
| Current Smoker | 12% | 18% | 10% | 14% | 9.25% | 12% |
| Current postmenopausal hormone therapy user | 53% | 55% | 56% | 58% | 63% | 65% |
| Current multivitamin user | 31% | 32% | 29% | 29% | 25% | 24% |
| Parental history of myocardial infarction before age 60 years | 12% | 14% | 14% | 16% | 13% | 16% |
The MetS was defined as having ≥ 3 of the following metabolic factors: (1). Blood pressure ≥135/85 mm Hg; (2). High density lipoprotein cholesterol <50 mg/dL; (3). Triglycerols >=150 mg/dL; and (4). The diagnosis of incident type 2 diabetes mellitus during follow-up;
Data are expressed as mean±SD for continuous variables and percentage for categorical variables.
During a median of 10.2 years of follow-up (253,729 person-years), we identified 724 incident cases of CVD: 468 coronary heart disease cases, and 256 stroke cases. In the age and randomized treatment-adjusted model (Model 1), the presence of MS appeared to be more strongly associated with increased risk of CVD as compared with the absence of MS within each BMI category (Table 2). Such associations persisted even after further adjustment for traditional cardiovascular risk factors (Model 2). After additional adjustment for BMI, the relative risks for CVD became attenuated and remained significant for only women with the MS regardless of BMI. Similar associations were shown for coronary heart disease. Elevated risks of stroke were evident only among women with the MS but did not reach statistical significance. Further adjustment for CRP in the multivariate models did not appreciably alter these findings.
TABLE 2.
Adjusted relative risk of cardiovascular Events during 10 years of follow-up according to body mass index and the metabolic syndrome
| BMI (kg/m2) | <25 | 25 – 29.9 | ≥30 | |||
|---|---|---|---|---|---|---|
| Metabolic Syndrome | No (n=12,943) | Yes (n=583) | No (n=6730) | Yes (n=1104) | No (n=2925) | Yes (n=1341) |
| CVD | ||||||
| No. of cases | 278 | 46 | 163 | 78 | 77 | 82 |
| Model 1* | 1.00 | 2.58 (1.88–3.53) | 1.10 (0.91–1.33) | 2.72 (2.12–3.50) | 1.36 (1.06–1.75) | 2.85 (2.23–3.64) |
| Model 2† | 1.00 | 2.40 (1.71–3.37) | 1.08 (0.87–1.33) | 3.01 (2.30–3.94) | 1.58 (1.21–2.08) | 2.89 (2.19–3.80) |
| Model 2+BMI | 1.00 | 2.33 (1.66–3.28) | 0.91 (0.70–1.18) | 2.50 (1.82–3.43) | 1.05 (0.66–1.66) | 1.85 (1.13–3.02) |
| Model 2 + CRP | 1.00 | 2.32 (1.65–3.26) | 0.92 (0.71–1.20) | 2.54 (1.85–3.48) | 1.07 (0.68–1.70) | 1.87 (1.14–3.05) |
| CHD | ||||||
| No. of cases | 149 | 34 | 107 | 59 | 55 | 64 |
| Model 1* | 1.00 | 3.69 (2.53–5.37) | 1.35 (1.05–1.73) | 3.89 (2.87–5.26) | 1.80 (1.32–2.45) | 4.15 (3.10–5.56) |
| Model 2† | 1.00 | 3.36 (2.25–5.03) | 1.28 (0.98–1.69) | 3.92 (2.83–5.43) | 1.93 (1.38–2.69) | 3.93 (2.83–5.44) |
