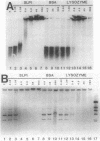
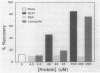
Abstract
The expression of the positively charged human protein secretory leukocyte protease inhibitor (SLPI) in Escherichia coli causes severe cellular toxicity. After induction of SLPI synthesis in a high-level-expression strain, SGE61, the growth of the strain is arrested and total protein and RNA synthesis rates decline by 60 to 70%. The mechanism of SLPI-mediated inhibition of macromolecular synthesis was examined in cell-free transcription-translation systems. SLPI proved to be a potent inhibitor of translation in vitro. When SLPI was added to translation reactions at SLPI/mRNA ratios attained during maximal SLPI accumulation in SGE61, translation of a test mRNA was inhibited by 75%. The mechanism of translation inhibition was deduced from in vitro experiments showing that SLPI bound to mRNA and interfered with the interaction of RNA-metabolizing enzymes, such as RNase. In addition, SLPI bound to DNA in vitro, but transcription was not inhibited as strongly in cell-free reactions as it was in SGE61. Similar nucleic acid-binding and translation inhibition properties were displayed in vitro by another basic protein, chicken egg white lysozyme, but were not displayed by the relatively acidic protein bovine serum albumin. On the basis of these results, we concluded that SLPI binds to nucleic acids via charge interactions and inhibits translation by competing with ribosomes for binding to mRNA. Since SLPI-mRNA and SLPI-DNA binding occurred at SLPI/mRNA and SLPI/DNA ratios existing in SGE61, nucleic acid binding may contribute to the toxicity of SLPI to E. coli. These results indicate that, in general, high-level expression of basic recombinant proteins in E. coli may be problematic.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J. Toxicity of an overproduced foreign gene product in Escherichia coli and its use in plasmid vectors for the selection of transcription terminators. Gene. 1984 Feb;27(2):161–172. doi: 10.1016/0378-1119(84)90137-9. [DOI] [PubMed] [Google Scholar]
- CANFIELD R. E., LIU A. K. THE DISULFIDE BONDS OF EGG WHITE LYSOZYME (MURAMIDASE). J Biol Chem. 1965 May;240:1997–2002. [PubMed] [Google Scholar]
- Dunn R. J., Hackett N. R., McCoy J. M., Chao B. H., Kimura K., Khorana H. G. Structure-function studies on bacteriorhodopsin. I. Expression of the bacterio-opsin gene in Escherichia coli. J Biol Chem. 1987 Jul 5;262(19):9246–9254. [PubMed] [Google Scholar]
- Emr S. D., Hall M. N., Silhavy T. J. A mechanism of protein localization: the signal hypothesis and bacteria. J Cell Biol. 1980 Sep;86(3):701–711. doi: 10.1083/jcb.86.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grütter M. G., Fendrich G., Huber R., Bode W. The 2.5 A X-ray crystal structure of the acid-stable proteinase inhibitor from human mucous secretions analysed in its complex with bovine alpha-chymotrypsin. EMBO J. 1988 Feb;7(2):345–351. doi: 10.1002/j.1460-2075.1988.tb02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Inducible high level synthesis of mature human fibroblast interferon in Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4677–4688. doi: 10.1093/nar/11.14.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Caravaggio T. Isoelectric points and molecular weights of proteins. J Chromatogr. 1976 Apr 21;127(11):1–28. doi: 10.1016/s0021-9673(00)98537-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Stetler G., Brewer M. T., Thompson R. C. Isolation and sequence of a human gene encoding a potent inhibitor of leukocyte proteases. Nucleic Acids Res. 1986 Oct 24;14(20):7883–7896. doi: 10.1093/nar/14.20.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Ohlsson K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6692–6696. doi: 10.1073/pnas.83.18.6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowska M., Miller E. S., Karam J., Stormo G., Gold L. The bacteriophage T4 regA gene: primary sequence of a translational repressor. Nucleic Acids Res. 1984 Aug 10;12(15):5979–5993. doi: 10.1093/nar/12.15.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne S. R., Thomas C. M., Nugent M. E., Tacon W. C. Use of a modified Escherichia coli trpR gene to obtain tight regulation of high-copy-number expression vectors. Gene. 1986;46(1):103–112. doi: 10.1016/0378-1119(86)90172-1. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]