| Model 2+BMI | 1.00 | 3.26 (2.18–4.89) | 1.07 (0.78–1.49) | 3.22 (2.20–4.72) | 1.25 (0.72–2.19) | 2.46 (1.37–4.42) |
| Model 2 + CRP | 1.00 | 3.24 (2.16–4.85) | 1.09 (0.79–1.52) | 3.28 (2.24–4.80) | 1.29 (0.74–2.25) | 2.50 (1.39–4.47) |
| Total Stroke ‡ | ||||||
| No. of cases | 129 | 12 | 56 | 19 | 22 | 18 |
| Model 1* | 1.00 | 1.35 (0.74–2.45) | 0.81 (0.59–1.11) | 1.35 (0.83–2.19) | 0.85 (0.54–1.33) | 1.33 (0.81–2.17) |
| Model 2† | 1.00 | 1.24 (0.64–2.39) | 0.83 (0.58–1.18) | 1.74 (1.05–2.87) | 1.13 (0.70–1.82) | 1.49 (0.86–2.58) |
| Model 2+BMI | 1.00 | 1.21 (0.63–2.34) | 0.72 (0.46–1.14) | 1.50 (0.83–2.71) | 0.82 (0.35–1.91) | 1.05 (0.42–2.66) |
| Model 2 + CRP | 1.00 | 1.21 (0.63–2.33) | 0.73 (0.46–1.15) | 1.51 (0.84–2.74) | 0.83 (0.36–1.93) | 1.06 (0.42–2.67) |
Model 1: adjusted for age and randomized treatment assignment (vitamin E and aspirin) in the Women’s Health Study;
Model 2: model 1 with additional adjustment for smoking, exercise, alcohol intake, total calorie intake, postmenopausal hormone use, multivitamin use, and parental history of myocardial infarction before 60 years;
Total stroke included ischemic stroke, hemorrhagic stroke, and stroke of unknown type.
When further stratified by plasma CRP concentrations (≤ 3 mg/L and > 3 mg/dl), the magnitude of the risk of CVD was higher in those with high CRP than in those with low CRP, although their 95% CIs were quite large and overlapping due to relatively small sample sizes (Figure 1). Overall, CRP added more prognostic values beyond BMI and the MS, which has been carefully addressed in previous report in our cohort 14. Alternatively, within the same category of CRP, the multivariate relative risks of CVD were significantly increased by the presence of MS but not those without the MS. At every level of BMI, women with the presence of either each individual risk factor or the number of MS components (≥1 and ≥2 risk factors) tended to have an elevated risk of CVD compared with same-weight women without them, but there was no obvious increasing trend in CVD risk across increasing BMI categories for women without these metabolic risk factors (Figure 2).
Figure 1.
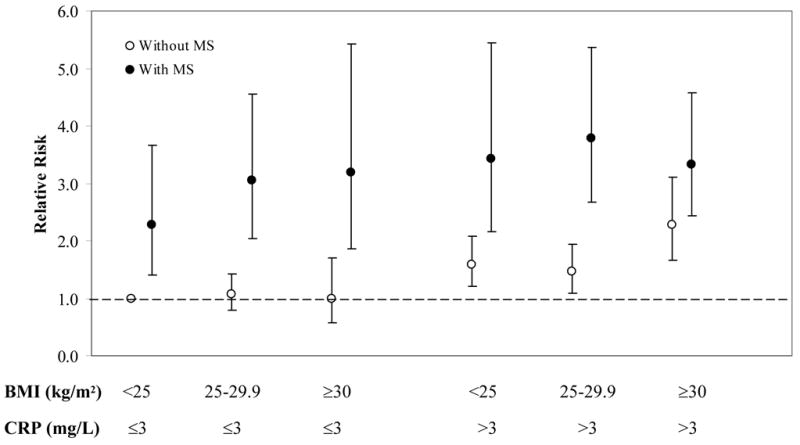
Multivariate-adjusted relative risks of CVD during 10 years of follow-up according to BMI (<25, 25–29.9, ≥30), the presence of MS (yes vs no), and plasma CRP concentrations (≤3 mg/L and >3 mg/L) at baseline. RRs were adjusted for age, randomized treatment assignment (aspirin and vitamin E), smoking status, exercise, alcohol intake, total calorie intake, postmenopausal hormone use, multivitamin use, and parental history of myocardial infarction before 60 years.
Figure 2.
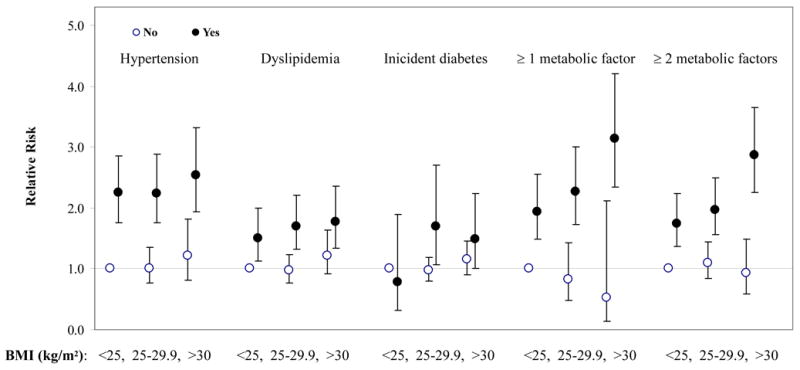
Multivariate-adjusted relative risks of CVD according to BMI (<25, 25–29.9, ≥30) and the presence of individual metabolic factors (yes vs no) including baseline hypertension, dyslipidemia (defined as low high density lipoprotein cholesterol and/or high triglycerols), and incident diabetes, or at least 1 or at least 2 factors. Analyses were adjusted for age, randomized treatment assignment, smoking, exercise, total calories, alcohol use, multivitamin use, postmenopausal hormone use, and parental history of myocardial infarction before 60 y. Adjustment for dyslipidemia and incident diabetes were further made in the models for joint associations with hypertension, baseline hypertension and dyslipidemia were controlled for joint associations with incident diabetes, and hypertension and incident diabetes were adjusted in the models for joint associations with dyslipidemia.
Discussion
In this large prospective study of middle-aged and older US women followed over 10 years, we found that the presence of traditional MS-associated risk factors, either individually or jointly, predicted an increased risk of developing CVD independent of BMI. The excess risk of CVD attributable to the MS was independent of other conventional CVD risk factors and was not explained by elevated CRP concentrations.
Our study indicates that the cluster of metabolic risk factors as an entity largely reflects the underlying metabolic consequences of adiposity that increase long-term risk of CVD. Prospective data on the importance of BMI compared with the MS to predict CVD are very limited and the results have been inconsistent. In a prospective study of 780 women referred to angiography, the MS but not BMI significantly predicted 3-year risk of cardiovascular death 6. This cohort was at higher risk than the cohort in the present study, with suspicion for myocardial ischemia and a 33% prevalence of the MS compared to the 24% prevalence of the MS in our cohort. Recent data from the Framingham Offspring Study of 2902 individuals free of diabetes or CVD followed for 11 years, Meigs et al. reported that individuals with MS or insulin resistance, regardless of BMI, were at an approximately 1.6- to 2-fold increased risk of CVD relative to those with normal weight and without MS or insulin resistance 16. Their findings from both women and men are consistent with our results, although the prevalence of MS among those with normal weight (7%) and those with obesity (37%) in the Framingham community were higher than those in our WHS cohort of female health professionals (4% and 31%) 16. In contrast, in a 10-year prospective study of 19173 men with a relatively low prevalence of MS (19.5%), Katzmarzyk et al. found that a higher risk of CVD mortality was evident in obese men (BMI ≥30) either with or without MS as compared with normal weight men (BMI: 18.5–24.9) without the MS 7. The discrepant findings from these studies may reflect population differences in the distributions of some lifestyle and genetic factors as well as baseline anthropometric variables.
There are also several alternative explanations for the observed excess risk for CVD associated with the MS independent of BMI. First, the MS may be a more important determinant in the prediction of risk of CVD than is the assessment of overall obesity especially among older people. Overall adiposity defined by BMI may not be a as strong a marker for metabolic risk factors as body fat distribution because excess accumulation of visceral abdominal adipose tissue was more strongly correlated with a higher prevalence of the MS across all BMI groups for both men and women 17. Moreover, insulin resistance is thought to play a central role in the development of the MS and is a possible link between adiposity and CVD. Overall adiposity defined by BMI may not be an optimal marker for insulin resistance. Also, systemic inflammation is a common correlate of the insulin resistance and is directly linked to the MS 18,19. Central obesity has been suggested as a major determinant of CRP concentrations in individuals with the MS 20 because visceral fat distribution rather than overall adiposity may largely contribute to the production and release of cytokines from adipose tissue. The MS has been more consistently associated with CRP concentrations than BMI. Nevertheless, while CRP concentrations added additional prognostic information beyond BMI and the MS, CRP did not fully account for the observed high risk of CVD associated with MS in our study.
Of note, we found that about 4% of normal weight women had the MS while 31% of obese women had the MS in our cohort. On the one hand, it is likely that underlying adiposity might have been misclassified using BMI. On the other hand, there are some women who are truly lean and have the MS while some women are indeed obese but do not have the MS. They may be genetically predisposed or resistant to the development of MS and subsequent CVD, although the precise mechanisms underlying the pathogenesis of metabolic abnormalities remain to be determined. Given the natural history of obesity, individual with true obesity may inevitably develop adverse metabolic consequences as they age. To fully address the temporal sequences of BMI and adverse health consequences, further longitudinal data on weight assessment and metabolic biomarker measurements at different time points are needed.
The strengths of our study include the prospective study design, large sample size, high follow-up rate, long follow-up duration, reliable assessment of CVD endpoints, and detailed assessment of lifestyle variables, metabolic and cardiovascular risk factors. Several limitations should be considered, however. First, our reliance on all single measures of baseline markers may introduce random measurement errors and may also not accurately reflect changes in metabolic disorders over long periods. Second, the lack of baseline data on waist and hip circumferences has limited our ability to determine the true metabolic risk profiles due to body fat distribution. Third, our analyses may have relatively low statistical power to detect any association with CVD death and stroke subtypes. Fourth, the lack of information on fasting glucose or insulin concentrations at baseline did not allow for a standardized definition for the MS. We also acknowledge that any existing MS definitions cannot reflect the predictive values of homostatic, thrombolytic, or endothelial factors, which are not incorporated into the MS definition but may confer additional prognostic values. Finally, because our study population included solely female health professionals who were mostly white, results from the present study may not be generalizable to men or to other ethnic groups.
Acknowledgments
We are indebted to the 39,876 dedicated and committed participants of the WHS. We also acknowledge the contributions of the entire staff of the WHS.
This study was supported by grants DK66401, CA-47988, and HL-43851 from the National Institutes of Health, Bethesda, MD. Dr. Meigs is supported by an American Diabetes Association Career Development Award.
References
- 1.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 2.Rexrode KM, Buring JE, Manson JE. Abdominal and total adiposity and risk of coronary heart disease in men. Int J Obes Relat Metab Disord. 2001;25:1047–1056. doi: 10.1038/sj.ijo.0801615. [DOI] [PubMed] [Google Scholar]
- 3.Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Bonadonna RC, Muggeo M. Carotid atherosclerosis and coronary heart disease in the metabolic syndrome: prospective data from the Bruneck study. Diabetes Care. 2003;26:1251–1257. doi: 10.2337/diacare.26.4.1251. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 6.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 7.Katzmarzyk PT, Church TS, Janssen I, Ross R, Blair SN. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 8.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buring JE, Hennekens CH. The Women’s Health Study: summary of the study design. J Myocard Ischemia. 1992;4:27–29. [Google Scholar]
- 10.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 11.Atiya M, Kurth T, Berger K, Buring JE, Kase CS. Interobserver agreement in the classification of stroke in the Women’s Health Study. Stroke. 2003;34:565–567. doi: 10.1161/01.str.0000054159.21017.7c. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 15.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women’s health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 16.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, D’Agostino RB. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 18.Festa A, D’Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 20.Santos AC, Lopes C, Guimaraes JT, Barros H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int J Obes (Lond) 2005;29:1452–1456. doi: 10.1038/sj.ijo.0803035. [DOI] [PubMed] [Google Scholar]


